Stimulation Duration in Patients with Early Oocyte Maturation Triggering Criteria Does Not Impact IVF-ICSI Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
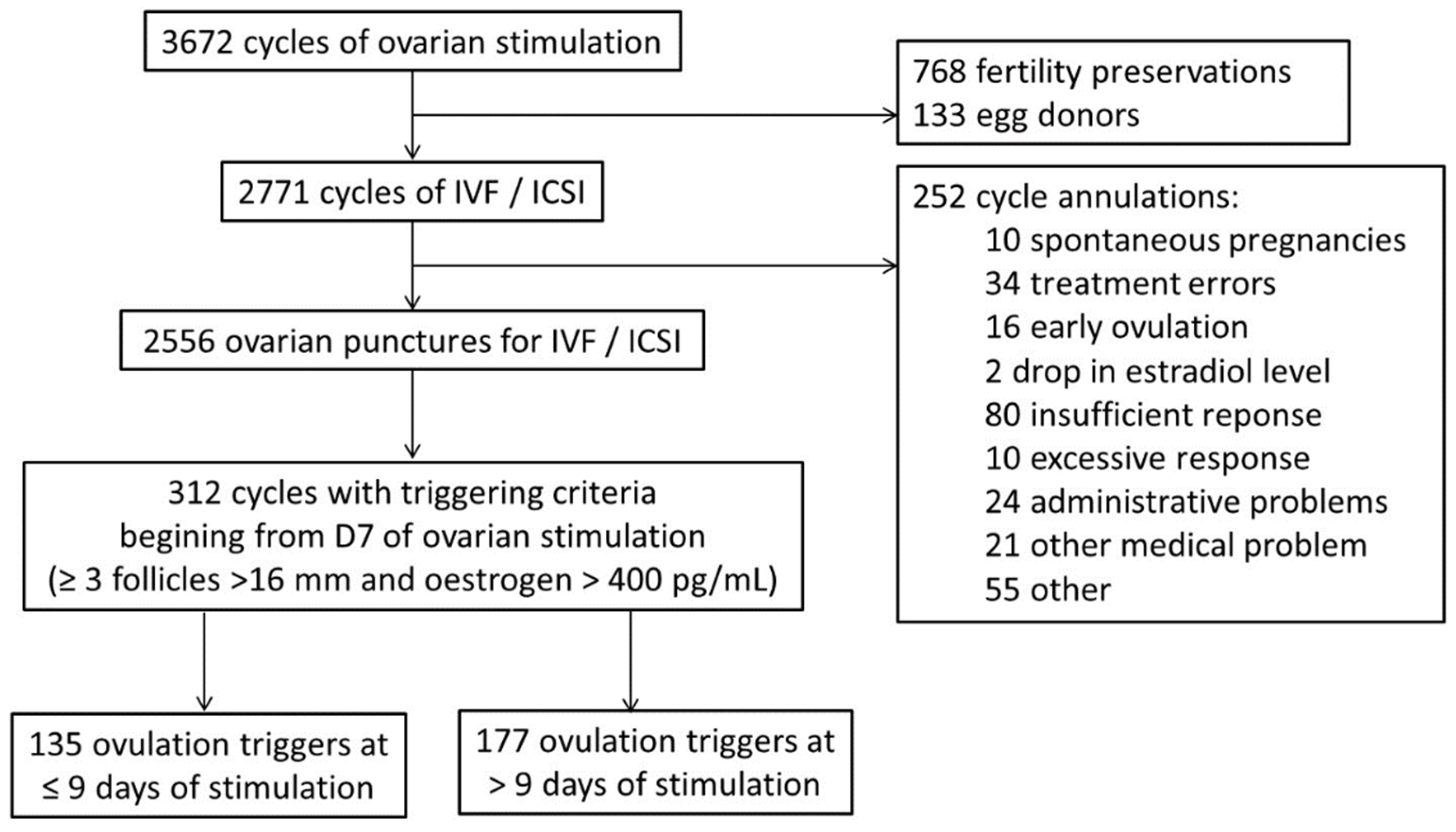
2.2. Procedure
2.3. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. IVF/ICSI Outcomes According to the Day of Ovulation Trigger
3.3. Predictive Factors of Live Births in the D ≤ 9 Group
3.4. Predictive Factors of Live Births in the D > 9 Group
3.5. Patient Characteristics Associated with an Optimal Time for Ovulation Triggering
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Revelli, A.; Martiny, G.; Delle Piane, L.; Benedetto, C.; Rinaudo, P.; Tur-Kaspa, I. A critical review of bi-dimensional and three-dimensional ultrasound techniques to monitor follicle growth: Do they help improving IVF outcome? Reprod. Biol. Endocrinol. 2014, 12, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovarian Stimulation, T.E.G.G.O.; Bosch, E.; Broer, S.; Griesinger, G.; Grynberg, M.; Humaidan, P.; Kolibianakis, E.; Kunicki, M.; La Marca, A.; Lainas, G.; et al. ESHRE guideline: Ovarian stimulation for IVF/ICSI†. Hum. Reprod. Open 2020, 2020, hoaa009. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.R.; Mahutte, N.G.; Arici, A.; Sakkas, D. Impact of duration and dose of gonadotrophins on IVF outcomes. Reprod. Biomed. Online 2006, 13, 645–650. [Google Scholar] [CrossRef]
- Mardešič, T.; Mannaerts, B.; Abuzeid, M.; Levy, M.; Witjes, H.; Fauser, B.C.J.M. Engage investigators short follicular phase of stimulation following corifollitropin alfa or daily recombinant fsh treatment does not compromise clinical outcome: A retrospective analysis of the engage trial. Reprod. Biomed. Online 2014, 28, 462–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, M.; Zapantis, A.; Taylor, M.; Jindal, S.K.; Neal-Perry, G.S.; Lieman, H.J.; Polotsky, A.J. Prolonged gonadotropin stimulation is associated with decreased ART success. J. Assist. Reprod. Genet. 2010, 27, 711–717. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, P.; Ying, L.; Plosker, S.; Mayer, J.; Ying, Y.; Imudia, A.N. Duration of ovarian stimulation is predictive of in-vitro fertilization outcomes. Minerva Ginecol. 2019, 71, 419–426. [Google Scholar] [CrossRef]
- Wang, R.; Lin, S.; Wang, Y.; Qian, W.; Zhou, L. Comparisons of GnRH antagonist protocol versus GnRH agonist long protocol in patients with normal ovarian reserve: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0175985. [Google Scholar] [CrossRef] [Green Version]
- ALPHA Scientists In Reproductive Medicine. ESHRE special interest group embryology istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Reprod. Biomed. Online 2011, 22, 632–646. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-C.; Li, Y.-P.; Pan, S.-P.; Chao, K.-H.; Chang, C.-H.; Yang, J.-H.; Chen, S.-U. The different impact of stimulation duration on oocyte maturation and pregnancy outcome in fresh cycles with gnrh antagonist protocol in poor responders and normal responders. Taiwan. J. Obstet. Gynecol. 2019, 58, 471–476. [Google Scholar] [CrossRef]
- Depalo, R.; Lorusso, F.; Palmisano, M.; Bassi, E.; Totaro, I.; Vacca, M.; Trerotoli, P.; Masciandaro, P.; Selvaggi, L. Follicular growth and oocyte maturation in gnrh agonist and antagonist protocols for in vitro fertilisation and embryo transfer. Gynecol. Endocrinol. 2009, 25, 328–334. [Google Scholar] [CrossRef]
- Yoldemir, T. Does the duration of gonadotropin stimulation affect embryo quality on post-retrieval day 3? Gynecol. Endocrinol. 2011, 27, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Kolibianakis, E.M.; Bourgain, C.; Papanikolaou, E.G.; Camus, M.; Tournaye, H.; Van Steirteghem, A.C.; Devroey, P. Prolongation of follicular phase by delaying HCG administration results in a higher incidence of endometrial advancement on the day of oocyte retrieval in GnRH antagonist cycles. Hum. Reprod. 2005, 20, 2453–2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catherino, W.H.; Leondires, M.; McKeeby, J.; Cruess, D.; Segars, J.H.; Armstrong, A. Prolonged ovarian stimulation in ART cycles does not improve clinical pregnancy rates in poor responders. Fertil. Steril. 2002, 78, S49. [Google Scholar] [CrossRef]
- Tao, T.; Robichaud, A.; Nadeau, S.; Savoie, R.; Gallant, B.; Ouellette, R.J. Optimized hormonal stimulation is critical for production of viable embryos and establishment of subsequent implantation. J. Assist. Reprod. Genet. 2006, 23, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Chen, Q.; Wang, L.; Lu, X.; Lyu, Q.; Wang, Y.; Kuang, Y. Live birth rates in the first complete IVF cycle among 20 687 women using a freeze-all strategy. Hum. Reprod. 2018, 33, 924–929. [Google Scholar] [CrossRef]
- Khader, A.; Lloyd, S.M.; McConnachie, A.; Fleming, R.; Grisendi, V.; La Marca, A.; Nelson, S.M. External validation of anti-müllerian hormone based prediction of live birth in assisted conception. J. Ovarian Res. 2013, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Metello, J.L.; Tomás, C.; Ferreira, P. Can we predict the IVF/ICSI live birth rate? JBRA Assist. Reprod. 2019, 23, 402–407. [Google Scholar] [CrossRef]
- Sunkara, S.K.; Rittenberg, V.; Raine-Fenning, N.; Bhattacharya, S.; Zamora, J.; Coomarasamy, A. Association between the number of eggs and live birth in IVF treatment: An analysis of 400 135 treatment cycles. Hum. Reprod. 2011, 26, 1768–1774. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.; Wan, F.; Huang, K.; Zhang, H. Does the number of oocytes retrieved influence pregnancy after fresh embryo transfer? PLoS ONE 2013, 8, e56189. [Google Scholar] [CrossRef]
- Steward, R.G.; Lan, L.; Shah, A.A.; Yeh, J.S.; Price, T.M.; Goldfarb, J.M.; Muasher, S.J. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: An analysis of 256,381 in vitro fertilization cycles. Fertil. Steril. 2014, 101, 967–973. [Google Scholar] [CrossRef]
- Abuzeid, M.I.; Bolonduro, O.; La Chance, J.; Abozaid, T.; Urich, M.; Ullah, K.; Ali, T.; Ashraf, M.; Khan, I. Cumulative live birth rate and assisted reproduction: Impact of female age and transfer day. Facts Views Vis. Obgyn 2014, 6, 145–149. [Google Scholar] [PubMed]
| D ≤ 9 Group n = 135 (43.3%) | D > 9 Group n = 177 (56.7%) | p-Value | |
|---|---|---|---|
| Age, years; mean (SD) | 34.94 (4.48) | 34.6 (4.22) | 0.49 |
| BMI kg/m2; mean (SD) | 23.95 (3.99) | 24.5 (4.31) | 0.25 |
| Type of infertility | 0.28 | ||
| Primary, n (%) | 92 (68.1) | 108 (61.0) | |
| Secondary, n (%) | 43 (31.9) | 67 (37.9) | |
| Cause of infertility | 0.72 | ||
| Endometriosis, n (%) | 12 (8.9) | 12 (6.8) | |
| DOR, n (%) | 10 (7.4) | 16 (9.0) | |
| Male factor, n (%) | 37 (27.4) | 56 (31.6) | |
| Mix/Other, n (%) | 76 (56.3) | 93 (52.5) | |
| AMH, ng/mL, mean (SD) | 2.94 (2.41) | 3 (2.03) | 0.81 |
| Stimulation protocol | 0.26 | ||
| GnRH-antagonist, n (%) | 83 (61.5) | 119 (67.2) | |
| Long GnRH-agonist, n (%) | 39 (28.9) | 49 (27.7) | |
| Short GnRH-agonist, n (%) | 13 (9.6) | 9 (5.1) | |
| D ≤ 9 Group n = 135 (43.3%) | D > 9 Group n = 177 (56.7%) | p-Value | |
|---|---|---|---|
| E2 level on D8, pg/mL mean (SD) | 1703 (872) | 1434 (692) | 0.004 |
| E2 level > 1000 pg/mL on D8, n (%) | 39 (28.9) | 64 (36.2) | 0.18 |
| >3 follicles of 12–15 mm on D8, n (%) | 73 (54.1) | 142 (80.2) | 0.27 |
| Number of oocytes retrieved, mean (SD) | 10.8 (6.3) | 13.5 (7.4) | 0.001 |
| Number of mature oocytes, mean (SD) | 7.3 (3.7) | 10.3 (5.0) | <0.001 |
| Number of transferable embryos, mean (SD) | 1.9 (1.6) | 3.0 (2.7) | <0.001 |
| Number of frozen embryos, mean (SD) | 0.9 (1.5) | 2.0 (2.8) | <0.001 |
| Fresh transfers with blastocyst, n/total fresh embryo transfers (%) | 52/93 (56) | 68/120 (57) | 1 |
| Frozen transfers with blastocyst, n/total frozen embryo transfers (%) | 44/50 (88) | 82/105 (78) | 0.14 |
| Number of clinical pregnancies after fresh and frozen embryo transfers, n (%) | 50 (37.0) | 83 (46.9) | 0.10 |
| Number of live births after fresh and frozen embryo transfers, n (%) | 26 (19.3) | 50 (28.2) | 0.09 |
| D ≤ 9 without Live Birth n = 109 | D ≤ 9 with Live Birth n = 26 | p-Value | |
|---|---|---|---|
| Age, years; mean (SD) | 35.0 (4.6) | 34.6 (4.1) | 0.68 |
| BMI, kg/m2; mean (SD) | 23.96 (3.94) | 23.87 (4.3) | 0.13 |
| Type of infertility | 0.64 | ||
| Primary, n (%) | 73 (67.0) | 19 (73.1) | |
| Secondary, n (%) | 36 (33.0) | 7 (26.9) | |
| Cause of infertility | 0.55 | ||
| Endometriosis, n (%) | 10 (9.2) | 2 (7.7) | |
| DOR, n (%) | 8 (7.3) | 2 (7.7) | |
| Male factor, n (%) | 27 (24.8) | 10 (38.5) | |
| Mix/Other, n (%) | 64 (58.7) | 12 (46.2) | |
| AMH ng/mL, mean (SD) | 2.96 (2.56) | 2.87 (1.73) | 0.82 |
| Low AMH level a, n (%) | 8 (7.3) | 1 (3.8) | 1.00 |
| E2 level on D8, pg/mL mean (SD) | 1669 (855) | 1848 (943) | 0.35 |
| E2 level < 1000 pg/mL on D8, n (%) | 31 (28.4) | 8 (30.8) | 0.81 |
| Number of retrieved oocytes, mean (SD) | 10.2 (6.2) | 13.2 (6.4) | 0.03 |
| Number of mature oocytes, mean (SD) | 6.8 (4.3) | 9.4 (4.3) | 0.03 |
| Number of obtained embryos, mean (SD) | 5.0 (3.8) | 7.7 (4.9) | 0.01 |
| D > 9 without Live Birth n = 127 | D > 9 with Live Birth n = 50 | p-Value | |
|---|---|---|---|
| Age, years; mean (SD) | 35.2 (4.2) | 33.0 (4.0) | 0.001 |
| BMI, kg/m2; mean (SD) | 24.9 (4.6) | 23.5 (3.4) | 0.03 |
| Type of infertility | 0.73 | ||
| Primary, n (%) | 76 (60.8) | 32 (64) | |
| Secondary, n (%) | 49 (39.2) | 18 (36) | |
| Cause of infertility | 0.06 | ||
| Endometriosis, n (%) | 6 (4.72) | 6 (12) | |
| DOR, n (%) | 14 (11.0) | 2 (4) | |
| Male factor, n (%) | 36 (28.3) | 20 (40) | |
| Mix/Other, n (%) | 71 (55.9) | 22 (44) | |
| AMH ng/mL, mean (SD) | 2.95 (2.14) | 3.13 (1.74) | 0.60 |
| Low AMH level a, n (%) | 4 (3.1) | 2 (4.0) | 0.67 |
| E2 level on D8, pg/mL mean (SD) | 1487 (688) | 1302 (693) | 0.11 |
| E2 level < 1000 pg/mL on D8, n (%) | 40 (31.5) | 24 (48.0) | 0.055 |
| Number of oocytes retrieved, mean (SD) | 12.8 (7.6) | 15.2 (6.6) | 0.055 |
| Number of mature oocytes, mean (SD) | 9.7 (5.2) | 11.8 (9.7) | 0.04 |
| Number of embryos obtained, mean (SD) | 8.2 (5.8) | 9.7 (4.8) | 0.07 |
| D ≤ 9 Group with Live Birth n = 26 | D > 9 Group with Live Birth n = 50 | p-Value | |
|---|---|---|---|
| Age, mean (SD) | 34.6 (4.1) | 33.0 (4.0) | 0.1 |
| BMI, mean (SD) | 23.87 (4.30) | 23.49 (3.35) | 0.68 |
| Type of infertility | 0.45 | ||
| Primary, n (%) | 19 (73.1) | 32 (64) | |
| Secondary, n (%) | 7 (26.9) | 18 (36) | |
| Cause of infertility | 0.86 | ||
| Endometriosis, n (%) | 2 (7.7) | 6 (12) | |
| IOP, n (%) | 2 (7.7) | 2 (4) | |
| Masculine, n (%) | 10 (38.5) | 20 (40) | |
| Mix/Other, n (%) | 12 (46.2) | 22 (44) | |
| AMH ng/mL, mean (SD) | 2.87 (1.73) | 3.13 (1.74) | 0.53 |
| Low AMH level a, n (%) | 1 (3.8) | 2 (4.0) | 1.00 |
| E2 level on D8, pg/mL mean (SD) | 1848 (943) | 1302 (693) | 0.01 |
| E2 level < 1000 pg/mL on D8, n (%) | 8 (30.8) | 24 (48.0) | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stout, S.; Dabi, Y.; Dupont, C.; Selleret, L.; Touboul, C.; Chabbert-Buffet, N.; Daraï, E.; Mathieu d’Argent, E.; Kolanska, K. Stimulation Duration in Patients with Early Oocyte Maturation Triggering Criteria Does Not Impact IVF-ICSI Outcomes. J. Clin. Med. 2022, 11, 2330. https://doi.org/10.3390/jcm11092330
Stout S, Dabi Y, Dupont C, Selleret L, Touboul C, Chabbert-Buffet N, Daraï E, Mathieu d’Argent E, Kolanska K. Stimulation Duration in Patients with Early Oocyte Maturation Triggering Criteria Does Not Impact IVF-ICSI Outcomes. Journal of Clinical Medicine. 2022; 11(9):2330. https://doi.org/10.3390/jcm11092330
Chicago/Turabian StyleStout, Sophie, Yohann Dabi, Charlotte Dupont, Lise Selleret, Cyril Touboul, Nathalie Chabbert-Buffet, Emile Daraï, Emmanuelle Mathieu d’Argent, and Kamila Kolanska. 2022. "Stimulation Duration in Patients with Early Oocyte Maturation Triggering Criteria Does Not Impact IVF-ICSI Outcomes" Journal of Clinical Medicine 11, no. 9: 2330. https://doi.org/10.3390/jcm11092330





