Impact of Whole Body Vibration and Zoledronic Acid on Femoral Structure after Ovariectomy: Morphological Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Method of Ovariectomy in the Rats
2.3. Whole-Body Vibration (WBV)
2.4. Bone Preparation, PSR Staining, and Histomorphometry
2.5. Immunohistochemical Staining
2.6. Statistical Analysis
3. Results
3.1. Histomorphometry
3.2. Collagen 1 Immunoreaction in Femoral Bone
3.3. Osteoprotegerin (OPG) Immunoreaction in Femoral Bone
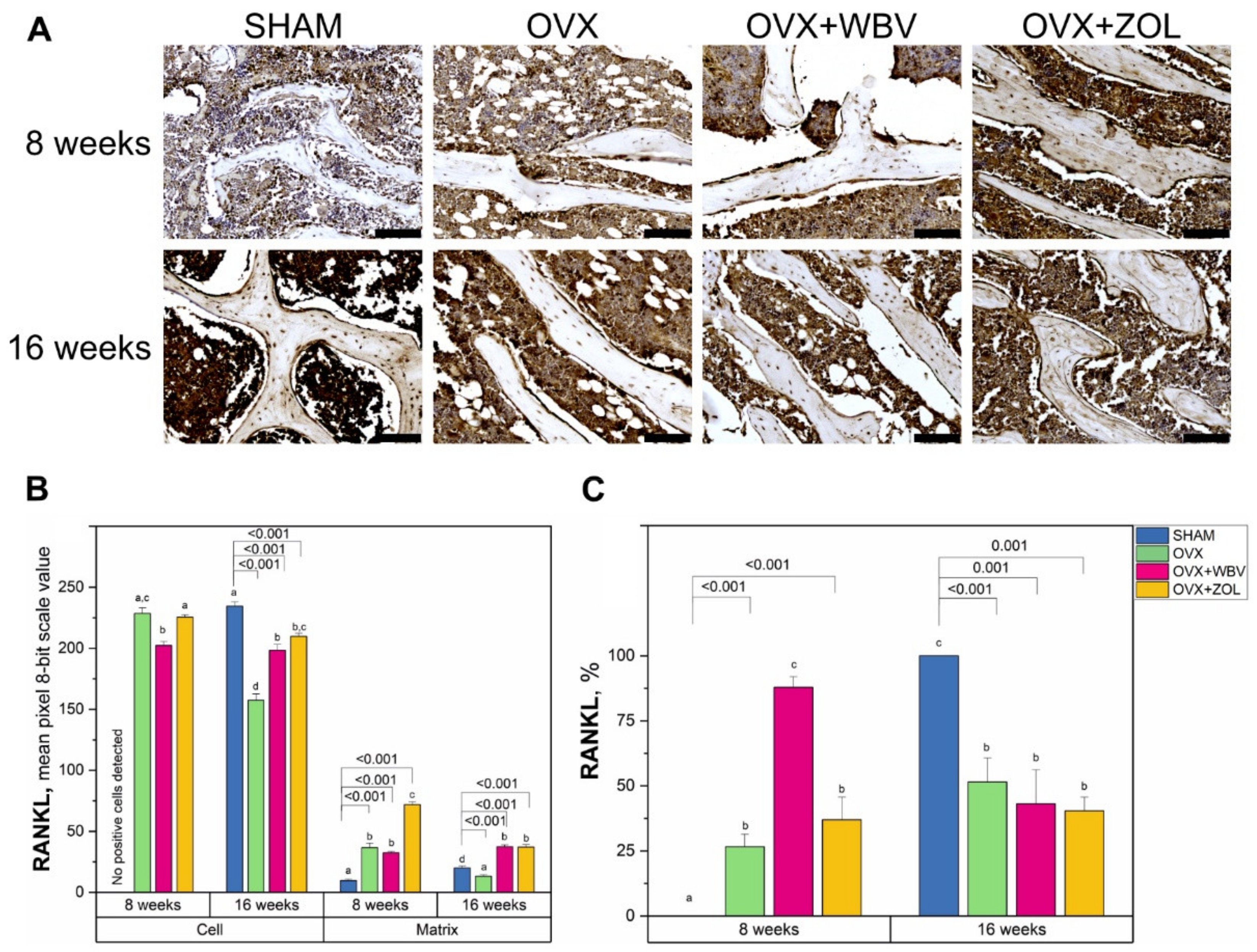
3.4. Receptor Activator of Nuclear Factor Kappa-Β Ligand (RANKL) Immunoreaction in Femoral Bone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akkawi, I.; Zmerly, H. Osteoporosis: Current concepts. Joints 2018, 6, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, C.; Melton, L.J., 3rd. Epidemiology of osteoporosis. Trends Endocrinol. Metab. 1992, 3, 224–229. [Google Scholar] [CrossRef]
- Khosla, S.; Monroe, D.G. Regulation of Bone Metabolism by Sex Steroids. Cold Spring Harb. Perspect. Med. 2018, 8, a031211. [Google Scholar] [CrossRef] [Green Version]
- Singh-Ospina, N.; Maraka, S.; Rodriguez-Gutierrez, R.; Davidge-Pitts, C.; Nippoldt, T.B.; Prokop, L.J.; Murad, M.H. Effect of sex steroids on the bone health of transgender individuals: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2017, 102, 3904–3913. [Google Scholar] [CrossRef] [Green Version]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Tadano, S.; Giri, B. X-ray diffraction as a promising tool to characterize bone nanocomposites. Sci. Technol. Adv. Mater. 2012, 12, 064708. [Google Scholar] [CrossRef]
- Hart, N.; Newton, R.; Tan, J.; Rantalainen, T.; Chivers, P.; Siafarikas, A.; Nimphius, S. Biological basis of bone strength: Anatomy, physiology and measurement. J. Musculoskelet. Neuronal Interact. 2020, 20, 347–371. [Google Scholar] [PubMed]
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar]
- Eastell, R.; O’Neill, T.W.; Hofbauer, L.C.; Langdahl, B.; Reid, I.R.; Gold, D.T.; Cummings, S.R. Postmenopausal Osteoporosis. Nat. Rev. Dis. Primers 2016, 2, 16069. [Google Scholar] [CrossRef]
- Alswat, K.A. Gender disparities in osteoporosis. J. Clin. Med. Res. 2017, 9, 382–387. [Google Scholar] [CrossRef] [Green Version]
- Langdahl, B.L. Osteoporosis in premenopausal women. Curr. Opin. Rheumatol. 2017, 29, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Hammad, L.F.; Benajiba, N. Lifestyle factors influencing bone health in young adult women in Saudi Arabia. Afr. Health Sci. 2017, 17, 524–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiyasatkulkovit, W.; Promruk, W.; Rojviriya, C.; Pakawanit, P.; Chaimongkolnukul, K.; Kengkoom, K.; Teerapornpuntakit, J.; Panupinthu, N.; Charoenphandhu, N. Impairment of bone microstructure and upregulation of osteoclastogenic markers in spontaneously hypertensive rats. Sci. Rep. 2019, 9, 12293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudyk, H.; Tomaszewska, E.; Kotsyumbas, I.; Muszyński, S.; Tomczyk-Warunek, A.; Szymańczyk, S.; Dobrowolski, P.; Wiącek, D.; Kamiński, D.; Brezvyn, O. Bone homeostasis in experimental fumonisins intoxication of rats. Ann. Anim. Sci. 2019, 19, 403–419. [Google Scholar] [CrossRef] [Green Version]
- Moe, S.M.; Chen, N.X.; Newman, C.L.; Gattone, V.H., 2nd; Organ, J.M.; Chen, X.; Allen, M.R. A comparison of calcium to zoledronic acid for improvement of cortical bone in an animal model of CKD. J. Bone Miner. Res. 2014, 29, 902–910. [Google Scholar] [CrossRef] [Green Version]
- Khajuria, D.K.; Vasireddi, R.; Trebbin, M.; Karasik, D.; Razdan, R. Novel therapeutic intervention for osteoporosis prepared with strontium hydroxyapatite and zoledronic acid: In vitro and pharmacodynamic evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 698–708. [Google Scholar] [CrossRef]
- Olçar, H.A.; Halıcı, M.; Kafadar, İ.H.; Karaman, İ.; Lekesizcan, A.; Gönen, Z.B. Can injection of adipose stem cells to non-union zone increase bone union? Experimental rat study. Jt. Dis. Relat. Surg. 2020, 31, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Pócs, L.; Janovszky, Á.; Ocsovszki, I.; Kaszaki, J.; Piffkó, J.; Szabó, A. Microcirculatory consequences of limb ischemia/reperfusion in ovariectomized rats treated with zoledronic acid. J. Orthop. Surg. Res. 2019, 14, 95. [Google Scholar] [CrossRef]
- Schanda, J.E.; Keibl, C.; Heimel, P.; Monforte, X.; Tangl, S.; Feichtinger, X.; Teuschl, A.; Baierl, A.; Muschitz, C.; Redl, H.; et al. Zoledronic acid substantially improves bone microarchitecture and biomechanical properties after rotator cuff repair in a rodent chronic defect model. Am. J. Sports Med. 2020, 48, 2151–2160. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, W.; Wang, Y.; Zhao, Z.; Zhao, C.; Wang, S.; Gao, C. Prior administration of vitamin K2 improves the therapeutic effects of zoledronic acid in ovariectomized rats by antagonizing zoledronic acid-induced inhibition of osteoblasts proliferation and mineralization. PLoS ONE 2018, 13, e0202269. [Google Scholar] [CrossRef]
- Cunha, V.V.; Silva, P.B.; Lemos, J.M.; Martins, J.L.; Freitas, M.O.; Avelar, R.L. Evaluation of a collagen matrix in a mandible defect in rats submitted to the use of bisphosphonates. Acta Cir. Bras. 2020, 35, e202001005. [Google Scholar] [CrossRef] [PubMed]
- Raina, D.B.; Matuszewski, L.M.; Vater, C.; Bolte, J.; Isaksson, H.; Lidgren, L.; Tägil, M.; Zwingenberger, S. A facile one-stage treatment of critical bone defects using a calcium sulfate/hydroxyapatite biomaterial providing spatiotemporal delivery of bone morphogenic protein-2 and zoledronic acid. Sci. Adv. 2020, 6, eabc1779. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D.; Pannacciulli, N.; Brown, J.P.; Czerwinski, E.; Nedergaard, B.S.; Bolognese, M.A.; Malouf, J.; Bone, H.G.; Reginster, J.Y.; Singer, A.; et al. Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J. Clin. Endocrinol. Metab. 2016, 101, 3163–3170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhillon, S. Zoledronic acid (Reclast®, Aclasta®): A review in osteoporosis. Drugs 2016, 76, 1683–1697. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.L.; Frederico, É.H.; Guimarães, C.A.; Guedes-Aguiar, E.O.; Moreira-Marconi, E.; Paineiras-Domingos, L.L.; Sá-Caputo, D.C.; Bernardo-Filho, M.; Asad, N.R. Long-term effects of mechanical vibration stimulus on the bone formation of Wistar rats: An assessment method based on X-rays images. Acad. Radiol. 2021, 28, e240–e245. [Google Scholar] [CrossRef]
- Ferreira, G.Z.; Zen Filho, E.V.; Rubira-Bullen, I.R.F.; Garlet, G.P.; Santos, C.F.; da Silva Santos, P.S. Delayed alveolar bone repair and osteonecrosis associated with Zoledronic acid therapy in rats: Macroscopic, microscopic and molecular analysis. J. Appl. Oral Sci. 2020, 28, e20200204. [Google Scholar] [CrossRef]
- Skiba, G.; Raj, S.; Sobol, M.; Kowalczyk, P.; Grela, E.R. Role of polyphenols in the metabolism of the skeletal system in humans and animals—A review. Ann. Anim. Sci. 2021, 21, 1275–1300. [Google Scholar] [CrossRef]
- Kostyshy, N.; Kulyk, Y.; Kostyshyn, L.; Gzhegotskyi, M. Metabolic and structural response of bone to whole-body vibration in obesity and sedentary rat models for osteopenia. Rom. J. Diabetes Nutr. Metab. Dis. 2020, 27, 200–208. [Google Scholar]
- Pang, M.Y.; Lau, R.W.; Yip, S.P. The effects of whole-body vibration therapy on bone turnover, muscle strength, motor function, and spasticity in chronic stroke: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2013, 49, 439–450. [Google Scholar]
- Minematsu, A.; Nishii, Y.; Imagita, H.; Sakata, S. Whole body vibration at low-frequency can increase trabecular thickness and width in adult rats. J. Musculoskelet. Neuronal Interact. 2019, 19, 169–177. [Google Scholar]
- McGee-Lawrence, M.E.; Wenger, K.H.; Misra, S.; Davis, C.L.; Pollock, N.K.; Elsalanty, M.; Ding, K.; Isales, C.M.; Hamrick, M.W.; Wosiski-Kuhn, M.; et al. Whole-body vibration mimics the metabolic effects of exercise in male leptin receptor–deficient mice. Endocrinology 2017, 158, 1160–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.C.; Tseng, T.L.; Huang, W.C.; Chung, Y.H.; Cguang, H.L.; Wu, J.H. Whole-body vibration training effect on physical performance and obesity in mice. Int. J. Med. Sci. 2014, 11, 1218–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellia, A.; Sallì, M.; Lombardo, M.; D’Adamo, M.; Guglielmi, V.; Tirabasso, C.; Giordani, L.; Federici, M.; Lauro, D.; Foti, C.; et al. Effects of whole body vibration plus diet on insulin-resistance in middle-aged obese subjects. Int. J. Sports Med. 2014, 35, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Maddalozzo, G.F.; Iwaniec, U.T.; Turner, R.T.; Rosen, C.J.; Widrick, J.J. Whole-body vibration slows the acquisition of fat in mature female rats. Int. J. Obes. 2008, 32, 1348–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 2631-1:1997; Mechanical Vibration and Shock—Evaluation of Human Exposure to Whole-Body Vibration—Part 1: General Requirements. International Organization for Standardization (ISO): Geneva, Switzerland, 1997. Available online: https://www.iso.org/standard/7612.html (accessed on 7 March 2022).
- ISO 2631-5:2018; Mechanical Vibration and Shock—Evaluation of Human Exposure to Whole-Body Vibration—Part 5: Method for Evaluation of Vibration Containing Multiple Shocks. International Organization for Standardization (ISO): Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/50905.html (accessed on 7 March 2022).
- Singh, I.; Nigam, S.P.; Saran, V.H. Effect of backrest inclination on sitting subjects exposed to WBV. Proc. Technol. 2016, 23, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Paschold, H.W.; Mayton, A.G. Whole-body vibration. Prof. Saf. 2011, 56, 30–35. [Google Scholar]
- Prisby, R.D.; Lafage-Proust, M.H.; Malaval, L.; Belli, A.; Vico, L. Effects of whole body vibration on the skeleton and other organ systems in man and animal models: What we know and what we need to know. Ageing Res. Rev. 2008, 7, 319–329. [Google Scholar] [CrossRef]
- Pasqualini, M.; Lavet, C.; Elbadaoui, M.; Vanden-Bossche, A.; Laroche, N.; Gnyubkin, V.; Vico, L. Skeletal site-specific effects of whole body vibration in mature rats: From deleterious to beneficial frequency-dependent effects. Bone 2013, 55, 69–77. [Google Scholar] [CrossRef]
- Xie, P.; Tang, Z.; Qing, F.; Chen, X.; Zhu, X.; Fan, Y.; Yang, X.; Zhang, X. Bone mineral density, microarchitectural and mechanical alterations of osteoporotic rat bone under long-term whole-body vibration therapy. J. Mech. Behav. Biomed. Mater. 2016, 53, 341–349. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Chen, C.S. Mechanotransduction in development: A growing role for contractility. Nat. Rev. Mol. Cell Biol. 2009, 10, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Gao, B.; Fan, Y.; Liu, Y.; Feng, S.; Cong, Q.; Zhang, X.; Zhou, Y.; Yadav, P.S.; Lin, J.; et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ss-catenin. Elife 2020, 9, e52779. [Google Scholar] [CrossRef]
- Chen, X.; Wanggou, S.; Bodalia, A.; Zhu, M.; Dong, W.; Fan, J.J.; Yin, W.C.; Min, H.K.; Hu, M.; Draghici, D.; et al. A feedforward mechanism mediated by mechanosensitive ion channel PIEZO1 and tissue mechanics promotes glioma aggression. Neuron 2018, 100, 799–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrickx, G.; Fischer, V.; Liedert, A.; von Kroge, S.; Haffner-Luntzer, M.; Brylka, L.; Pawlus, E.; Schweizer, M.; Yorgan, T.; Baranowsky, A.; et al. Piezo1 inactivation in chondrocytes impairs trabecular bone formation. J. Bone Miner. Res. 2021, 36, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-Z.; Zhou, T.; Xu, J.-Q.; Wang, Y.-X.; Sun, M.-M.; He, Y.-J.; Pan, S.-W.; Xiong, W.; Peng, Z.-K.; Gao, X.-H.; et al. Structure, kinetic properties and biological function of mechanosensitive Piezo channels. Cell Biosci. 2021, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Kostyshyn, N.; Grzegotsky, M.; Servetnyk, M. Assessment of structural and functional condition of rats bone tissue under the influence of various parameters of vibration. Curr. Issues Pharm. Med. Sci. 2018, 31, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Rubin, C.T.; Mcleod, K.J. Promotion of bony ingrowth by frequency-specific, low-amplitude mechanical strain. Clin. Orthop. Relat. Res. 1994, 298, 165–174. [Google Scholar] [CrossRef]
- Rubin, C.T.; Lanyon, L.E. Regulation of bone formation by applied dynamic loads. J. Bone Jt. Surg. Am. 1984, 66, 397–402. [Google Scholar] [CrossRef]
- Rubin, C.T.; Lanyon, L.E. Regulation of bone mass by mechanical strain magnitude. Calcif. Tissue Int. 1985, 37, 411–417. [Google Scholar] [CrossRef]
- Severs, W.B.; Keil, L.C.; Klase, P.A.; Deen, K.C. Urethane anesthesia in rats. Pharmacology 1981, 22, 209–226. [Google Scholar] [CrossRef]
- Xiao, Y. Structural, Mechanical and Viscoelastic Changes in Ovariectomized Rat upon Drug Treatment and Vibration Therapy. Ph.D. Thesis, National University of Singapore, Singapore, 2012. [Google Scholar]
- Flieger, J.; Karachalios, T.; Khaldi, L.; Raptoou, P.; Lyritis, G. Mechanical stimulation in the form of vibration prevents postmenopausal bone loss in ovariectomized rats. Calcif. Tissue Int. 1998, 63, 510–514. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Puzio, I.; Donaldson, J.; Muszyński, S. Acrylamide-induced prenatal programming of bone structure in mammal model. Ann. Anim. Sci. 2020, 20, 1257–1287. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hułas-Stasiak, M.; Jakubowicz-Gil, J.; Dobrowolski, P.; Grzesiak, M.; Muszyński, S.; Świątkiewicz, M.; Tomaszewska, E. Regulation of folliculogenesis by growth factors in piglet ovary exposed prenatally to β-hydroxy-β-methylbutyrate (HMB). Ann. Anim. Sci. 2020, 20, 899–917. [Google Scholar] [CrossRef]
- Festing, M.F.W. On determining sample size in experiments involving laboratory animals. Lab. Anim. 2018, 52, 341–350. [Google Scholar] [CrossRef]
- Rocabado, J.M.R.; Kaku, M.; Nozaki, K.; Ida, T.; Kitami, M.; Aoyagi, Y.; Uoshima, K. A multi-factorial analysis of bone morphology and fracture strength of rat femur in response to ovariectomy. J. Orthop. Surg. Res. 2018, 13, 318. [Google Scholar] [CrossRef]
- Bagi, C.M.; Wilkie, D.; Georgelos, K.; Williams, D.; Bertolini, D. Morphological and structural characteristics of the proximal femur in human and rat. Bone 1997, 21, 261–267. [Google Scholar] [CrossRef]
- Singh, I.J.; Gunberg, D.L. Quantitative histology of changes with age in rat bone cortex. J. Morphol. 1971, 133, 241–251. [Google Scholar] [CrossRef]
- Liu, X.L.; Li, C.L.; Lu, W.W.; Cai, W.X.; Zheng, L.W. Skeletal site-specific response to ovariectomy in a rat model: Change in bone density and microarchitecture. Clin. Oral Implant. Res. 2015, 26, 392–398. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, K.H.; Lee, Y.M.; Ku, Y.; He, S.J.; Rhyu, I.C.; Seol, Y.J. Ovariectomy and timing of impaired maxillary alveolar bone regeneration: An experimental study in rats. J. Periodontol. 2020, 91, 1357–1366. [Google Scholar] [CrossRef]
- Kakihata, C.M.M.; Peretti, A.L.; Tavares, A.L.F.; Wutzke, M.L.S.; de Ribeiro, L.F.C.; Costa, R.M.; Bertolini, G.R. Morphometric effects of whole-body vibration on the bone in a rat model of postmenopausal osteoporosis. J. Manip. Physiol. Ther. 2020, 43, 551–557. [Google Scholar] [CrossRef]
- Hashimoto, K.; Matsumoto, T.; Shimizu, R. Effects of rest insertion combined with whole-body vibration on bone healing in ovariectomized mice. Trans. Jpn. Soc. Med. Biol. Eng. 2018, 58, s373. [Google Scholar]
- Runge, W.O.; Ruppert, D.S.; Marcellin-Little, D.J.; Dahners, L.E.; Harrysson, O.L.; Weinhold, P.S. Bone changes after short-term whole body vibration are confined to cancellous bone. J. Musculoskelet. Neuronal Interact. 2018, 18, 485–492. [Google Scholar] [PubMed]
- Walsh, M.C.; Choi, Y. Biology of the RANKL–RANK–OPG system in immunity, bone, and beyond. Front. Immunol. 2014, 5, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyce, B.F.; Xing, L. The RANKL/RANK/OPG pathway. Curr. Osteoporos. Rep. 2007, 5, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, S.; Ishii, K.; Amizuka, N.; Li, M.; Kobayashi, T.; Kohno, K.; Ito, M.; Takeshita, S.; Ikeda, K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007, 5, 464–475. [Google Scholar] [CrossRef] [Green Version]
- Chesnut, C.; Majumdar, S.; Gardner, J.; Shields, A.; Newitt, D.C.; Erickson, E.; Glott, M.; Kriegman, A.; Mindeholm, L. Assessment of bone quality, quantity, and turnover with multiple methodologies at multiple skeletal sites. In Noninvasive Assessment of Trabecular Bone Architecture and the Competence of Bone; Majumdar, S., Bay, B.K., Eds.; Springer: Boston, MA, USA, 2001; pp. 95–97. [Google Scholar]
- Khosla, S. Minireview: The OPG/RANKL/RANK system. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef]
- Azizieh, F.Y.; Shehab, D.; Jaralleh, K.A.; Gupta, R.; Raghupathy, R. Circulatory levels of RANKL, OPG, and oxidative stress markers in postmenopausal women with normal or low bone mineral density. Biomark. Insights 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG pathway: A mechanism involved in exercise-induced bone remodeling. Biomed. Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Han, L.; Nookaew, I.; Mannen, E.; Silva, M.J.; Almeida, M.; Xiong, J. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife 2019, 8, e49631. [Google Scholar] [CrossRef]
- Oxlund, B.S.; Ørtoft, G.; Andreassen, T.T.; Oxlund, H. Low-intensity, high-frequency vibration appears to prevent the decrease in strength of the femur and tibia associated with ovariectomy of adult rats. Bone 2003, 32, 69–77. [Google Scholar] [CrossRef]
- Sehmisch, S.; Galal, R.; Kolios, L.; Tezval, M.; Dullin, C.; Zimmer, S.; Stuermer, K.M.; Stuermer, E.K. Effects of low-magnitude, high-frequency mechanical stimulation in the rat osteopenia model. Osteoporos. Int. 2009, 20, 1999–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komarova, M.; Sehmisch, S.; Tezval, M.; Ammon, J.; Lieberwirth, P.; Sauerhoff, C.; Trautmann, L.; Wicke, M.; Dullin, C.; Stuermer, K.M.; et al. Identification of a vibration regime favorable for bone healing and muscle in estrogen-deficient rats. Calcif. Tissue Int. 2013, 92, 509–520. [Google Scholar]
- Dionello, C.F.; Sá-Caputo, D.; Pereira, H.V.F.S.; Souca-Gonçalves, C.R.; Maiworm, A.I.; Morel, D.S.; Moreira-Marconi, E.; Paineiras-Domingos, L.L.; Bemben, D.; Bernardo-Filho, M. Effects of whole body vibration exercises on bone mineral density of women with postmenopausal osteoporosis without medications: Novel findings and literature review. J. Musculoskelet. Neuronal. Interact. 2016, 16, 193–203. [Google Scholar] [PubMed]




| Factor | BV/TV, % | Tb.Th, mm | Tb.Thmax, mm | Tb.Sp, mm | Tb.Spmax, mm | Tb.N, 1/mm | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Treatment | Mean ± SE | Dunnett | Mean ± SE | Dunnett | Mean ± SE | Dunnett | Mean ± SE | Dunnett | Mean ± SE | Dunnett | Mean ± SE | Dunnett |
| 8 weeks | SHAM | 30.5 ± 2.4 abc | - | 28.5 ± 3.4 a | - | 51.4 ± 4.5 a | - | 56.0 ± 3.9 a | - | 89.6 ± 3.9 | - | 11.0 ± 0.8 | - |
| OVX | 29.4 ± 1.2 bc | 0.995 | 27.6 ± 1.1 a | 0.999 | 56.5 ± 6.8 a | 0.997 | 65.3 ± 6.7 ab | 0.908 | 98.0 ± 8.5 | 0.945 | 10.7 ± 0.6 | 0.999 | |
| OVX + WBV | 24.4 ± 1.2 c | 0.032 | 31.7 ± 2.8 ab | 0.931 | 56.0 ± 12.6 a | 0.999 | 85.0 ± 7.6 b | 0.004 | 118.8 ± 9.34 | 0.043 | 7.9 ± 0.5 | 0.021 | |
| OVX + ZOL | 35.3 ± 1.6 bc | 0.147 | 43.8 ± 3.2 bc | 0.002 | 77.5 ± 9.1 b | 0.023 | 62.4 ± 3.7 ab | 0.996 | 92.7 ± 7.2 | 0.999 | 8.2 ± 0.6 | 0.049 | |
| 16 weeks | SHAM | 36.9 ± 1.5 a | - | 47.3 ± 2.9 c | - | 86.7 ± 11.4 b | - | 72.1 ± 3.3 ab | - | 108.1 ± 6.0 | - | 7.9 ± 0.3 | - |
| OVX | 35.0 ± 1.3 ab | 0.907 | 43.8 ± 4.0 bc | 0.904 | 58.7 ± 0.9 a | 0.049 | 60.4 ± 3.8 a | 0.880 | 91.3 ± 2.9 | 0.441 | 8.4 ± 1.1 | 0.992 | |
| OVX + WBV | 28.8 ± 1.0 bc | 0.003 | 35.6 ± 1.1 abc | 0.024 | 60.0 ± 4.9 a | 0.051 | 77.8 ± 6.1 ab | 0.317 | 104.9 ± 9.3 | 0.999 | 8.1 ± 0.4 | 0.999 | |
| OVX + ZOL | 26.6 ± 1.0 c | <0.001 | 28.5 ± 1.9 a | <0.001 | 58.2 ± 6.5 a | 0.048 | 58.7 ± 2.8 a | 0.611 | 85.7 ± 9.0 | 0.177 | 9.7 ± 1.1 | 0.335 | |
| ANOVA p-value | |||||||||||||
| Main factors | |||||||||||||
| Time | <0.001 | 0.495 | 0.021 | <0.001 | 0.056 | 0.128 | |||||||
| Treatment | 0.075 | 0.004 | 0.332 | 0.846 | 0.664 | 0.068 | |||||||
| Interaction | |||||||||||||
| Time × Treatment | <0.001 | <0.001 | 0.054 | 0.178 | 0.153 | 0.088 | |||||||
| Factor | BV/TV, % | Tb.Th, mm | Tb.Thmax, mm | Tb.Sp, mm | Tb.Spmax, mm | Tb.N, 1/mm | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Treatment | Mean ± SE | Dunnett | Mean ± SE | Dunnett | Mean ± SE | Dunnett | Mean ± SE | Dunnett | Mean ± SE | Dunnett | Mean ± SE | Dunnett |
| 8 weeks | SHAM | 36.0 ± 3.3 ab | - | 25.6 ± 2.5 a | - | 52.7 ± 7.4 ab | - | 46.8 ± 3.9 a | - | 80.6 ± 6.8 a | - | 14.1 ± 0.4 a | - |
| OVX | 26.1 ± 1.8 ac | 0.041 | 25.1 ± 3.4 a | 0.999 | 43.8 ± 6.2 a | 0.865 | 63.3 ± 7.0 a | 0.152 | 95.0 ± 10.0 ab | 0.482 | 10.9 ± 1.0 abc | 0.041 | |
| OVX + WBV | 24.4 ± 0.8 c | 0.013 | 26.6 ± 1.3 a | 0.999 | 49.0 ± 3.1 ab | 0.999 | 75.2 ± 5.3 b | 0.003 | 98.8 ± 3.6 ab | 0.251 | 9.3 ± 0.5 bc | 0.001 | |
| OVX + ZOL | 28.4 ± 0.9 ac | 0.049 | 27.4 ± 0.9 a | 0.992 | 51.3 ± 4.8 ab | 0.999 | 56.5 ± 2.2 a | 0.644 | 87.2 ± 3.0 a | 0.969 | 10.4 ± 0.5 abc | 0.015 | |
| 16 weeks | SHAM | 43.4 ± 2.7 b | - | 44.2 ± 3.7 b | - | 75.3 ± 10.0 b | - | 53.5 ± 2.5 a | - | 82.3 ± 4.8 a | - | 10.0 ± 0.5 bc | - |
| OVX | 33.1 ± 3.9 abc | 0.031 | 33.2 ± 1.4 a | 0.012 | 60.1 ± 5.5 ab | 0.390 | 64.7 ± 6.3 a | 0.506 | 100.8 ± 5.8 ab | 0.235 | 10.2 ± 1.5 bc | 0.999 | |
| OVX + WBV | 23.9 ± 1.4 c | <0.001 | 30.3 ± 2.3 a | 0.001 | 52.7 ± 4.2 ab | 0.082 | 89.8 ± 6.8 b | <0.001 | 118.0 ± 8.3 b | 0.003 | 8.0 ± 0.5 c | 0.401 | |
| OVX + ZOL | 33.7 ± 3.2 abc | 0.048 | 26.4 ± 1.7 a | <0.001 | 60.8 ± 7.9 ab | 0.440 | 50.0 ± 5.6 a | 0.997 | 73.8 ± 6.9 a | 0.898 | 12.8 ± 1.0 ab | 0.106 | |
| ANOVA p-value | |||||||||||||
| Main factors | |||||||||||||
| Time | <0.001 | 0.010 | 0.171 | <0.001 | <0.001 | 0.001 | |||||||
| Treatment | 0.009 | <0.001 | 0.006 | 0.282 | 0.476 | 0.113 | |||||||
| Interaction | |||||||||||||
| Time × Treatment | 0.369 | 0.001 | 0.484 | 0.244 | 0.115 | 0.004 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostyshyn, N.M.; Świetlicka, I.; Tomaszewska, E.; Dobrowolski, P.; Muszyński, S. Impact of Whole Body Vibration and Zoledronic Acid on Femoral Structure after Ovariectomy: Morphological Evaluation. J. Clin. Med. 2022, 11, 2441. https://doi.org/10.3390/jcm11092441
Kostyshyn NM, Świetlicka I, Tomaszewska E, Dobrowolski P, Muszyński S. Impact of Whole Body Vibration and Zoledronic Acid on Femoral Structure after Ovariectomy: Morphological Evaluation. Journal of Clinical Medicine. 2022; 11(9):2441. https://doi.org/10.3390/jcm11092441
Chicago/Turabian StyleKostyshyn, Nazar M., Izabela Świetlicka, Ewa Tomaszewska, Piotr Dobrowolski, and Siemowit Muszyński. 2022. "Impact of Whole Body Vibration and Zoledronic Acid on Femoral Structure after Ovariectomy: Morphological Evaluation" Journal of Clinical Medicine 11, no. 9: 2441. https://doi.org/10.3390/jcm11092441








