Multimodality Imaging Approach to Spontaneous Coronary Artery Dissection
Abstract
1. Introduction
2. Epidemiology
2.1. Sex, Age, Ethnicity
2.2. Pregnancy-Associated SCAD
2.3. Inflammatory Conditions
2.4. Inheritance and Genetics
2.5. Risk Factors for Ischemic Heart Disease
3. Pathophysiology
4. Clinical Presentation
- Extent of dissection;
- Extent of the reduction of the vessel lumen;
- Site.
5. Diagnosis
- SCA on an atherosclerotic basis;
- Coronary spasm;
- Takotsubo syndrome;
- Coronary thromboembolism;
- MINOCA.
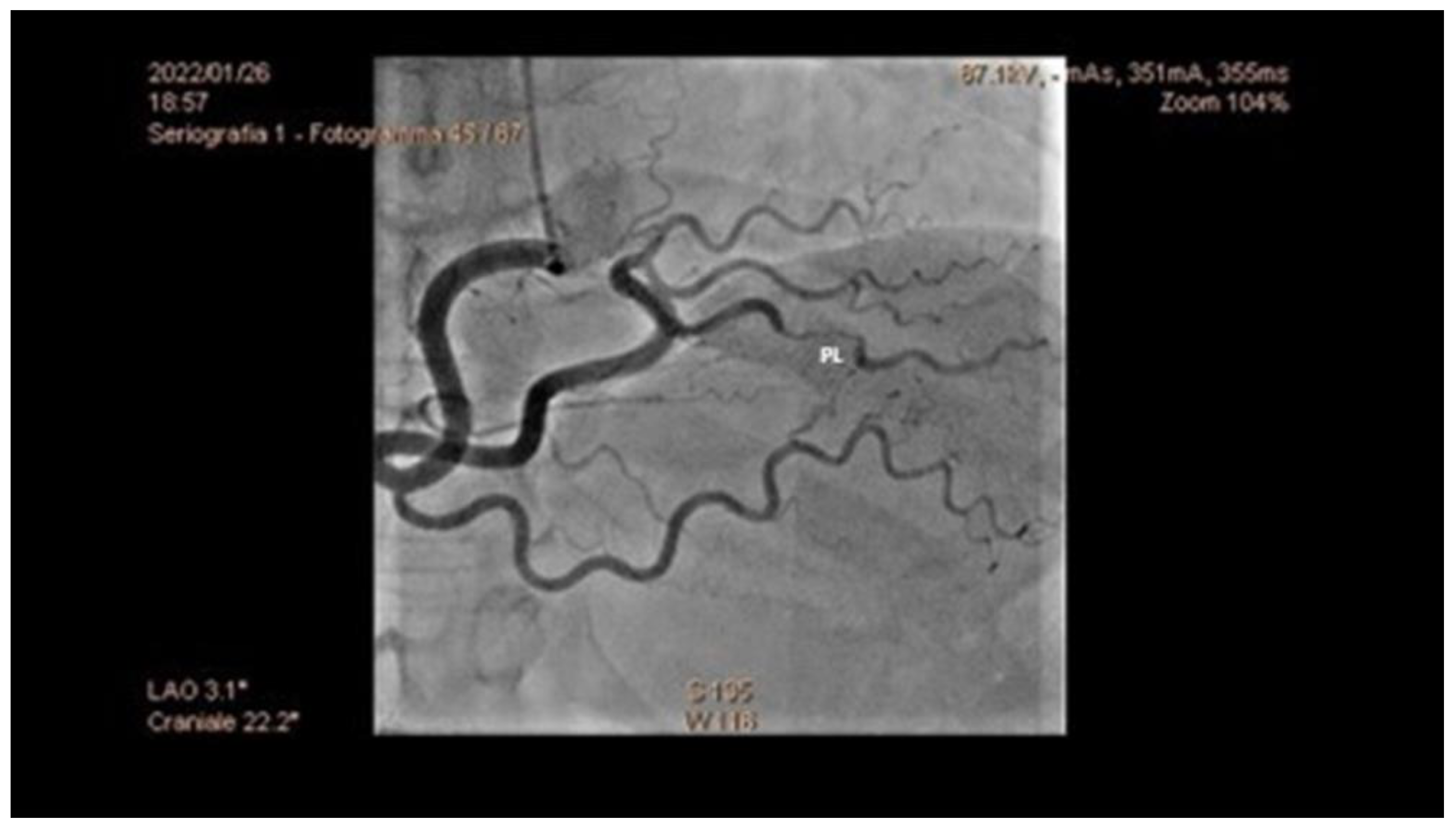
5.1. Angiography
- Increased coronary tortuosity;
- Predilection for more distal coronary segments;
- Predominant involvement of the left anterior descending coronary artery;
- False lumen starting and/or ending at a side branch;
- Absence or reduced incidence of co-existent atherosclerosis;
- Association with FMD;
- Association of sites of dissection with myocardial bridging.
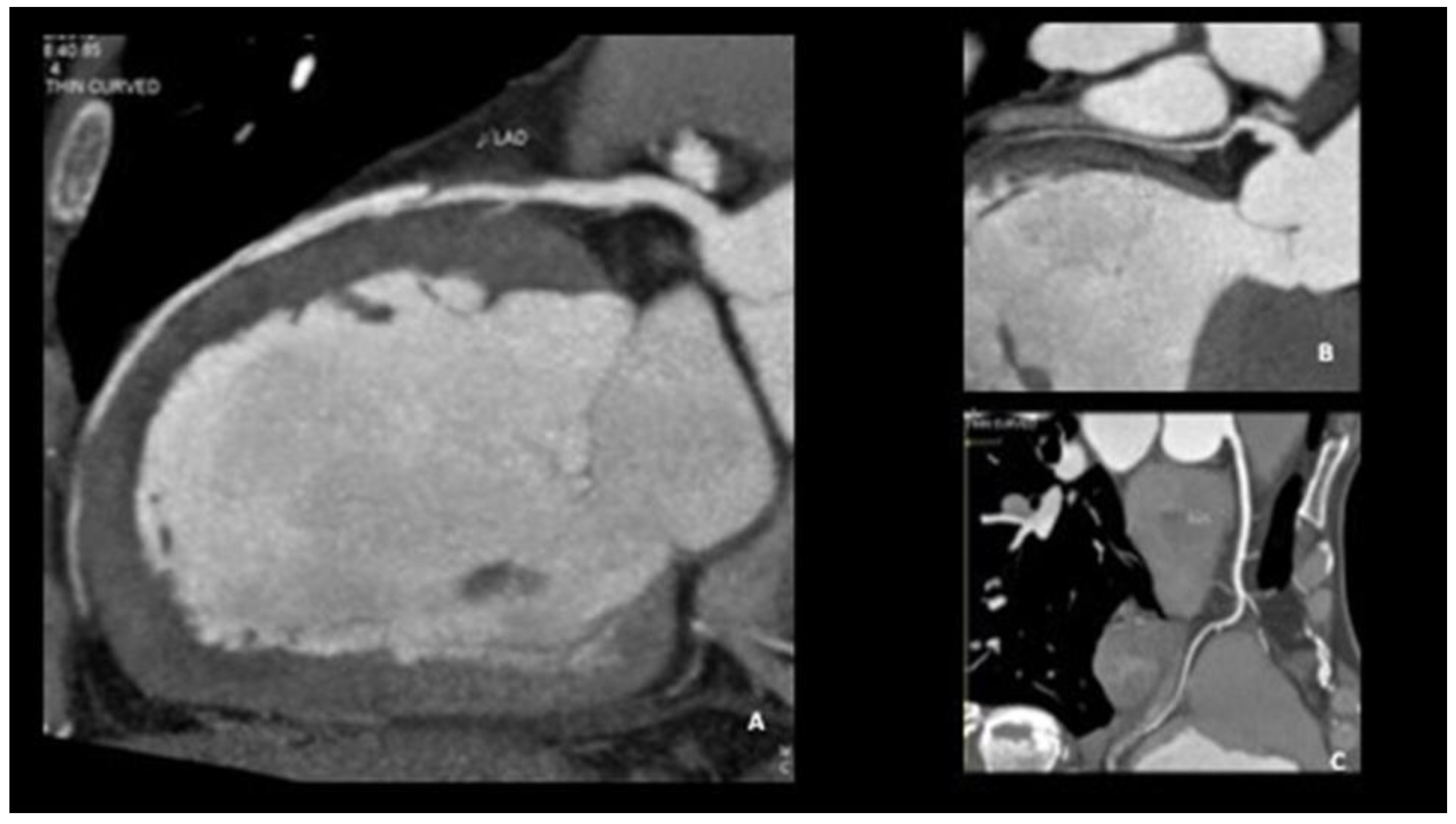
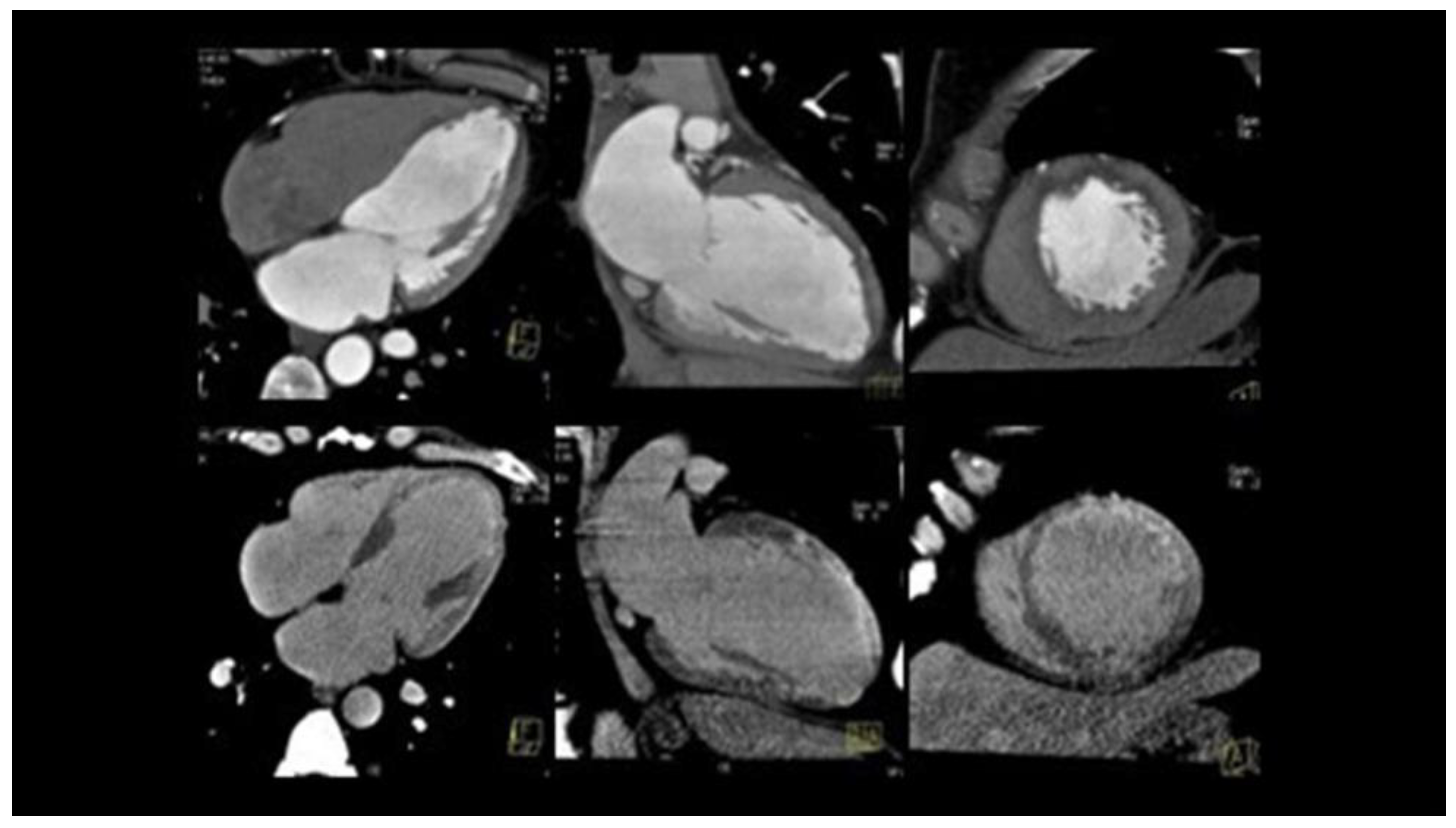
5.2. Challenging Diagnoses: Role of Intravascular and Non-Invasive Imaging
5.3. Gray Diagnostic Zones
- Streaming of contrast: it can give an image similar to a linear type I dissection, but it disappears as the injection pressure of the contrast medium is increased.
- Coronary vasospasm: it disappears with an injection of nitroglycerin ic.
- Iatrogenic dissections: in this case, the differential diagnosis between SCAD and iatrogenic dissection is given by the ECG and symptoms preceding the chorus; in the case of iatrogenic dissection, symptoms and ECG changes appear during coronary angiography.
- Coronary embolism: Type 4 SCAD and coronary embolic occlusion can look very similar. The truncation of multiple branches due to a shower of embolic material can be a diagnostic feature but needs to be carefully distinguished from multivessel SCAD. Non-occlusive embolic clots can sometimes cause a filling defect, giving an apparent dual lumen appearance which mimics Type 1 SCAD.
- Rupture or erosion of atherosclerotic plaque with the penetration of contrast medium inside the plaque. These may include non-obstructive sites of plaque erosion and rupture where contrast penetration of ruptured plaque or a tongue of thrombus can give a linear appearance; sometimes embolization of thrombus into the distal vessel can give a dual lumen appearance distally.
- Takotsubo syndrome: the apical left anterior descending coronary artery is a common site for SCAD. This can lead to an apical regional wall motion abnormality similar in appearance to Takotsubo syndrome. Both conditions can also lead to the complete recovery of heart function.
- Reconsider the pre-test probability.
- Check for true lumen thrombus. SCAD does not appear to be highly thrombogenic and the presence of substantial true lumen thrombus should raise suspicion of an alternative diagnosis.
- Consider intracoronary (OCT) imaging.
- Assess pre-demission or evidence of atherosclerosis with follow-up CT Coronary Angiography.
- Consider other tests to assess the probability of paradoxical embolism or intracardiac thrombus [1].
5.4. Diagnostic Algorithm
6. Management
6.1. Acute Management
- Clinical presentation of onset;
- Hemodynamic picture of the patient;
- Angiographic characteristics of the lesion;
- Extension of the myocardial territory at risk.
6.2. Short-Term Management
7. Cardiac Rehabilitation and Physical Activity after SCAD
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adlam, D.; Fernando, A.; Maas, A.; Persu, A.; Vrints, C. Part III — Spontaneous Coronary Artery Dissections. In The PCR-EAPCI Textbook, 2nd ed.; PCR editor, 2012; Available online: https://www.escardio.org/Education/Textbooks/interventional-cardiology (accessed on 1 October 2022).
- Adlam, D.; Alfonso, F.; Maas, A.; Vrints, C.; Al-Hussaini, A.; Bueno, H.; Capranzano, P.; Gevaert, S.; Hoole, S.P.; Johnson, T.; et al. European Society of Cardiology, acute cardiovascular care association, SCAD study group: A position paper on spontaneous coronary artery dissection. Eur. Heart J. 2018, 39, 3353–3368. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.H. Spontaneous Coronary-Artery Dissection. N. Enlg. J. Med. 2020, 383, 2358–2370. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.N.; Kim, E.S.H.; Saw, J.; Adlam, D.; Arslanian-Engoren, C.; Economy, K.E.; Ganesh, S.K.; Gulati, R.; Lindsay, M.E.; Mieres, J.H.; et al. Spontaneous Coronary Artery Dissection: Current State of the Science A Scientific Statement From the American Heart Association. Circulation 2018, 137, e523–e557. [Google Scholar] [CrossRef]
- Hayes, S.N.; Tweet, M.S.; Adlam, D.; Kim, E.S.H.; Gulati, R.; Price, J.E.; Rose, C.H. Spontaneous Coronary Artery Dissection: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 961–984. [Google Scholar] [CrossRef] [PubMed]
- Kok, S.N.; Hayes, S.N.; Cutrer, F.M.; Raphael, C.E.; Gulati, R.; Best, P.J.M.; Tweet, M.S. Prevalence and Clinical Factors of Migraine in Patients With Spontaneous Coronary Artery Dissection. J. Am. Heart Assoc. 2018, 7, e010140. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, C.; Zavalloni, D.; Rossini, R.; Morici, N.; Ettori, F.; Leonzi, O.; Latib, A.; Ferlini, M.; Trabattoni, D.; Colombo, P.; et al. Management and Long-Term Prognosis of Spontaneous Coronary Artery Dissection. Am. J. Cardiol. 2015, 116, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Starovoytov, A.; Humphries, K.; Sheth, T.; So, D.; Minhas, K.; Brass, N.; Lavoie, A.; Bishop, H.; Lavi, S.; et al. Canadian spontaneous coronary artery dissection cohort study: In-hospital and 30-day outcomes. Eur. Heart J. 2019, 40, 1188–1197. [Google Scholar] [CrossRef]
- Hassan, S.; Prakash, R.; Starovoytov, A.; Saw, J. Natural History of Spontaneous Coronary Artery Dissection With Spontaneous Angiographic Healing. JACC Cardiovasc. Interv. 2019, 12, 518–527. [Google Scholar] [CrossRef]
- Tweet, M.S.; Hayes, S.N.; Codsi, E.; Gulati, R.; Rose, C.H.; Best, P.J. Spontaneous Coronary Artery Dissection Associated with Pregnancy. J. Am. Coll. Cardiol. 2017, 70, 426–435. [Google Scholar] [CrossRef]
- Al-Hussaini, A.; Abdelaty, A.; Gulsin, G.S.; Arnold, J.R.; Garcia-Guimaraes, M.; Premawardhana, D.; Budgeon, C.; Wood, A.; Natarajan, N.; Mangion, K.; et al. Chronic infarct size after spontaneous coronary artery dissection: Implications for pathophysiology and clinical management. Eur. Heart J. 2020, 41, 2197–2205. [Google Scholar] [CrossRef]
- Faden, M.S.; Bottega, N.; Benjamin, A.; Brown, R.N. A nationwide evaluation of spontaneous coronary artery dissection in pregnancy and the puerperium. Heart 2016, 102, 1974–1979. [Google Scholar] [CrossRef] [PubMed]
- Goel, K.; Tweet, M.; Olson, T.M.; Maleszewski, J.J.; Gulati, R.; Hayes, S.N. Familial spontaneous coronary artery dissection: Evidence for genetic susceptibility. JAMA Intern. Med. 2015, 175, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Carss, K.J.; Baranowska, A.A.; Armisen, J.; Webb, T.R.; Hamby, S.E.; Premawardhana, D.; Al-Hussaini, A.; Wood, A.; Wang, Q.; Deevi, S.V.V.; et al. Spontaneous Coronary Artery Dissection: Insights on Rare Genetic Variation From Genome Sequencing. Circ. Genom. Precis. Med. 2020, 13, e003030. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Starovoytov, A.; Aymong, E.; Inohara, T.; Alfadhel, M.; McAlister, C.; Samuel, R.; Grewal, T.; Parolis, J.A.; Sheth, T.; et al. Canadian Spontaneous Coronary Artery Dissection Cohort Study: 3-Year Outcomes. J. Am. Coll. Cardiol. 2022, 80, 1585–1597. [Google Scholar] [CrossRef] [PubMed]
- Adlam, D.; García-Guimaraes, M.; Maas, A.H.E.M. Spontaneous coronary artery dissection: No longer a rare disease. Eur. Heart J. 2019, 40, 1198–1201. [Google Scholar] [CrossRef]
- Sharma, S.; Kaadan, M.I.; Duran, J.M.; Ponzini, F.; Mishra, S.; Tsiaras, S.V.; Scott, N.S.; Weinberg, I.; Ghoshhajra, B.; Lindsay, M.; et al. Risk Factors, Imaging Findings, and Sex Differences in Spontaneous Coronary Artery Dissection. Am. J. Cardiol. 2019, 123, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Lettieri, C.; Caico, S.I.; Musumeci, G.; Rossini, R.; Castiglioni, B. Le dissezioni coronariche spontanee. G. Ital. Cardiol. 2018, 19, 488–494. [Google Scholar]
- Saw, J.; Aymong, E.; Mancini, G.J.; Sedlak, T.; Starovoytov, A.; Ricci, D. Nonatherosclerotic Coronary Artery Disease in Young Women. Can. J. Cardiol. 2014, 30, 814–819. [Google Scholar] [CrossRef]
- Saw, J.; Aymong, E.; Sedlak, T.; Buller, C.E.; Starovoytov, A.; Ricci, D.; Robinson, S.; Vuurmans, T.; Gao, M.; Humphries, K.; et al. Spontaneous Coronary Artery Dissection. Circ. Cardiovasc. Interv. 2014, 7, 645–655. [Google Scholar] [CrossRef]
- Waterbury, T.M.; Tweet, M.S.; Hayes, S.N.; Eleid, M.F.; Bell, M.R.; Lerman, A.; Singh, M.; Best, P.J.; Lewis, B.R.; Rihal, C.S.; et al. Early Natural History of Spontaneous Coronary Artery Dissection. Circ. Cardiovasc. Interv. 2018, 11, e006772. [Google Scholar] [CrossRef]
- Waterbury, T.M.; Tarantini, G.; Vogel, B.; Mehran, R.; Gersh, B.J.; Gulati, R. Non-atherosclerotic causes ofacute coronary syndromes. Nat. Rev. Cardiol. 2020, 17, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, F.; Paulo, M.; Lennie, V.; Dutary, J.; Bernardo, E.; Jiménez-Quevedo, P.; Gonzalo, N.; Escaned, J.; Bañuelos, C.; Pérez-Vizcayno, M.J.; et al. Spontaneous coronary artery dissection: Long-term follow-up of a large series of patients prospectively managed with a "conservative" therapeutic strategy. J. Am. Coll. Cardiol. Interv. 2012, 5, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.; Al-Hussaini, A.; Joseph, S.; van Soest, G.; Wood, A.; Macaya, F.; Gonzalo, N.; Cade, J.; Caixeta, A.; Hlinomaz, O.; et al. Spontaneous coronary artery dissection: Pathophysiological insights from optical coherence tomography. J. Am. Coll. Cardiol. Imaging 2019, 12, 2475–2488. [Google Scholar] [CrossRef] [PubMed]
- Persu, A.; Touze, E.; Mousseaux, E.; Barral, X.; Joffre, F.; Plouin, P.-F. Diagnosis and management of fibromuscular dysplasia: An expert consensus. Eur. J. Clin. Investig. 2011, 42, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Toggweiler, S.; Puck, M.; Thalhammer, C.; Manka, R.; Wyss, M.; Bilecen, D.; Corti, R.; Amann-Vesti, B.R.; Lüscher, T.F.; Wyss, C.A. Associated vascular lesions in patients with spontaneous coronary artery dissection. Swiss Med. Wkly. 2012, 142, w13538. [Google Scholar] [CrossRef]
- Kadian-Dodov, D.; Gornik, H.L.; Gu, X.; Froehlich, J.; Bacharach, J.M.; Chi, Y.-W.; Gray, B.H.; Jaff, M.R.; Kim, E.S.H.; Mace, P.; et al. Dissection and aneurysm in patients with fibromuscular dysplasia: Findings from the U.S. Registry for FMD. J. Am. Coll. Cardiol. 2016, 68, 176–185. [Google Scholar] [CrossRef]
- Eleid, M.F.; Guddeti, R.R.; Tweet, M.S.; Lerman, A.; Singh, M.; Best, P.J.; Vrtiska, T.J.; Prasad, M.; Rihal, C.S.; Hayes, S.N.; et al. Coronary artery tortuosity in spontaneous coronary artery dissection: Angiographic characteristics and clinical implications. Circ. Cardiovasc. Interv. 2014, 7, 656–662. [Google Scholar] [CrossRef]
- Yang, C.; Alfadhel, M.; Saw, J. Spontaneous Coronary Artery Dissection: Latest Developments and New Frontiers. Curr. Atheroscler. Rep. 2020, 22, 49. [Google Scholar] [CrossRef]
- Prasad, M.; Tweet, M.S.; Hayes, S.N.; Leng, S.; Liang, J.J.; Eleid, M.F.; Gulati, R.; Vrtiska, T.J. Prevalence of extracoronary vascular ab-normalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am. J. Cardiol. 2015, 115, 16727. [Google Scholar] [CrossRef]
- Ricci, F.; Neumann, J.T.; Rübsamen, N.; Sörensen, N.A.; Ojeda, F.; Cataldo, I.; Zeller, T.; Schäfer, S.; Hartikainen, T.S.; Golato, M.; et al. High-sensitivity troponin I with or without ultra-sensitive copeptin for the instant rule-out of acute myocardial infarction. Front. Cardiovasc. Med. 2022, 9, 895421. [Google Scholar] [CrossRef]
- Ricci, F.; Sutton, R.; Palermi, S.; Tana, C.; Renda, G.; Gallina, S.; Melander, O.; Caterina, R.; Fedorowski, A. Prognostic significance of noncardiac syncope in the general population: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2018, 29, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Sperlongano, S.; Russo, V.; D’Ascenzi, F.; Benfari, G.; Renon, F.; Palermi, S.; Ilardi, F.; Giallauria, F.; Limongelli, G.; et al. The Role of Multimodality Imaging in Athlete’s Heart Diagnosis: Current Status and Future Directions. J. Clin. Med. 2021, 10, 5126. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, P.; Prakash, R.; Starovoytov, A.; Boone, R.; Saw, J. Pre-Disposing and Precipitating Factors in Men With Spontaneous Coronary Artery Dissection. JACC: Cardiovasc. Interv. 2016, 9, 866–868. [Google Scholar] [CrossRef]
- Smaardijk, V.R.; Mommersteeg, P.M.; Kop, W.J.; Pellegrini, D.; van Geuns, R.-J.; Maas, A.H. Psychological and clinical characteristics of patients with spontaneous coronary artery dissection: A case-control study. Int. J. Cardiol. 2020, 323, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.; Starovoytov, A.; Heydari, M.; Mancini, G.J.; Saw, J. Catheter-Induced Iatrogenic Coronary Artery Dissection in Patients With Spontaneous Coronary Artery Dissection. JACC Cardiovasc. Interv. 2016, 9, 1851–1853. [Google Scholar] [CrossRef] [PubMed]
- Saw, J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter. Cardiovasc. Interv. 2013, 84, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Tweet, M.S.; Gulati, R.; Williamson, E.E.; Vrtiska, T.J.; Hayes, S.N. Multimodality Imaging for Spontaneous Coronary Artery Dissection in Women. JACC Cardiovasc. Imaging 2016, 9, 436–450. [Google Scholar] [CrossRef]
- Nepal, S.; Bishop, M.A. Spontaneous Coronary Artery Dissection. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tweet, M.S.; Pellikka, P.; Oh, J.; Hayes, S. Echocardiographic findings in acute spontaneous coronary artery dissection. J. Am. Coll. Cardiol. 2021, 77, 220. [Google Scholar] [CrossRef]
- Finn, A.V.; Chandrashekhar, Y.; Narula, J. IVUS and OCT: Either or survivor. J. Am. Coll. Cardiol. Imaging 2011, 4, 1047–1049. [Google Scholar] [CrossRef]
- Aoki, T.; Rodriguez-Porcel, M.; Matsuo, Y.; Cassar, A.; Kwon, T.-G.; Franchi, F.; Gulati, R.; Kushwaha, S.S.; Lennon, R.J.; Lerman, L.O.; et al. Evaluation of coronary adventitial vasa vasorum using 3D optical coherence tomography—Animal and human studies. Atherosclerosis 2015, 239, 203–208. [Google Scholar] [CrossRef]
- Leipsic, J.; Abbara, S.; Achenbach, S.; Cury, R.; Earls, J.P.; Mancini, G.J.; Nieman, K.; Pontone, G.; Raff, G.L. SCCT guidelines for the interpretation and reporting of coronary CT angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J. Cardiovasc. Comput. Tomogr. 2014, 8, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Aung, N.; Gallina, S.; Zemrak, F.; Fung, K.; Bisaccia, G.; Paiva, J.M.; Khanji, M.Y.; Mantini, C.; Palermi, S.; et al. Cardiovascular magnetic resonance reference values of mitral and tricuspid annular dimensions: The UK Biobank cohort. J. Cardiovasc. Magn. Reson. 2020, 23, 5. [Google Scholar] [CrossRef] [PubMed]
- Tweet, M.S.; Eleid, M.F.; Best, P.J.; Lennon, R.J.; Lerman, A.; Rihal, C.S.; Holmes, D.R., Jr.; Hayes, S.N.; Gulati, R. Spontaneous coronary artery dissection: Revascularization versus conservative therapy. Circ. Cardiovasc. Interv. 2014, 7, 777–786. [Google Scholar] [CrossRef]
- Kotecha, D.; Garcia-Guimaraes, M.; Premawardhana, D.; Pellegrini, D.; Oliver-Williams, C.; Bountziouka, V.; Wood, A.; Natarajan, N.; Jackson, R.; Chan, N.; et al. Risks and benefits of percutaneous coronary intervention in spontaneous coronary artery dissection. Heart 2021, 107, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.R.; Hoffman, M.; Marshall, E.S. Clopidogrel Use Throughout Pregnancy in a Patient With a Drug-Eluting Coronary Stent. Obstet. Gynecol. 2011, 118, 432–433. [Google Scholar] [CrossRef] [PubMed]
- Briggs, G.G.; Freeman, R.K. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk, 10th ed.; Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, PA, USA, 2014. [Google Scholar]
- Thomas, R.J.; King, M.; Lui, K.; Oldridge, N.; Pina, I.L.; Spertus, J. AACVPR/ACCF/AHA 2010 update: Performance measures on cardiac rehabilitation for referral to cardiac rehabilitation/secondary prevention services: A report of the American Association of Cardiovascular and Pulmonary Rehabilitation and the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures. Circulation 2010, 122, 1342–1350. [Google Scholar] [PubMed]
- Compagno, S.; Palermi, S.; Pescatore, V.; Brugin, E.; Sarto, M.; Marin, R.; Calzavara, V.; Nizzetto, M.; Scevola, M.; Aloi, A.; et al. Physical and psychological reconditioning in long COVID syndrome: Results of an out-of-hospital exercise and psychological-based rehabilitation program. IJC Heart Vasc. 2022, 41, 101080. [Google Scholar] [CrossRef] [PubMed]
- Palermi, S.; Bragazzi, N.L.; Cular, D.; Ardigò, L.P.; Padulo, J. How chest press-based exercises can alleviate the burden of cardiovascular diseases. Hum. Mov. 2022, 23, 88–98. [Google Scholar] [CrossRef]



| Predisposing Conditions | Precipitating Conditions |
|---|---|
Disorders of the connective tissue:
| Intense physical exercise (isometric or aerobic activity) Intense emotional stress Childbirth and labor of childbirth Intense Valsalva-like activity (retching, vomiting, coughing, bowel movements) Drugs (cocaine, amphetamines, methamphetamines) Hormonal therapies (injection of β-chorionic gonadotropins, corticosteroid injections) |
| Clinical Presentation | Incidence (%) |
|---|---|
| Chest pain | 91–100% |
| Dyspnea | 25–57% |
| Syncope | 23% |
| Malignant ventricular arrhythmias | 14% |
| Bradyarrhythmia | Rare |
| Cardiogenic shock | Rare |
| Unexpected death | Rare |
| Asymptomatic with random findings on coronary angiography | Rare |
| Coronarography | IVUS | OCT | CCTA | |
|---|---|---|---|---|
| Advantages | Easily executable Images suggestive of SCAD in most cases | Visualization of the intramural hematoma and the flap | Visualization of the vessel wall, the intimate, the false light, intramural hematoma, and any flaps and areas of communication between the true and false lumen | No possibility of iatrogenic damage Image of the presence of contrast in the false lumen Visualization of intramural hematoma |
| Limitations | Invasive Image of the vase light but not the wall | Passage of the guide in the coronary artery No identification of the middle intimal membrane No identification of small areas of connection between false and true lumen | Passage of catheter in coronary artery and injection of contrast at high pressure, with the danger of expansion of the dissection itself | Limited data on its use in acute Absence of specific diagnostic criteria for EXP Frequency-dependent motion artifacts Limited utility for pots < 2.5 mm in diameter Difficulty in distinguishing between non calcified atherosclerotic plaques and intramural hematoma |
| Use | Diagnosis of SCAD | Confirmation of diagnosis | The gold standard method for the confirmation of SCAD | Follow up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrazzo, G.; Palermi, S.; Pastore, F.; Ragni, M.; De Luca, M.; Gambardella, M.; Quaranta, G.; Messalli, G.; Riegler, L.; Pergola, V.; et al. Multimodality Imaging Approach to Spontaneous Coronary Artery Dissection. J. Clin. Med. 2023, 12, 154. https://doi.org/10.3390/jcm12010154
Marrazzo G, Palermi S, Pastore F, Ragni M, De Luca M, Gambardella M, Quaranta G, Messalli G, Riegler L, Pergola V, et al. Multimodality Imaging Approach to Spontaneous Coronary Artery Dissection. Journal of Clinical Medicine. 2023; 12(1):154. https://doi.org/10.3390/jcm12010154
Chicago/Turabian StyleMarrazzo, Gemma, Stefano Palermi, Fabio Pastore, Massimo Ragni, Mariarosaria De Luca, Michele Gambardella, Gaetano Quaranta, Giancarlo Messalli, Lucia Riegler, Valeria Pergola, and et al. 2023. "Multimodality Imaging Approach to Spontaneous Coronary Artery Dissection" Journal of Clinical Medicine 12, no. 1: 154. https://doi.org/10.3390/jcm12010154
APA StyleMarrazzo, G., Palermi, S., Pastore, F., Ragni, M., De Luca, M., Gambardella, M., Quaranta, G., Messalli, G., Riegler, L., Pergola, V., Manto, A., & D’Andrea, A. (2023). Multimodality Imaging Approach to Spontaneous Coronary Artery Dissection. Journal of Clinical Medicine, 12(1), 154. https://doi.org/10.3390/jcm12010154








