Changes in Choroidal Thickness and Retinal Activity with a Myopia Control Contact Lens
Abstract
1. Introduction
2. Methods
2.1. Study Design and Recruitment
2.2. Protocol
2.3. Material and Procedures
2.4. Statistics Analysis
3. Results
3.1. Sample Characteristics and Visual Acuity
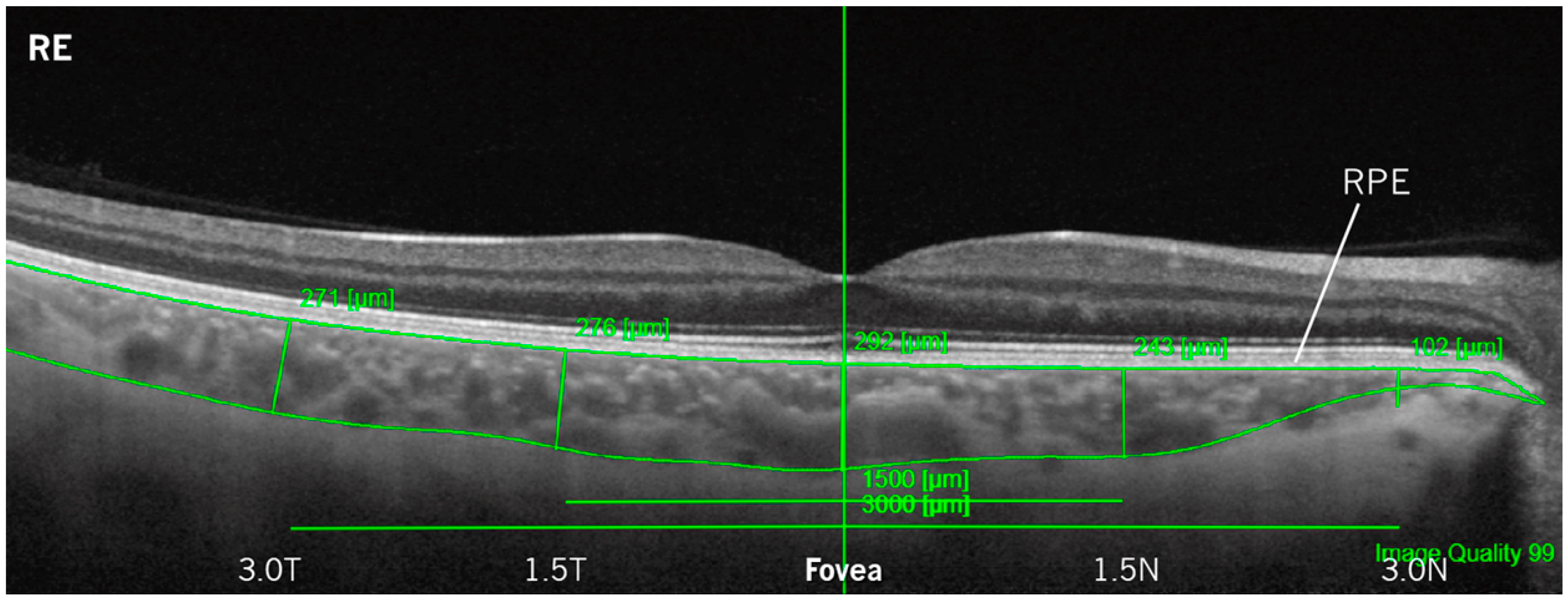
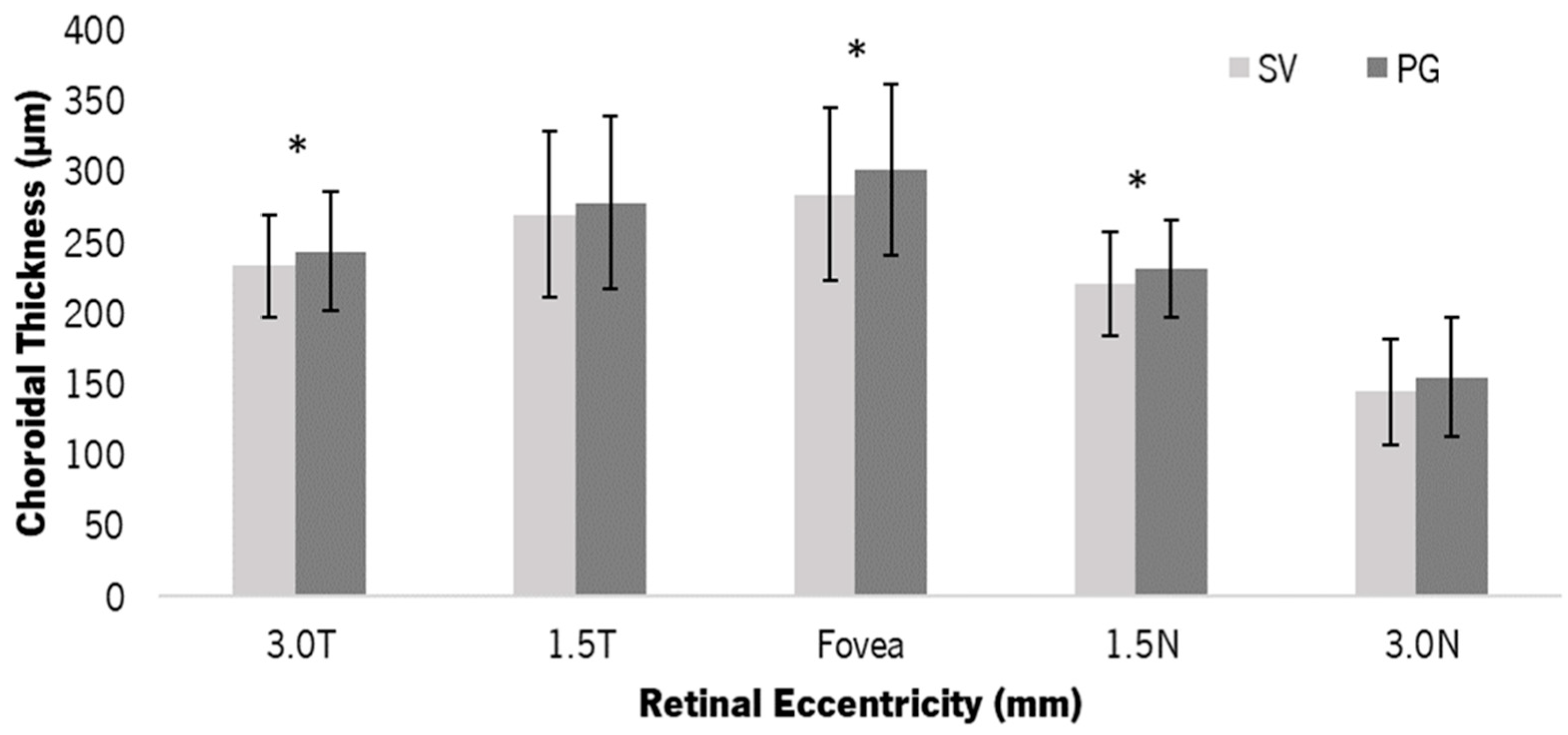
3.2. Choroidal Thickness
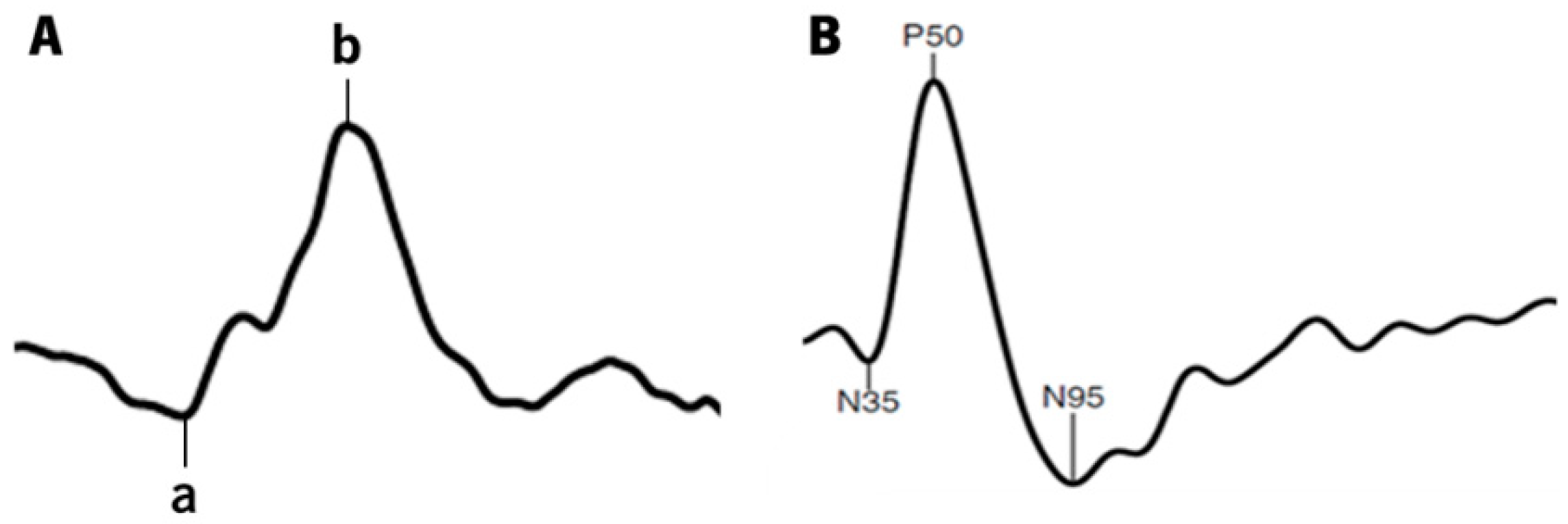
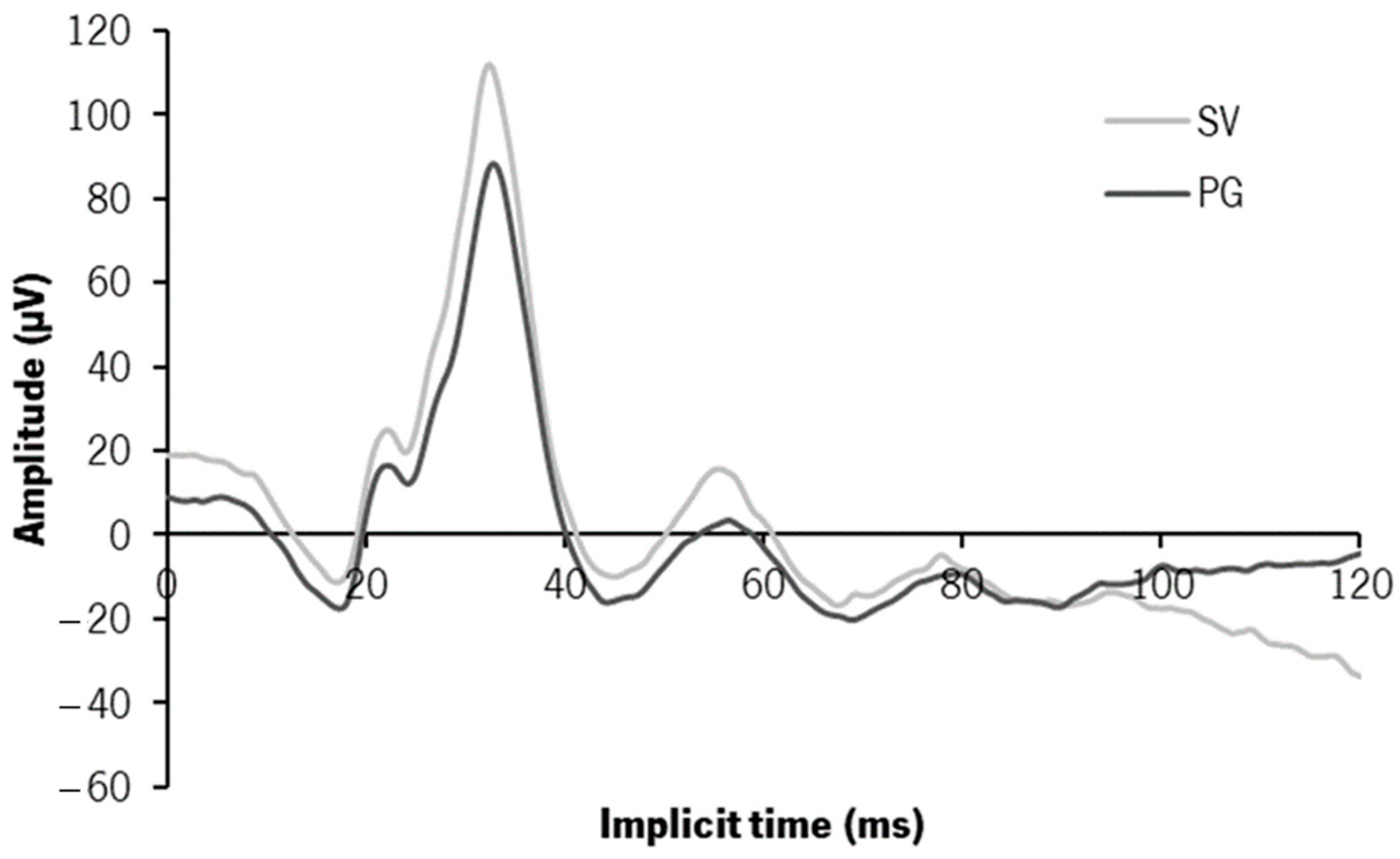
3.3. Electrophysiology—ffERG—Photopic 3.0 Response
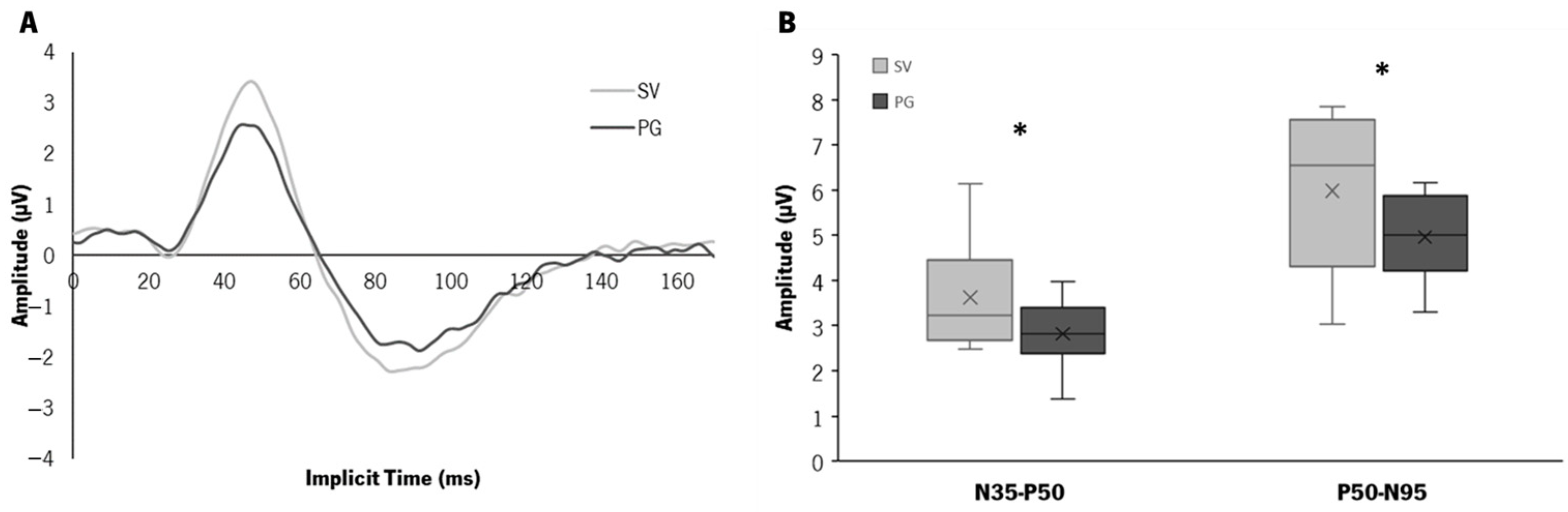
3.4. Electrophysiology—Pattern ERG Response
3.5. Choroidal Thickness and Electroretinogram
4. Discussion
4.1. Structural Changes—Choroidal Thickness
4.2. Functional Changes—ERG Findings
4.3. Relationship between Structural and Functional Changes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jonas, J.B.; Ang, M.; Cho, P.; Guggenheim, J.A.; He, M.G.; Jong, M.; Logan, N.S.; Liu, M.; Morgan, I.; Ohno-Matsui, K.; et al. IMI Prevention of Myopia and Its Progression. Investig. Ophthalmol. Vis. Sci. 2021, 62, 6. [Google Scholar] [CrossRef]
- Chua, S.Y.L.; Sabanayagam, C.; Cheung, Y.B.; Chia, A.; Valenzuela, R.K.; Tan, D.; Wong, T.Y.; Cheng, C.Y.; Saw, S.M. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol. Opt. 2016, 36, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Resnikoff, S.; Jonas, J.B.; Friedman, D.; He, M.; Jong, M.; Nichols, J.J.; Ohno-Matsui, K.; Smith, I.I.I.E.L.; Wildsoet, C.F.; Taylor, H.R.; et al. Myopia–A 21st Century Public Health Issue. Investig. Ophthalmol. Vis. Sci. 2019, 60, Mi–Mii. [Google Scholar] [CrossRef] [PubMed]
- Nickla, D.L.; Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef]
- Jin, P.; Zou, H.; Xu, X.; Ta, C.C.; Zhu, J.; Deng, J.; Lv, M.; Jin, J.; Sun, S.; Wang, L.; et al. Longitudinal Changes in Choroidal and Retinal Thicknesses in Children with Myopic Shift. Retina 2019, 39, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Xu, Y.; Pang, Z.; Mu, G. The influence of the choroid on the onset and development of myopia: From perspectives of choroidal thickness and blood flow. Acta Ophthalmol. 2021, 99, 730–738. [Google Scholar] [CrossRef]
- Fontaine, M.; Gaucher, D.; Sauer, A.; Speeg-Schatz, C. Choroidal thickness and ametropia in children: A longitudinal study. Eur. J. Ophthalmol. 2017, 27, 730–734. [Google Scholar] [CrossRef]
- Read, S.A.; Fuss, J.A.; Vincent, S.J.; Collins, M.J.; Alonso-Caneiro, D. Choroidal changes in human myopia: Insights from optical coherence tomography imaging. Clin. Exp. Optom. 2019, 102, 270–285. [Google Scholar] [CrossRef]
- Jin, P.; Zou, H.; Zhu, J.; Xu, X.; Jin, J.; Chang, T.C.; Lu, L.; Yuan, H.; Sun, S.; Yan, B.; et al. Choroidal and Retinal Thickness in Children With Different Refractive Status Measured by Swept-Source Optical Coherence Tomography. Am. J. Ophthalmol. 2016, 168, 164–176. [Google Scholar] [CrossRef]
- Read, S.A.; Collins, M.J.; Vincent, S.J.; Alonso-Caneiro, D. Choroidal Thickness in Myopic and Nonmyopic Children Assessed With Enhanced Depth Imaging Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7578. [Google Scholar] [CrossRef]
- Wei, W.B.; Xu, L.; Jonas, J.B.; Shao, L.; Du, K.F.; Wang, S.; Chen, C.X.; Xu, J.; Wang, Y.X.; Zhou, J.Q.; et al. Subfoveal Choroidal Thickness: The Beijing Eye Study. Ophthalmology 2013, 120, 175–180. [Google Scholar] [CrossRef]
- Wallman, J.; Winawer, J. Homeostasis of Eye Growth and the Question of Myopia. Neuron 2004, 43, 447–468. [Google Scholar] [CrossRef]
- Wallman, J.; Wildsoet, C.; Xu, A.; Gottlieb, M.D.; Nickla, D.L.; Marran, L.; Krebs, W.; Christensen, A.M. Moving the retina: Choroidal modulation of refractive state. Vis. Res. 1995, 35, 37–50. [Google Scholar] [CrossRef]
- Hung, L.F.; Wallman, J.; Smith, E., 3rd. Vision-dependent changes in the choroidal thickness of macaque monkeys. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1259–1269. [Google Scholar]
- Read, S.A.; Collins, M.J.; Sander, B.P. Human Optical Axial Length and Defocus. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6262. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.T.H.; Phillips, J.R.; Backhouse, S. Effect of retinal image defocus on the thickness of the human choroid. Ophthalmic Physiol. Opt. 2015, 35, 405–413. [Google Scholar] [CrossRef]
- Nava, D.R.; Antony, B.; Zhang, L.I.; Abràmoff, M.D.; Wildsoet, C.F. Novel method using 3-dimensional segmentation in spectral domain-optical coherence tomography imaging in the chick reveals defocus-induced regional and time-sensitive asymmetries in the choroidal thickness. Vis. Neurosci. 2016, 33, E010. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.T.H.; Phillips, J.R. Effect of Atropine Eye Drops on Choroidal Thinning Induced by Hyperopic Retinal Defocus. J. Ophthalmol. 2018, 2018, 8528315. [Google Scholar] [CrossRef]
- Swarbrick, H.A.; Alharbi, A.; Watt, K.; Lum, E.; Kang, P. Myopia Control during Orthokeratology Lens Wear in Children Using a Novel Study Design. Ophthalmology 2015, 122, 620–630. [Google Scholar] [CrossRef]
- Chen, Z.; Xue, F.; Zhou, J.; Qu, X.; Zhou, X. Effects of Orthokeratology on Choroidal Thickness and Axial Length. Optom. Vis. Sci. 2016, 93, 1064–1071. [Google Scholar] [CrossRef]
- Lee, J.H.; Hong, I.H.; Lee, T.Y.; Han, J.R.; Jeon, G.S. Choroidal Thickness Changes after Orthokeratology Lens Wearing in Young Adults with Myopia. Ophthalmic Res. 2021, 64, 121–127. [Google Scholar] [CrossRef]
- Li, Z.; Cui, D.; Hu, Y.; Ao, S.; Zeng, J.; Yang, X. Choroidal thickness and axial length changes in myopic children treated with orthokeratology. Contact Lens Anterior Eye 2017, 40, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Breher, K.; García, M.G.; Ohlendorf, A.; Wahl, S. The effect of the optical design of multifocal contact lenses on choroidal thickness. PLoS ONE 2018, 13, e0207637. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Brown, B.; Schmid, K.L. Delayed mfERG responses in myopia. Vis. Res. 2006, 46, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.C.; Kee, C.S.; Chan, H.H.L. Myopia progression in children is linked with reduced foveal mfERG response. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5320–5325. [Google Scholar] [CrossRef]
- Koh, V.; Tan, C.; Nah, G.; Zhao, P.; Yang, A.; Lin, S.T.; Wong, T.Y.; Saw, S.M.; Chia, A. Correlation of structural and electrophysiological changes in the retina of young high myopes. Ophthalmic Physiol. Opt. 2014, 34, 658–666. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.H.; Park, T.K.; Ohn, Y.H. Evaluation of structural and functional changes in non-pathologic myopic fundus using multifocal electroretinogram and optical coherence tomography. Doc. Ophthalmol. 2013, 126, 199–210. [Google Scholar] [CrossRef]
- Kader, M.A. Electrophysiological study of myopia. Saudi J. Ophthalmol. 2012, 26, 91–99. [Google Scholar] [CrossRef]
- Sachidanandam, R.; Ravi, P.; Sen, P. Effect of axial length on full-field and multifocal electroretinograms. Clin. Exp. Optom. 2017, 100, 668–675. [Google Scholar] [CrossRef]
- Field, G.D.; Gauthier, J.L.; Sher, A.; Greschner, M.; Machado, T.A.; Jepson, L.H.; Shlens, J.; Gunning, D.E.; Mathieson, K.; Dabrowski, W.; et al. Functional connectivity in the retina at the resolution of photoreceptors. Nature 2010, 467, 673–677. [Google Scholar] [CrossRef]
- Luu, C.D.; Foulds, W.S.; Tan, D.T.H. Features of the Multifocal Electroretinogram May Predict the Rate of Myopia Progression in Children. Ophthalmology 2007, 114, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Si, J.K.; Tang, K.; Bi, H.S.; Guo, D.D.; Guo, J.G.; Wang, X.R. Orthokeratology for Myopia Control. Optom. Vis. Sci. 2015, 92, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, P.; Peixoto-de-Matos, S.; Logan, N.; Ngo, C.; Jones, D.; Young, G. A 3-year Randomized Clinical Trial of MiSight Lenses for Myopia Control. Optom. Vis. Sci. 2019, 96, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Read, S.A.; Collins, M.J. Monocular myopic defocus and daily changes in axial length and choroidal thickness of human eyes. Exp. Eye Res. 2012, 103, 47–54. [Google Scholar] [CrossRef]
- Hyman, L.; Gwiazda, J.; Hussein, M. Relationship of Age, Sex, and Ethnicity With Myopia Progression and Axial Elongation in the Correction of Myopia Evaluation Trial. Arch. Ophthalmol. 2005, 123, 977–987. [Google Scholar] [CrossRef]
- Pauné, J.; Queiros, A.; Quevedo, L.; Neves, H.; Lopes-Ferreira, D.; González-Méijome, J.M. Peripheral myopization and visual performance with experimental rigid gas permeable and soft contact lens design. Contact Lens Anterior Eye 2014, 37, 455–460. [Google Scholar] [CrossRef]
- McCulloch, D.L.; Marmor, M.F.; Brigell, M.G.; Hamilton, R.; Holder, G.E.; Tzekov, R.; Bach, M. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc. Ophthalmol. 2015, 130, 1–12. [Google Scholar] [CrossRef]
- Bach, M.; Brigell, M.G.; Hawlina, M.; Holder, G.E.; Johnson, M.A.; McCulloch, D.L.; Meigen, T.; Viswanathan, S. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc. Ophthalmol. 2013, 126, 1–7. [Google Scholar] [CrossRef]
- Leipert, K.P.; Gottlob, I. Pattern electroretinogram: Effects of miosis, accommodation, and defocus. Doc. Ophthalmol. 1987, 67, 335–346. [Google Scholar] [CrossRef]
- Manjunath, V.; Taha, M.; Fujimoto, J.G.; Duker, J.S. Choroidal Thickness in Normal Eyes Measured Using Cirrus HD Optical Coherence Tomography. Am. J. Ophthalmol. 2010, 150, 325–329.e1. [Google Scholar] [CrossRef]
- Hoseini-Yazdi, H.; Vincent, S.J.; Collins, M.J.; Read, S.A. Regional alterations in human choroidal thickness in response to short-term monocular hemifield myopic defocus. Ophthalmic Physiol. Opt. 2019, 39, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Amorim-De-Sousa, A.; Faria-Ribeiro, M.; Pauné, J.; González-Méijome, J.M.; Queirós, A. Visual Performance and High-Order Aberrations with Different Contact Lens Prototypes with Potential for Myopia Control. Curr. Eye Res. 2020, 45, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Pauné, J.; Thivent, S.; Armengol, J.; Quevedo, L.; Faria-Ribeiro, M.; González-Méijome, J.M. Changes in Peripheral Refraction, Higher-Order Aberrations, and Accommodative Lag With a Radial Refractive Gradient Contact Lens in Young Myopes. Eye Contact Lens Sci. Clin. Pract. 2016, 42, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.K.; Wan, K.; Cheung, S.W.; Vincent, S.J.; Cho, P. Weekly changes in axial length and choroidal thickness in children during and following orthokeratology treatment with different compression factors. Transl. Vis. Sci. Technol. 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, Y.; Cui, D.; Long, W.; He, M.; Yang, X. Change in subfoveal choroidal thickness secondary to orthokeratology and its cessation: A predictor for the change in axial length. Acta Ophthalmol. 2019, 97, e454–e459. [Google Scholar] [CrossRef]
- Lam, B.L. Full-field Electroretinogram. In Electrophysiology of Vision: Clinical Testing and Applications; Lam, B.L., Ed.; Taylor & Francis: New York, NY, USA, 2005; pp. 1–64. [Google Scholar]
- Odom, J.V.; Maida, T.M.; Dawson, W.W. Pattern evoked retinal response (PERR) in human: Effects of spatial frequency, temporal frequency, luminance and defocus. Curr. Eye Res. 1982, 2, 99–108. [Google Scholar] [CrossRef]
- Ostrin, L.A.; Choh, V.; Wildsoet, C.F. The pattern ERG in chicks–Stimulus dependence and optic nerve section. Vis. Res. 2016, 128, 45–52. [Google Scholar] [CrossRef]
- Siegel, M.J.; Marx, M.S.; Bodis-Wollner, I.; Podos, S.M. The effect of refractive error on pattern electroretinograms in primates. Curr. Eye Res. 1986, 5, 183–187. [Google Scholar] [CrossRef]
- Bach, M.; Mathieu, M. Different effect of dioptric defocus vs. light scatter on the Pattern Electroretinogram (PERG). Doc. Ophthalmol. 2004, 108, 99–106. [Google Scholar] [CrossRef]
- Panorgias, A.; Aigbe, S.; Jeong, E.; Otero, C.; Bex, P.J.; Vera-Diaz, F.A. Retinal Responses to Simulated Optical Blur Using a Novel Dead Leaves ERG Stimulus. Investig. Ophthalmol. Vis. Sci. 2021, 62, 1. [Google Scholar] [CrossRef]
- Zhang, D.; Belenky, M.; Sollars, P.; Pickard, G.; McMahon, D. Melanopsin mediates retrograde visual signaling in the retina. PLoS ONE 2012, 7, e42647. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.E.; Kerschensteiner, D. Retrograde Plasticity and Differential Competition of Bipolar Cell Dendrites and Axons in the Developing Retina. Curr. Biol. 2014, 24, 2301–2306. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.J.; Paul, D.L.; Wu, S.M. Survey on Amacrine Cells Coupling to Retrograde-Identified Ganglion Cells in the Mouse Retina. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5151–5162. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.; Amorim-de-Sousa, A.; AWong, N.; Bahmani, H.; González-Méijome, J.M.; Fernandes, P. Increase in b-wave amplitude after light stimulation of the blind spot is positively correlated with the axial length of myopic individuals. Sci. Rep. 2022, 12, 4785. [Google Scholar] [CrossRef]
- Amorim-de-Sousa, A.; Schilling, T.; Fernandes, P.; Seshadri, Y.; Bahmani, H.; González-Méijome, J.M. Blue light blind-spot stimulation upregulates b-wave and pattern ERG activity in myopes. Sci. Rep. 2021, 11, 9273. [Google Scholar] [CrossRef]
- Pauné, J.; Morales, H.; Armengol, J.; Quevedo, L.; Faria-Ribeiro, M.; González-Méijome, J.M. Myopia Control with a Novel Peripheral Gradient Soft Lens and Orthokeratology: A 2-Year Clinical Trial. BioMed Res. Int. 2015, 2015, 507572. [Google Scholar] [CrossRef]
- Speros, P.; Price, J. Oscillatory potentials. History, techniques and potential use in the evaluation of disturbances of retinal circulation. Surv. Ophthalmol. 1981, 25, 237–252. [Google Scholar] [CrossRef]

| SV | PG | diff p-Value | ||
|---|---|---|---|---|
| Implicit time (ms) | a-wave | 17.32 [1.25] | 17.47 [1.18] | 0.15 [1.20] 0.512 |
| b-wave | 32.29 [1.47] | 33.03 [1.32] | 0.50 [0.60] 0.049 | |
| Amplitude (µV) | a-wave | 38.39 [16.71] | 28.20 [10.67] | 0.25 [15.75] 0.093 |
| b-wave | 112.41 [63.24] | 105.62 [44.85] | 11.80 [30.55] 0.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorim-de-Sousa, A.; Pauné, J.; Silva-Leite, S.; Fernandes, P.; Gozález-Méijome, J.M.; Queirós, A. Changes in Choroidal Thickness and Retinal Activity with a Myopia Control Contact Lens. J. Clin. Med. 2023, 12, 3618. https://doi.org/10.3390/jcm12113618
Amorim-de-Sousa A, Pauné J, Silva-Leite S, Fernandes P, Gozález-Méijome JM, Queirós A. Changes in Choroidal Thickness and Retinal Activity with a Myopia Control Contact Lens. Journal of Clinical Medicine. 2023; 12(11):3618. https://doi.org/10.3390/jcm12113618
Chicago/Turabian StyleAmorim-de-Sousa, Ana, Jaume Pauné, Sara Silva-Leite, Paulo Fernandes, José Manuel Gozález-Méijome, and António Queirós. 2023. "Changes in Choroidal Thickness and Retinal Activity with a Myopia Control Contact Lens" Journal of Clinical Medicine 12, no. 11: 3618. https://doi.org/10.3390/jcm12113618
APA StyleAmorim-de-Sousa, A., Pauné, J., Silva-Leite, S., Fernandes, P., Gozález-Méijome, J. M., & Queirós, A. (2023). Changes in Choroidal Thickness and Retinal Activity with a Myopia Control Contact Lens. Journal of Clinical Medicine, 12(11), 3618. https://doi.org/10.3390/jcm12113618










