Lifestyle Modification and Atrial Fibrillation: Critical Care for Successful Ablation
Abstract
:1. Introduction
2. Rhythm Control for Atrial Fibrillation
3. Modifiable Risk Factors for Atrial Fibrillation
4. Risk Factor Modification before and in Conjunction with AF Ablation
5. Obesity and Ablation
6. Physical Inactivity and Ablation
7. Hypertension and Ablation
8. Diabetes Mellitus and Ablation
9. Obstructive Sleep Apnoea and Ablation
10. Alcohol Consumption and Ablation
11. Smoking and Ablation
12. Comprehensive Risk Factor Modification
13. Structure of Risk Factor Modification Clinics
- Patient centred, individually tailored care;
- A lifestyle journal including regular recordings of blood pressure, exercise and dietary intake, which is regularly reviewed, with feedback provided;
- Incremental, goal-directed approach;
- Flexible follow-up, with access to care between appointments;
- Surveillance and titration of medication and other treatments;
- Screening of all patients with AF for the presence of OSA and review of any CPAP treatment data to ensure efficacy.
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.-H.; McAnulty, J.H., Jr.; Zheng, Z.-J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.; McManus, D.D.; Newton-Cheh, C.; Lubitz, S.A.; Magnani, J.W.; Ellinor, P.; et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015, 386, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, C.; Hendriks, J.M.; Giles, L.; Karnon, J.; Pham, C.; Elliott, A.D.; Middeldorp, M.; Mahajan, R.; Lau, D.H.; Sanders, P.; et al. Increasing trends in hospitalisations due to atrial fibrillation in Australia from 1993 to 2013. Heart 2019, 105, 1358–1363. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T., Jr.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T., Jr.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of cardiology/American heart association task force on practice guidelines and the heart rhythm society. Circulation 2014, 130, e199–e267. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Abed, H.S.; Samuel, C.S.; Lau, D.H.; Kelly, D.J.; Royce, S.G.; Alasady, M.; Mahajan, R.; Kuklik, P.; Zhang, Y.; Brooks, A.G.; et al. Obesity results in progressive atrial structural and electrical remodeling: Implications for atrial fibrillation. Heart Rhythm 2013, 10, 90–100. [Google Scholar] [CrossRef]
- Mahajan, R.; Lau, D.H.; Brooks, A.G.; Shipp, N.J.; Manavis, J.; Wood, J.P.; Finnie, J.W.; Samuel, C.S.; Royce, S.G.; Twomey, D.; et al. Electrophysiological, Electroanatomical, and Structural Remodeling of the Atria as Consequences of Sustained Obesity. J. Am. Coll. Cardiol. 2015, 66, 1–11. [Google Scholar] [CrossRef]
- Munger, T.M.; Dong, Y.-X.; Masaki, M.; Oh, J.K.; Mankad, S.V.; Borlaug, B.A.; Asirvatham, S.J.; Shen, W.-K.; Lee, H.-C.; Bielinski, S.J.; et al. Electrophysiological and hemodynamic characteristics associated with obesity in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2012, 60, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Lau, D.H.; Mackenzie, L.; Kelly, D.J.; Psaltis, P.J.; Brooks, A.G.; Worthington, M.; Rajendram, A.; Kelly, D.R.; Zhang, Y.; Kuklik, P.; et al. Hypertension and atrial fibrillation: Evidence of progressive atrial remodeling with electrostructural correlate in a conscious chronically instrumented ovine model. Heart Rhythm 2010, 7, 1282–1290. [Google Scholar] [CrossRef]
- Lau, D.H.; Mackenzie, L.; Kelly, D.J.; Psaltis, P.J.; Worthington, M.; Rajendram, A.; Kelly, D.R.; Nelson, A.J.; Zhang, Y.; Kuklik, P.; et al. Short-term hypertension is associated with the development of atrial fibrillation substrate: A study in an ovine hypertensive model. Heart Rhythm 2010, 7, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.H.; Mackenzie, L.; Rajendram, A.; Psaltis, P.J.; Kelly, D.R.; Spyropoulos, P.; Zhang, Y.; Olakkengil, S.A.; Russell, C.H.; Brooks, A.G.; et al. Characterization of cardiac remodeling in a large animal one-kidney, one-clip hypertensive model. Blood Press. 2010, 19, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Middeldorp, M.; Meredith, M.; Mehta, A.; Mahajan, R.; Wong, C.; Twomey, D.; Elliott, A.D.; Kalman, J.M.; Abhayaratna, W.; et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J. Am. Coll. Cardiol. 2015, 65, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Elliott, A.; Middeldorp, M.E.; Meredith, M.; Mehta, A.B.; Mahajan, R.; Hendriks, J.M.; Twomey, D.; Kalman, J.M.; Abhayaratna, W.P.; et al. Impact of CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals With Atrial Fibrillation: The CARDIO-FIT Study. J. Am. Coll. Cardiol. 2015, 66, 985–996. [Google Scholar] [CrossRef]
- Middeldorp, M.; Pathak, R.K.; Meredith, M.; Mehta, A.; Elliott, A.D.; Mahajan, R.; Twomey, D.; Gallagher, C.; Hendriks, J.M.L.; Linz, D.; et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: The REVERSE-AF study. Europace 2018, 20, 1929–1935. [Google Scholar] [CrossRef]
- Wyse, D.G.; Waldo, A.L.; DiMarco, J.P.; Domanski, M.J.; Rosenberg, Y.; Schron, E.B.; Kellen, J.C.; Greene, H.L.; Mickel, M.C.; Dalquist, J.E.; et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [Google Scholar] [CrossRef]
- de Denus, S.; Sanoski, C.A.; Carlsson, J.; Opolski, G.; Spinler, S.A. Rate vs. rhythm control in patients with atrial fibrillation: A meta-analysis. Arch. Intern. Med. 2005, 165, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Poole, J.E.; Noseworthy, P.A.; Rosenberg, Y.D.; Jeffries, N.; Mitchell, L.B.; et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019, 321, 1261–1274. [Google Scholar] [CrossRef]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; Van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Ganesan, A.N.; Shipp, N.J.; Brooks, A.G.; Kuklik, P.; Lau, D.H.; Lim, H.S.; Sullivan, T.; Roberts-Thomson, K.C.; Sanders, P. Long-term outcomes of catheter ablation of atrial fibrillation: A systematic review and meta-analysis. J. Am. Heart Assoc. 2013, 2, e004549. [Google Scholar] [CrossRef] [Green Version]
- Gaita, F.; Scaglione, M.; Battaglia, A.; Matta, M.; Gallo, C.; Galatà, M.; Caponi, D.; Di Donna, P.; Anselmino, M. Very long-term outcome following transcatheter ablation of atrial fibrillation. Are results maintained after 10 years of follow up? Europace 2018, 20, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Teh, A.W.; Kistler, P.; Lee, G.; Medi, C.; Heck, P.M.; Spence, S.J.; Morton, J.B.; Sanders, P.; Kalman, J.M. Long-term effects of catheter ablation for lone atrial fibrillation: Progressive atrial electroanatomic substrate remodeling despite successful ablation. Heart Rhythm 2012, 9, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.X.; Sullivan, T.; Sun, M.T.; Mahajan, R.; Pathak, R.; Middeldorp, M.; Twomey, D.; Ganesan, A.N.; Rangnekar, G.; Roberts-Thomson, K.C.; et al. Obesity and the Risk of Incident, Post-Operative, and Post-Ablation Atrial Fibrillation: A Meta-Analysis of 626,603 Individuals in 51 Studies. JACC Clin. Electrophysiol. 2015, 1, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Furberg, C.D.; Psaty, B.M.; Siscovick, D. Physical activity and incidence of atrial fibrillation in older adults: The cardiovascular health study. Circulation 2008, 118, 800–807. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, E.J.; Levy, D.; Vaziri, S.M.; D’Agostino, R.B.; Belanger, A.J.; Wolf, P.A. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994, 271, 840–844. [Google Scholar] [CrossRef]
- Youssef, I.; Kamran, H.; Yacoub, M.; Patel, N.; Goulbourne, C.; Kumar, S.; Kane, J.; Hoffner, H.; Salifu, M.; McFarlane, S.I. Obstructive Sleep Apnea as a Risk Factor for Atrial Fibrillation: A Meta-Analysis. J. Sleep Disord. Ther. 2018, 7, 282. [Google Scholar] [CrossRef]
- Aune, D.; Feng, T.; Schlesinger, S.; Janszky, I.; Norat, T.; Riboli, E. Diabetes mellitus, blood glucose and the risk of atrial fibrillation: A systematic review and meta-analysis of cohort studies. J. Diabetes Complicat. 2018, 32, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, P.O.; Wu, C.F.; De La Cruz, C.; Weisse, A.B., Jr.; Ahmed, S.S.; Regan, T.J. Arrhythmias and the “Holiday Heart”: Alcohol-associated cardiac rhythm disorders. Am. Heart J. 1978, 95, 555–562. [Google Scholar] [CrossRef]
- Voskoboinik, A.; Prabhu, S.; Ling, L.-H.; Kalman, J.M.; Kistler, P.M. Alcohol and Atrial Fibrillation: A Sobering Review. J. Am. Coll. Cardiol. 2016, 68, 2567–2576. [Google Scholar] [CrossRef]
- Tu, S.J.; Gallagher, C.; Elliott, A.D.; Linz, D.; Pitman, B.M.; Hendriks, J.M.; Lau, D.H.; Sanders, P.; Wong, C.X. Risk Thresholds for Total and Beverage-Specific Alcohol Consumption and Incident Atrial Fibrillation. JACC Clin. Electrophysiol. 2021, 7, 1561–1569. [Google Scholar] [CrossRef]
- Chamberlain, A.M.; Agarwal, S.K.; Folsom, A.R.; Duval, S.; Soliman, E.Z.; Ambrose, M.; Eberly, L.; Alonso, A. Smoking and incidence of atrial fibrillation: Results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm 2011, 8, 1160–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, R.; Nelson, A.; Pathak, R.K.; Middeldorp, M.; Wong, C.X.; Twomey, D.; Carbone, A.; Teo, K.; Agbaedeng, T.; Linz, D.; et al. Electroanatomical Remodeling of the Atria in Obesity: Impact of Adjacent Epicardial Fat. JACC Clin. Electrophysiol. 2018, 4, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; Schotten, U.; Neuberger, H.-R.; Böhm, M.; Wirth, K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm 2011, 8, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Hjälm, H.H.; Fu, M.; Hansson, P.-O.; Zhong, Y.; Caidahl, K.; Mandalenakis, Z.; Morales, D.; Ergatoudes, C.; Rosengren, A.; Grote, L.; et al. Association between left atrial enlargement and obstructive sleep apnea in a general population of 71-year-old men. J. Sleep Res. 2018, 27, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.-K.; Kato, T.; Xiong, F.; Shi, Y.-F.; Naud, P.; Maguy, A.; Mizuno, K.; Tardif, J.-C.; Comtois, P.; Nattel, S. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. J. Am. Coll. Cardiol. 2014, 64, 2013–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voskoboinik, A.; Wong, G.; Lee, G.; Nalliah, C.; Hawson, J.; Prabhu, S.; Sugumar, H.; Ling, L.-H.; McLellan, A.; Morton, J.; et al. Moderate alcohol consumption is associated with atrial electrical and structural changes: Insights from high-density left atrial electroanatomic mapping. Heart Rhythm 2019, 16, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Marcus, G.M.; Dukes, J.W.; Vittinghoff, E.; Nah, G.; Badhwar, N.; Moss, J.D.; Lee, R.J.; Lee, B.K.; Tseng, Z.H.; Walters, T.E.; et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Intravenous Alcohol to Assess Changes in Atrial Electrophysiology. JACC Clin. Electrophysiol. 2021, 7, 662–670. [Google Scholar] [CrossRef]
- Mohanty, S.; Mohanty, P.; Tamaki, M.; Natale, V.; Gianni, C.; Trivedi, C.; Gokoglan, Y.; Di Biase, L.; Natale, A. Differential Association of Exercise Intensity with Risk of Atrial Fibrillation in Men and Women: Evidence from a Meta-Analysis. J. Cardiovasc. Electrophysiol. 2016, 27, 1021–1029. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.-S.; Kim, T.-H.; Jang, E.; Shin, H.; Kim, H.Y.; Yu, H.T.; Uhm, J.-S.; Kim, J.-Y.; Pak, H.-N.; et al. Ideal Blood Pressure in Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2018, 72, 1233–1245. [Google Scholar] [CrossRef]
- Kadhim, K.; Middeldorp, M.E.; Elliott, A.D.; Jones, D.; Hendriks, J.; Gallagher, C.; Arzt, M.; McEvoy, R.D.; Antic, N.A.; Mahajan, R.; et al. Self-Reported Daytime Sleepiness and Sleep-Disordered Breathing in Patients With Atrial Fibrillation: SNOozE-AF. Can. J. Cardiol. 2019, 35, 1457–1464. [Google Scholar] [CrossRef]
- Linz, D.; Brooks, A.G.; Elliott, A.D.; Nalliah, C.J.; Hendriks, J.; Middeldorp, M.; Gallagher, C.; Mahajan, R.; Kalman, J.M.; McEvoy, R.D.; et al. Variability of Sleep Apnea Severity and Risk of Atrial Fibrillation: The VARIOSA-AF Study. JACC Clin. Electrophysiol. 2019, 5, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-H.; Chuang, C.; Chan, C.-C.; Lee, H.-F.; Huang, Y.-C.; Huang, Y.-T.; Chang, S.-H.; Wang, C.-L.; Chao, T.-F.; Kuo, C.-T.; et al. Glycemic status and risks of thromboembolism and major bleeding in patients with atrial fibrillation. Cardiovasc. Diabetol. 2020, 19, 30. [Google Scholar] [CrossRef] [Green Version]
- Patlolla, S.H.; Lee, H.-C.; Noseworthy, P.A.; Wysokinski, W.E.; Hodge, D.O.; Greene, E.L.; Gersh, B.J.; Melduni, R.M. Impact of Diabetes Mellitus on Stroke and Survival in Patients With Atrial Fibrillation. Am. J. Cardiol. 2020, 131, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Drca, N.; Wolk, A. Alcohol consumption and risk of atrial fibrillation: A prospective study and dose-response meta-analysis. J. Am. Coll. Cardiol. 2014, 64, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, I.E.; Rasmussen, L.H.; Lane, D.A.; Overvad, T.F.; Skjøth, F.; Overvad, K.; Lip, G.Y.; Larsen, T.B. The impact of smoking on thromboembolism and mortality in patients with incident atrial fibrillation: Insights from the Danish Diet, Cancer, and Health study. Chest 2014, 145, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Annoura, M.; Ogawa, M.; Kumagai, K.; Zhang, B.; Saku, K.; Arakawa, K. Cholesterol paradox in patients with paroxysmal atrial fibrillation. Cardiology 1999, 92, 21–27. [Google Scholar] [CrossRef]
- Watanabe, H.; Tanabe, N.; Yagihara, N.; Watanabe, T.; Aizawa, Y.; Kodama, M. Association between lipid profile and risk of atrial fibrillation. Circ. J. 2011, 75, 2767–2774. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Tanabe, N.; Yagihara, N.; Watanabe, T.; Aizawa, Y.; Kodama, M. Prevalence of coronary artery disease assessed by multislice computed tomography coronary angiography in patients with paroxysmal or persistent atrial fibrillation. Circ. Cardiovasc. Imaging 2009, 2, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Kralev, S.; Schneider, K.; Lang, S.; Süselbeck, T.; Borggrefe, M. Incidence and severity of coronary artery disease in patients with atrial fibrillation undergoing first-time coronary angiography. PLoS ONE 2011, 6, e24964. [Google Scholar] [CrossRef]
- Voskoboinik, A.; Kalman, J.M.; De Silva, A.; Nicholls, T.; Costello, B.; Nanayakkara, S.; Prabhu, S.; Stub, D.; Azzopardi, S.; Vizi, D.; et al. Alcohol Abstinence in Drinkers with Atrial Fibrillation. N. Engl. J. Med. 2020, 382, 20–28. [Google Scholar] [CrossRef]
- Donnellan, E.; Wazni, O.M.; Elshazly, M.; Kanj, M.; Hussein, A.A.; Baranowski, B.; Kochar, A.; Trulock, K.; Aminian, A.; Schauer, P.; et al. Impact of Bariatric Surgery on Atrial Fibrillation Type. Circ. Arrhythm Electrophysiol. 2020, 13, e007626. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Evans, M.; Middeldorp, M.; Mahajan, R.; Mehta, A.B.; Meredith, M.; Twomey, D.; Wong, C.X.; Hendriks, J.M.; Abhayaratna, W.; et al. Cost-Effectiveness and Clinical Effectiveness of the Risk Factor Management Clinic in Atrial Fibrillation: The CENT Study. JACC Clin. Electrophysiol. 2017, 3, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Letsas, K.P.; Siklódy, C.H.; Korantzopoulos, P.; Weber, R.; Bürkle, G.; Mihas, C.C.; Kalusche, D.; Arentz, T. The impact of body mass index on the efficacy and safety of catheter ablation of atrial fibrillation. Int. J. Cardiol. 2013, 164, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Chilukuri, K.; Dalal, D.; Gadrey, S.; Marine, J.E.; MacPherson, E.; Macpherson, E.; Cheng, A.; Nazarian, S.; Sinha, S.; Spragg, D.; et al. A prospective study evaluating the role of obesity and obstructive sleep apnea for outcomes after catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2010, 21, 521–525. [Google Scholar] [CrossRef]
- Glover, B.M.; Hong, K.L.; Dagres, N.; Arbelo, E.; Laroche, C.; Riahi, S.; Bertini, M.; Mikhaylov, E.; Galvin, J.; Kiliszek, M.; et al. Impact of body mass index on the outcome of catheter ablation of atrial fibrillation. Heart 2019, 105, 244–250. [Google Scholar] [CrossRef]
- Sivasambu, B.; Balouch, M.A.; Zghaib, T.; Bajwa, R.J.; Chrispin, J.; Berger, R.D.; Ashikaga, H.; Nazarian, S.; Marine, J.E.; Calkins, H.; et al. Increased rates of atrial fibrillation recurrence following pulmonary vein isolation in overweight and obese patients. J. Cardiovasc. Electrophysiol. 2018, 29, 239–245. [Google Scholar] [CrossRef]
- Gessler, N.; Willems, S.; Steven, D.; Aberle, J.; Akbulak, R.O.; Gosau, N.; Hoffmann, B.A.; Meyer, C.; Sultan, A.; Tilz, R.; et al. Supervised Obesity Reduction Trial for AF Ablation Patients: Results from the SORT-AF trial. Europace 2021, 23, 1548–1558. [Google Scholar] [CrossRef]
- Mohanty, S.; Mohanty, P.; Natale, V.; Trivedi, C.; Gianni, C.; Burkhardt, J.D.; Sanchez, J.E.; Horton, R.; Gallinghouse, G.J.; Hongo, R.; et al. Impact of weight loss on ablation outcome in obese patients with longstanding persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2018, 29, 246–253. [Google Scholar] [CrossRef]
- Rostock, T.; Salukhe, T.V.; Steven, D.; Drewitz, I.; Hoffmann, B.A.; Bock, K.; Servatius, H.; Müllerleile, K.; Sultan, A.; Gosau, N.; et al. Long-term single- and multiple-procedure outcome and predictors of success after catheter ablation for persistent atrial fibrillation. Heart Rhythm 2011, 8, 1391–1397. [Google Scholar] [CrossRef]
- Donnellan, E.; Wazni, O.M.; Harb, S.; Kanj, M.; Saliba, W.I.; Jaber, W.A. Higher baseline cardiorespiratory fitness is associated with lower arrhythmia recurrence and death after atrial fibrillation ablation. Heart Rhythm 2020, 17, 1687–1693. [Google Scholar] [CrossRef]
- Risom, S.S.; Zwisler, A.-D.; Rasmussen, T.B.; Sibilitz, K.L.; Madsen, T.L.; Svendsen, J.H.; Gluud, C.; Lindschou, J.; Winkel, P.; Berg, S.K. Cardiac rehabilitation versus usual care for patients treated with catheter ablation for atrial fibrillation: Results of the randomized CopenHeartRFA trial. Am. Heart J. 2016, 181, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Risom, S.S.; Zwisler, A.-D.; Sibilitz, K.L.; Rasmussen, T.B.; Taylor, R.S.; Thygesen, L.C.; Madsen, T.S.; Svendsen, J.H.; Berg, S.K. Cardiac Rehabilitation for Patients Treated for Atrial Fibrillation With Ablation Has Long-Term Effects: 12-and 24-Month Follow-up Results From the Randomized CopenHeartRFA Trial. Arch. Phys. Med. Rehabil. 2020, 101, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Berruezo, A.; Tamborero, D.; Mont, L.; Benito, B.; Tolosana, J.M.; Sitges, M.; Vidal, B.; Arriagada, G.; Méndez, F.; Matiello, M.; et al. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur. Heart J. 2007, 28, 836–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letsas, K.P.; Weber, R.; Bürkle, G.; Mihas, C.C.; Minners, J.; Kalusche, D.; Arentz, T. Pre-ablative predictors of atrial fibrillation recurrence following pulmonary vein isolation: The potential role of inflammation. Europace 2009, 11, 158–163. [Google Scholar] [CrossRef]
- Santoro, F.; Di Biase, L.; Trivedi, C.; Burkhardt, J.D.; Perini, A.P.; Sanchez, J.; Horton, R.; Mohanty, P.; Mohanty, S.; Bai, R.; et al. Impact of Uncontrolled Hypertension on Atrial Fibrillation Ablation Outcome. JACC Clin. Electrophysiol. 2015, 1, 164–173. [Google Scholar] [CrossRef]
- Zylla, M.M.; Hochadel, M.; Andresen, D.; Brachmann, J.; Eckardt, L.; Hoffmann, E.; Kuck, K.-H.; Lewalter, T.; Schumacher, B.; Spitzer, S.G.; et al. Ablation of Atrial Fibrillation in Patients with Hypertension-An Analysis from the German Ablation Registry. J. Clin. Med. 2020, 9, 2402. [Google Scholar] [CrossRef]
- Pokushalov, E.; Romanov, A.; Corbucci, G.; Artyomenko, S.; Baranova, V.; Turov, A.; Shirokova, N.; Karaskov, A.; Mittal, S.; Steinberg, J.S. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J. Am. Coll. Cardiol. 2012, 60, 1163–1170. [Google Scholar] [CrossRef]
- Steinberg, J.S.; Shabanov, V.; Ponomarev, D.; Losik, D.; Ivanickiy, E.; Kropotkin, E.; Polyakov, K.; Ptaszynski, P.; Keweloh, B.; Yao, C.J.; et al. Effect of Renal Denervation and Catheter Ablation vs Catheter Ablation Alone on Atrial Fibrillation Recurrence Among Patients With Paroxysmal Atrial Fibrillation and Hypertension: The ERADICATE-AF Randomized Clinical Trial. JAMA 2020, 323, 248–255. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Kandzari, D.E.; O’Neill, W.W.; D’Agostino, R.; Flack, J.M.; Katzen, B.T.; Leon, M.B.; Liu, M.; Mauri, L.; Negoita, M.; et al. A controlled trial of renal denervation for resistant hypertension. N. Engl. J. Med. 2014, 370, 1393–1401. [Google Scholar] [CrossRef] [Green Version]
- Parkash, R.; Wells, G.A.; Sapp, J.L.; Healey, J.S.; Tardif, J.-C.; Greiss, I.; Rivard, L.; Roux, J.-F.; Gula, L.; Nault, I.; et al. Effect of Aggressive Blood Pressure Control on the Recurrence of Atrial Fibrillation After Catheter Ablation: A Randomized, Open-Label Clinical Trial (SMAC-AF [Substrate Modification With Aggressive Blood Pressure Control]). Circulation 2017, 135, 1788–1798. [Google Scholar] [CrossRef]
- Wang, A.; Truong, T.; Black-Maier, E.; Green, C.; Campbell, K.B.; Barnett, A.S.; Febre, J.; Loring, Z.; Al-Khatib, S.M.; Atwater, B.D.; et al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus. Heart Rhythm O2 2020, 1, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Creta, A.; Providência, R.; Adragão, P.; de Asmundis, C.; Chun, J.; Chierchia, G.; Defaye, P.; Schmidt, B.; Anselme, F.; Finlay, M.; et al. Impact of Type-2 Diabetes Mellitus on the Outcomes of Catheter Ablation of Atrial Fibrillation (European Observational Multicentre Study). Am. J. Cardiol. 2020, 125, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Anselmino, M.; Matta, M.; D’Ascenzo, F.; Pappone, C.; Santinelli, V.; Bunch, T.J.; Neumann, T.; Schilling, R.J.; Hunter, R.J.; Noelker, G.; et al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus: A systematic review and meta-analysis. Europace 2015, 17, 1518–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogossian, H.; Frommeyer, G.; Brachmann, J.; Lewalter, T.; Hoffmann, E.; Kuck, K.H.; Andresen, D.; Willems, S.; Spitzer, S.G.; Deneke, T.; et al. Catheter ablation of atrial fibrillation and atrial flutter in patients with diabetes mellitus: Who benefits and who does not? Data from the German ablation registry. Int. J. Cardiol. 2016, 214, 25–30. [Google Scholar] [CrossRef]
- Donnellan, E.; Aagaard, P.; Kanj, M.; Jaber, W.; Elshazly, M.; Hoosien, M.; Baranowski, B.; Hussein, A.; Saliba, W.; Wazni, O. Association Between Pre-Ablation Glycemic Control and Outcomes Among Patients With Diabetes Undergoing Atrial Fibrillation Ablation. JACC Clin. Electrophysiol. 2019, 5, 897–903. [Google Scholar] [CrossRef]
- Jongnarangsin, K.; Chugh, A.; Good, E.; Mukerji, S.; Dey, S.; Crawford, T.; Sarrazin, J.-F.; Kühne, M.; Chalfoun, N.; Wells, D.; et al. Body mass index, obstructive sleep apnea, and outcomes of catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2008, 19, 668–672. [Google Scholar] [CrossRef]
- Tang, R.-B.; Dong, J.-Z.; Liu, X.-P.; Kang, J.-P.; Ding, S.-F.; Wang, L.; Long, D.-Y.; Yu, R.-H.; Liu, S.; Ma, C.-S.; et al. Obstructive sleep apnoea risk profile and the risk of recurrence of atrial fibrillation after catheter ablation. Europace 2009, 11, 100–105. [Google Scholar] [CrossRef]
- Matiello, M.; Nadal, M.; Tamborero, D.; Berruezo, A.; Montserrat, J.; Embid, C.; Rios, J.; Villacastín, J.; Brugada, J.; Mont, L. Low efficacy of atrial fibrillation ablation in severe obstructive sleep apnoea patients. Europace 2010, 12, 1084–1089. [Google Scholar] [CrossRef]
- Fein, A.S.; Shvilkin, A.; Shah, D.; Haffajee, C.I.; Das, S.; Kumar, K.; Kramer, D.B.; Zimetbaum, P.J.; Buxton, A.E.; Josephson, M.E.; et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J. Am. Coll. Cardiol. 2013, 62, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.Y.; Liu, T.; Shehata, M.; Stevens, S.; Chugh, S.S.; Wang, X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am. J. Cardiol. 2011, 108, 47–51. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; Li, J.; Ge, X.; Guo, L.-Z.; Wang, Y.; Guo, W.-H.; Jiang, C.-X.; Ma, C.-S. Efficacy of catheter ablation of atrial fibrillation in patients with obstructive sleep apnoea with and without continuous positive airway pressure treatment: A meta-analysis of observational studies. Europace 2014, 16, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, W.T.; bin Nasir, U.; Alqalyoobi, S.; O’Neal, W.; Mawri, S.; Sabbagh, S.; Soliman, E.Z.; Al-Mallah, M. Meta-Analysis of Continuous Positive Airway Pressure as a Therapy of Atrial Fibrillation in Obstructive Sleep Apnea. Am. J. Cardiol. 2015, 116, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Naruse, Y.; Tada, H.; Satoh, M.; Yanagihara, M.; Tsuneoka, H.; Hirata, Y.; Ito, Y.; Kuroki, K.; Machino, T.; Yamasaki, H.; et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: Clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013, 10, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Mohanty, P.; Di Biase, L.; Shaheen, M.; Lewis, W.R.; Quan, K.; Cummings, J.E.; Wang, P.; Al-Ahmad, A.; Venkatraman, P.; et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: The impact of continuous positive airway pressure. Circ. Arrhythm Electrophysiol. 2010, 3, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Takigawa, M.; Takahashi, A.; Kuwahara, T.; Takahashi, Y.; Okubo, K.; Nakashima, E.; Watari, Y.; Nakajima, J.; Yamao, K.; Osaka, Y.; et al. Impact of Alcohol Consumption on the Outcome of Catheter Ablation in Patients With Paroxysmal Atrial Fibrillation. J. Am. Heart Assoc. 2016, 5, e004149. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Shi, R.; Hou, B.; Wu, L.; Zheng, L.; Ding, L.; Chen, G.; Zhang, S.; Yao, Y. Impact of Alcohol Consumption on Substrate Remodeling and Ablation Outcome of Paroxysmal Atrial Fibrillation. J. Am. Heart Assoc. 2015, 4, e002349. [Google Scholar] [CrossRef] [Green Version]
- Barmano, N.; Charitakis, E.; Kronstrand, R.; Walfridsson, U.; Karlsson, J.-E.; Walfridsson, H.; Nystrom, F.H. The association between alcohol consumption, cardiac biomarkers, left atrial size and re-ablation in patients with atrial fibrillation referred for catheter ablation. PLoS ONE 2019, 14, e0215121. [Google Scholar] [CrossRef] [Green Version]
- Fukamizu, S.; Sakurada, H.; Takano, M.; Hojo, R.; Nakai, M.; Yuba, T.; Komiyama, K.; Tatsumoto, A.; Maeno, K.; Mizusawa, Y.; et al. Effect of Cigarette Smoking on the Risk of Atrial Fibrillation Recurrence after Pulmonary Vein Isolation. J. Arrhythmia 2010, 26, 21–29. [Google Scholar] [CrossRef]
- Cheng, W.-H.; Lo, L.-W.; Lin, Y.-J.; Chang, S.-L.; Hu, Y.-F.; Hung, Y.; Chung, F.-P.; Chang, T.-Y.; Huang, T.-C.; Yamada, S.; et al. Cigarette smoking causes a worse long-term outcome in persistent atrial fibrillation following catheter ablation. J. Cardiovasc. Electrophysiol. 2018, 29, 699–706. [Google Scholar] [CrossRef]
- Pathak, R.; Middeldorp, M.; Lau, D.H.; Mehta, A.; Mahajan, R.; Twomey, D.; Alasady, M.; Hanley, L.; Antic, N.A.; McEvoy, R.D.; et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: The ARREST-AF cohort study. J. Am. Coll. Cardiol. 2014, 64, 2222–2231. [Google Scholar] [CrossRef]
- Pokushalov, E.; Romanov, A.; Katritsis, D.G.; Artyomenko, S.; Bayramova, S.; Losik, D.; Baranova, V.; Karaskov, A.; Steinberg, J.S. Renal denervation for improving outcomes of catheter ablation in patients with atrial fibrillation and hypertension: Early experience. Heart Rhythm 2014, 11, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, E.; Wazni, O.M.; Kanj, M.; Baranowski, B.; Cremer, P.; Harb, S.; McCarthy, C.P.; McEvoy, J.W.; Elshazly, M.B.; Aagaard, P.; et al. Association between pre-ablation bariatric surgery and atrial fibrillation recurrence in morbidly obese patients undergoing atrial fibrillation ablation. Europace 2019, 21, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, E.; Wazni, O.; Kanj, M.; Hussein, A.; Baranowski, B.; Lindsay, B.; Aminian, A.; Jaber, W.; Schauer, P.; Saliba, W. Outcomes of Atrial Fibrillation Ablation in Morbidly Obese Patients Following Bariatric Surgery Compared with a Nonobese Cohort. Circ. Arrhythm. Electrophysiol. 2019, 12, e007598. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, J.M.; De Wit, R.; Crijns, H.J.; Vrijhoef, H.; Prins, M.H.; Pisters, R.; Pison, L.A.; Blaauw, Y.; Tieleman, R.G. Nurse-led care vs. usual care for patients with atrial fibrillation: Results of a randomized trial of integrated chronic care vs. routine clinical care in ambulatory patients with atrial fibrillation. Eur. Heart J. 2012, 33, 2692–2699. [Google Scholar] [CrossRef]
- Brieger, D.; Amerena, J.; Attia, J.; Bajorek, B.; Chan, K.H.; Connell, C.; Freedman, B.; Ferguson, C.; Hall, T.; Haqqani, H.; et al. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian Clinical Guidelines for the Diagnosis and Management of Atrial Fibrillation 2018. Heart Lung Circ. 2018, 27, 1209–1266. [Google Scholar] [CrossRef] [Green Version]
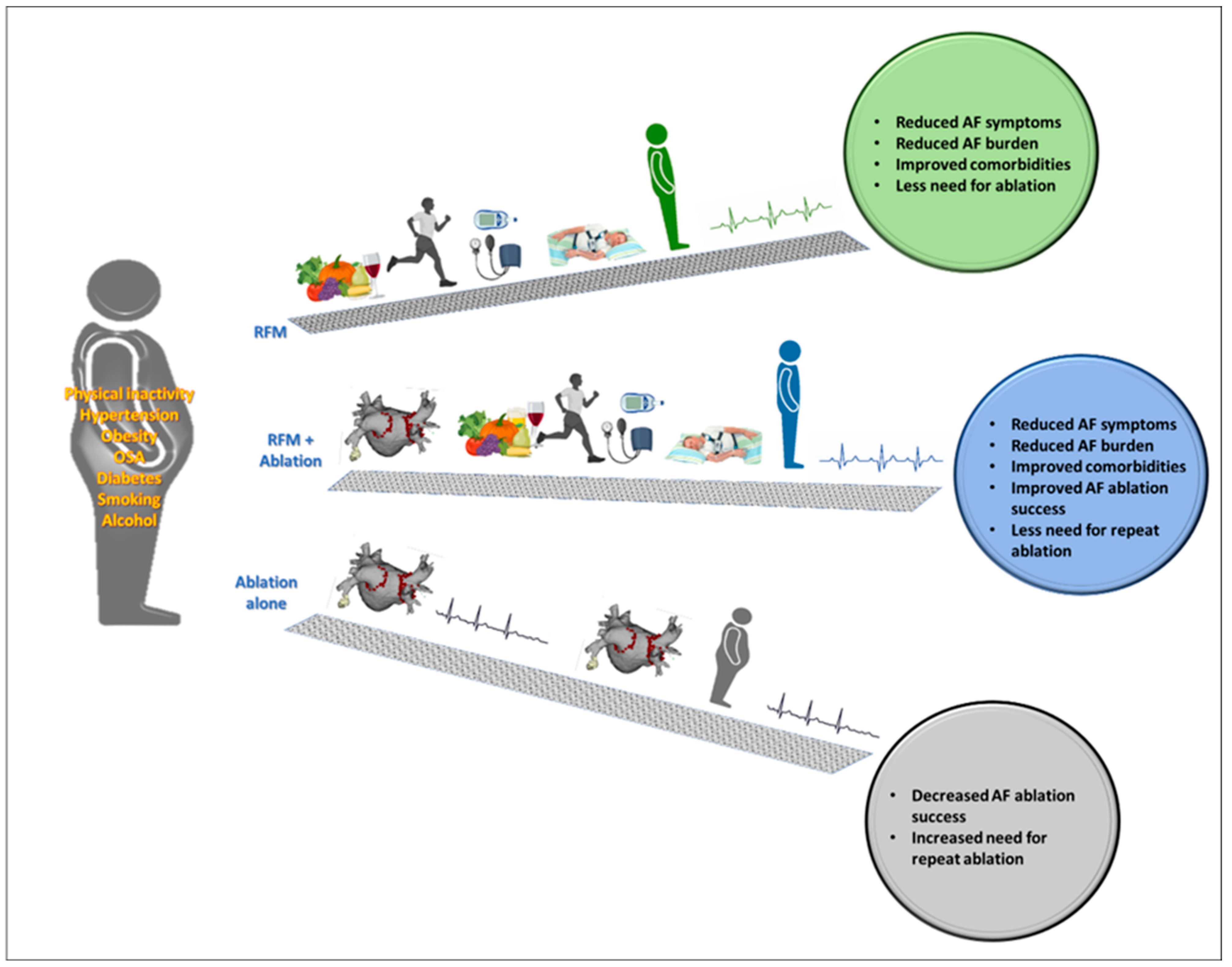
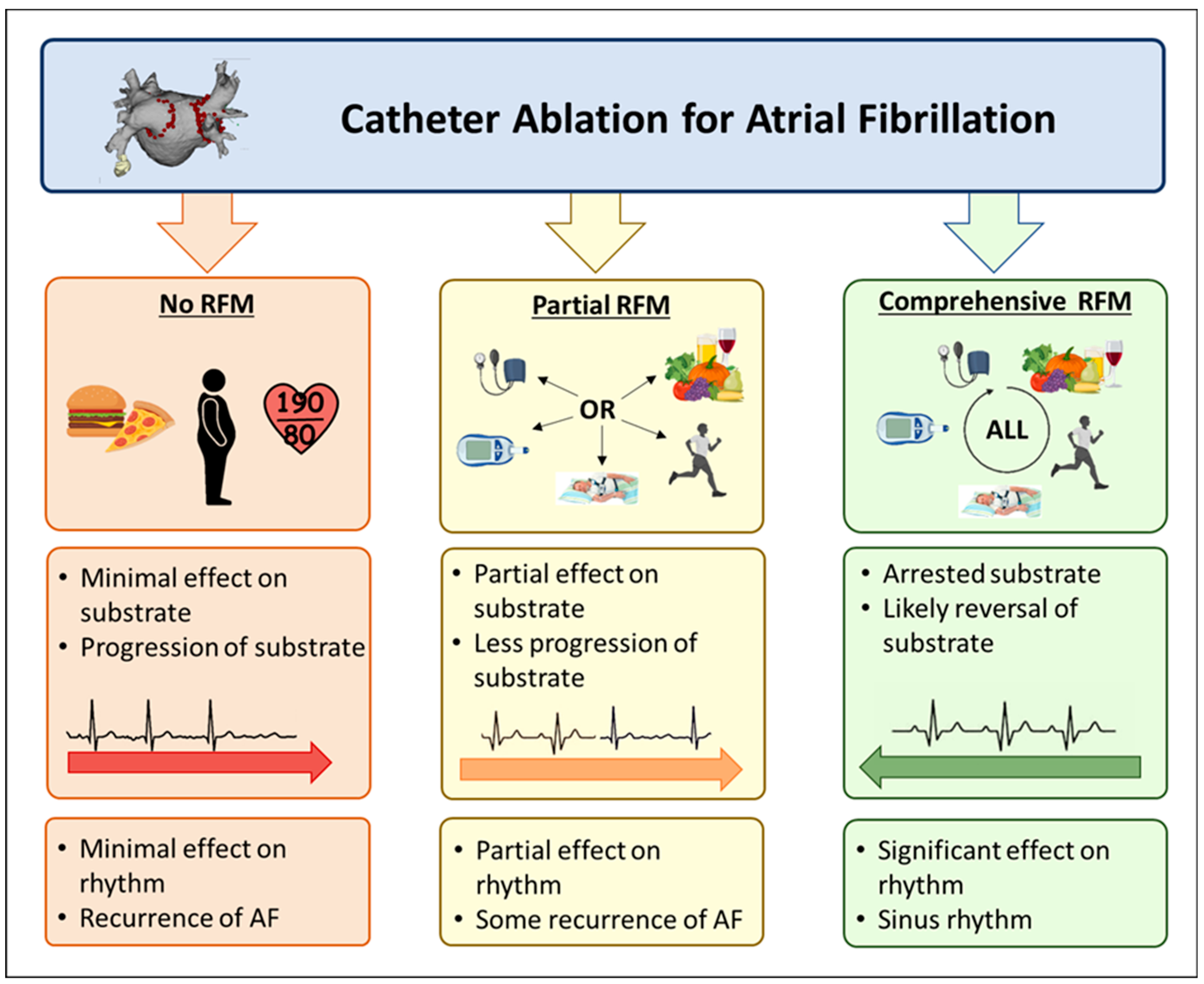
| Risk Factor | Effect |
|---|---|
| Obesity | Increased LA size, pressure and blood volume, increased central blood volume and systemic vascular resistance, increased epicardial and pericardial fat deposition, conduction slowing [7,8,9,32]. 29% increased risk for developing AF for each 5-point increase in BMI [23]. |
| Physical Inactivity | Higher risk of developing AF and association with poorer cardiovascular health and increasing obesity [38]. Up to 28% reduction in the risk of AF was associated with moderate-intensity physical activity in the Cardiovascular Health Study [24]. |
| Hypertension | Left ventricular hypertrophy and stiffening, reduced diastolic filling, increased left atrial volumes all associated with hypertensive increased afterload. Left atrial dilatation, adverse electrophysiological changes and circulating hormones such as angiotensin II linked with fibrosis and perpetuation of these changes [10,11,12]. 40 to 50% increased likelihood of developing AF in the Framingham Heart Study [25]. Blood pressure above 130/80 mmHg associated with significantly higher risk of major adverse cardiovascular events in a large cohort of nearly 300,000 patients with known atrial fibrillation [39]. |
| Obstructive Sleep Apnoea (OSA) | Repetitive interruption of ventilation via recurrent pharyngeal collapse leads to hypoxaemia-related atrial electrophysiological changes and altered haemodynamics that increase LA pressures, leading to left atrial enlargement [33,34]. Chronic OSA is associated with inflammatory and prothrombotic systemic changes [35]. The risk of developing AF doubles with the presence of OSA [26]. Degree of symptoms is a very poor indicator of the presence or severity of OSA [40]. Considerable night-to-night variability shown in the severity of sleep-disordered breathing [41]. Repeat testing in the case of an unexpectedly negative overnight oximetry study should be strongly considered where clinical suspicion remains high [41]. |
| Diabetes mellitus | One-third increase in the risk of developing AF has been independently attributed to diabetes mellitus [27]. In patients with AF, the presence of diabetes has been associated with increased thromboembolism and stroke risk [42,43]. |
| Alcohol consumption | Binge-drinking has been associated with increased risk of AF episodes, termed ‘Holiday Heart’ for many years [28]. Chronic alcohol use is associated with increased incidence and burden of AF, and regular moderate alcohol consumption is associated with reduced voltage and slowed conduction on electro-anatomical mapping at the time of AF ablation [29,36]. Chronic alcohol consumption is strongly associated with hypertension, obesity and OSA [29]. While more than seven standard drinks per week was associated with increased risk of developing AF, in a recent study of UK Biobank data, this risk may vary with the type of beverage consumed, with an increased risk of AF seen with reported beer or cider consumption, compared to wine or spirits [30]. Comprehensive review of previous data on alcohol and AF risk suggests that any alcohol at all increases the risk of AF and there is no benefit on AF from light alcohol consumption, which has been seen in studies related to ischaemic heart disease risk [29,44]. |
| Smoking | Observational cohort studies, such as the ARIC study, show up to double the risk of developing AF associated with smoking [31]. Mechanisms proposed include increased sympathetic tone, oxidative stress, inflammation and atrial fibrosis. In the presence of AF, smoking increases the risk of thromboembolism and mortality [45]. |
| Cholesterol levels | Observational studies have not shown a correlation with lower cholesterol, or particularly LDL and reduced AF, with, in fact a paradoxical relationship where less AF was seen with higher LDL levels [46,47]. The significance of this data is unclear though, and many patients with AF and hypercholesterolaemia have associated increased risk for cardiovascular events related to comorbidities such as hypertension or prior cardiovascular events. Adverse cardiovascular events, rather than AF-risk per se are associated with raised lipids in these instances [48,49]. |
| Study | Study Type | Number of Patients | Intervention or Risk Factor Studied * | Population | Change in Risk Factor(s) | Average Follow-Up Duration (Months) | Number of Procedures | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Pathak et al., 2014 (ARREST AF) [90] | Cohort | 149 | Aggressive comprehensive risk factor management | AF patients, BMI > 27 kg/m2 61 RFM 88 standard care | RFM group More weight loss Improved BP control Improved blood sugar control Reduced sleep apnoea | 41.9 | 1.6 ± 0.7 | Reduced AF symptom burden (p < 0.001) Improved arrhythmia-free survival: 87% arrhythmia free treatment group vs. 17% in control group (p < 0.001) |
| Gessler et al., 2021 (SORT-AF) [57] | Randomised controlled trial | 133 | 6 months of structured weight loss program | AF patients, BMI 34.9 67 weight loss 66 usual care | Weight loss group lost more weight (3.91%) | 12 | 1 17% had >1 ablation | AF burden reduced in both groups post-ablation (p < 0.001) but no difference between groups |
| Mohanty et al., 2018 [58] | Cohort | 90 | 1 year of weight loss intervention | Long-standing persistent AF patients, BMI 38 58 weight loss 32 standard care | Weight loss group Lost 24.9 kg cf control group 0.9 kg (p < 0.001) | 12 | 1 | No difference in AF symptoms by AFSS Improved physical (p = 0.013) and mental (p < 0.02) component scores of SF-36 in weight loss group compared to usual care |
| Pokushalov et al., 2012 [67] | Randomised controlled trial | 27 | Renal denervation in addition to pulmonary vein isolation versus pulmonary vein isolation alone | AF patients refractory to 2 AADs with drug-resistant hypertension, BMI 28 14 PVI only 13 PVI + renal denervation | Intervention group: BP improved from 181/97 to 156/87 | 12 | 1 | Intervention group: 69% arrhythmia-free Control group: 29% arrhythmia-free (p = 0.033) |
| Pokushalov et al., 2014 [91] | Meta-analysis of combined data from 2 randomised controlled trials | 80 | Renal denervation in addition to pulmonary vein isolation versus pulmonary vein isolation alone | AF patients BMI not stated 39 PVI only 41 PVI + renal denervation | Intervention group: BP | 12 | 1 | Intervention group: 63% AF-free Control group: 41% AF-free (p = 0.014) |
| Steinberg et al., 2020 (ERADICATE-AF) [68] | Randomised controlled trial | 302 | Renal denervation in addition to pulmonary vein isolation versus pulmonary vein isolation alone | Paroxysmal AF patients, BMI not stated, 16.8% obese 154 PVI + renal denervation 148 PVI alone | Intervention group: mean BP reduced 150–135 mmHg vs. control group 151–147 mmHg (p < 0.001) | 12 | 1 | Greater freedom from AF recurrence (72%) in treatment vs. (57%) control group (p = 0.006) |
| Parkash et al., 2017 (SMAC-AF) [70] | Randomised controlled trial | 184 | Aggressive BP treatment (target <120 mmHg) vs. standard BP treatment (target <140 mmHg) | AF patients, BMI 32, (57% paroxysmal) 92 aggressive BP treatment 92 standard BP treatment | Aggressive BP treatment group mean BP reduced 143–123 mmHg vs. control group 142–135 mmHg (p < 0.001) | 14 | 1 | Intervention group recurrence of AF/atrial tachycardia/atrial flutter not different to control group (both 61%) (p = 0.763) |
| Fein et al., 2013 [79] | Retrospective cohort | 62 | Treatment of obstructive sleep apnoea vs. non-treatment | AF patients, BMI 30, 53% persistent AF 32 with OSA on CPAP 30 with OSA no CPAP | Not specified | 12 | Not specified | Higher atrial tachyarrhythmia-free survival rate with CPAP than without (72% vs. 37%) (p = 0.01) |
| Patel et al., 2010 [84] | Retrospective cohort | 3000 | Treatment of obstructive sleep apnoea vs. non-treatment | AF patients, BMI 27, 53% paroxysmal 315 with OSA on CPAP 325 with OSA no CPAP | CPAP vs. no CPAP | 32 | 1 | Higher AF-free survival rate with CPAP than without (79% vs. 68%) (p = 0.001) |
| Naruse et al., 2013 [83] | Prospective case–control | 153 | Treatment of obstructive sleep apnoea vs. non-treatment | AF patients, BMI 25, 54% paroxysmal 82 with OSA on CPAP 34 with OSA no CPAP | CPAP vs. no CPAP | 19 | 1 | Lower AF recurrence with OSA + CPAP vs. OSA no CPAP (30% vs. 53%) (HR 0.41, CI 0.22–0.76, p < 0.01) |
| Jongnarangsin et al., 2008 [76] | Retrospective cohort | 324 | Treatment of obstructive sleep apnoea vs. non-treatment | AF patients, BMI 30, 72% paroxysmal 18 with OSA on CPAP 14 with OSA no CPAP | CPAP vs. no CPAP | 7 | 1 | Lower AF recurrence with OSA + CPAP vs. OSA no CPAP (50% vs. 71%) (underpowered for this outcome, p = 0.289) |
| Donnellan et al., 2019 [75] | Retrospective cohort | 298 | Pre-procedure HbA1c control <7% vs. poor control | AF patients with diabetes, BMI 34, 40% paroxysmal n = 298 | HbA1c controlled to <7% compared to >9% | 26 | Not specified | AF recurrence lower with HbA1c <7% (32.4%) vs. >9% (69%) (p < 0.0001) HbA1c trend in 12 months prior to ablation: 10% improvement showed lower (2%) recurrence vs. HbA1c worsening trend (91%) (p < 0.0001) |
| Donnellan et al., 2019 [92] | Retrospective observational cohort | 239 | Bariatric surgery vs. no bariatric surgery pre-AF ablation | AF patients, BMI 41, 39% paroxysmal 51 Bariatric surgery vs. 188 no Bariatric surgery | Bariatric surgery vs. no Bariatric surgery | 36 | 1.3 | Lower AF recurrence in surgery group vs. non-surgery group (20% vs. 61%) (p < 0.0001) Lower repeat procedure requirement with surgery group vs. non-surgery group (12% vs. 41%) (p < 0.0001) |
| Donnellan et al., 2019 [93] | Retrospective observational cohort | 255 | Bariatric surgery for morbid obesity pre-ablation vs. non-obese | AF patients, BMI 35, 41% paroxysmal 51 Bariatric Surgery BMI 37 vs. 102 no surgery BMI 43 vs. 102 non-obese BMI 25.6 | Bariatric surgery vs. no Bariatric surgery vs. non-obese | 29 | Not specified | Comparable AF recurrence in surgery group (20%) to non-obese group (24.5%), both significantly lower than non-surgery group (55%) (p < 0.0001) |
| Risom et al., 2016 (CopenHeartRFA) [61] | Randomised controlled trial | 210 | 12-weeks of cardiac rehabilitation | AF patients, BMI 28, 72% paroxysmal 105 Cardiac Rehabilitation vs. 105 usual care | Cardiac rehabilitation group improved VO2 max at 4 months compared with usual care | 6 | Not specified | VO2 max increased in cardiac rehabilitation group vs. controls, no significant difference in mental health or other SF-36 score components (p = 0.20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitzgerald, J.L.; Middeldorp, M.E.; Gallagher, C.; Sanders, P. Lifestyle Modification and Atrial Fibrillation: Critical Care for Successful Ablation. J. Clin. Med. 2022, 11, 2660. https://doi.org/10.3390/jcm11092660
Fitzgerald JL, Middeldorp ME, Gallagher C, Sanders P. Lifestyle Modification and Atrial Fibrillation: Critical Care for Successful Ablation. Journal of Clinical Medicine. 2022; 11(9):2660. https://doi.org/10.3390/jcm11092660
Chicago/Turabian StyleFitzgerald, John L., Melissa E. Middeldorp, Celine Gallagher, and Prashanthan Sanders. 2022. "Lifestyle Modification and Atrial Fibrillation: Critical Care for Successful Ablation" Journal of Clinical Medicine 11, no. 9: 2660. https://doi.org/10.3390/jcm11092660
APA StyleFitzgerald, J. L., Middeldorp, M. E., Gallagher, C., & Sanders, P. (2022). Lifestyle Modification and Atrial Fibrillation: Critical Care for Successful Ablation. Journal of Clinical Medicine, 11(9), 2660. https://doi.org/10.3390/jcm11092660





