Rupture of Splenic Artery Aneurysm in Patient with ACTN2 Mutation
Abstract
:1. Introduction
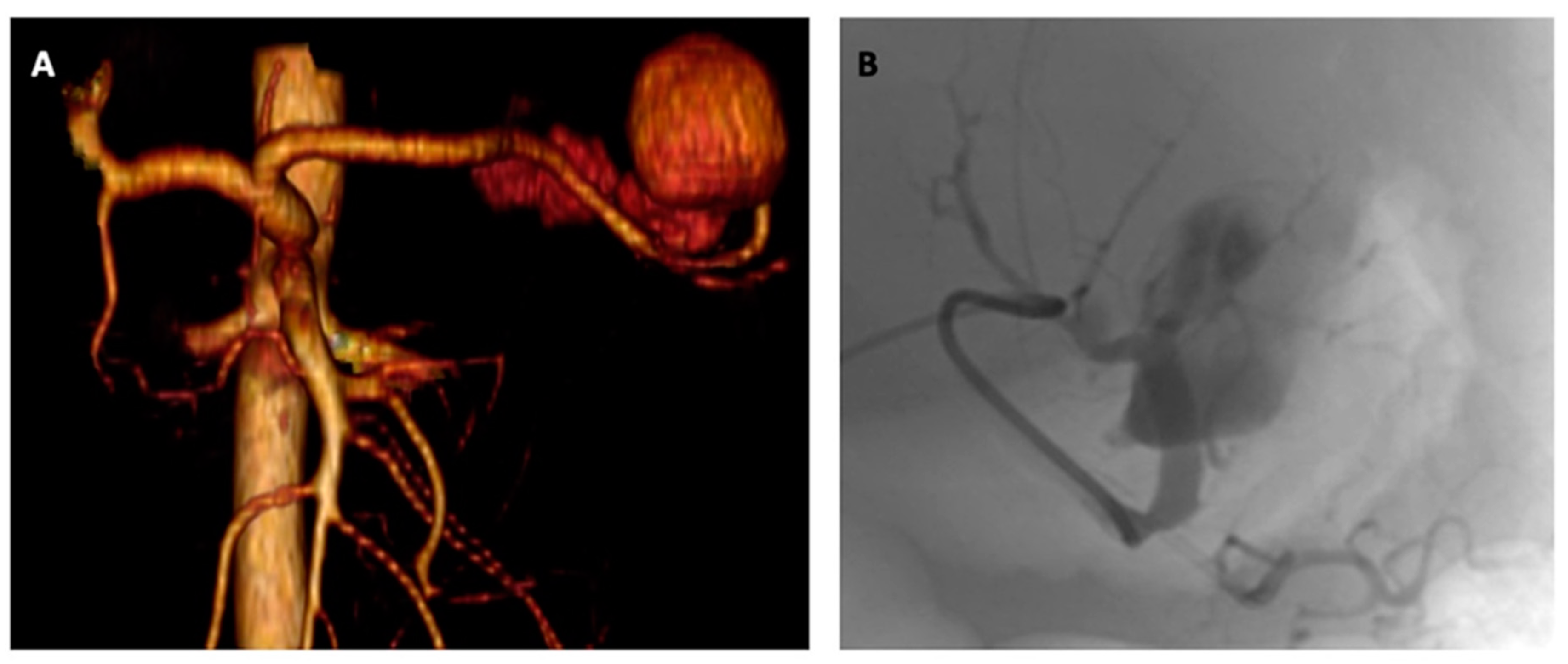
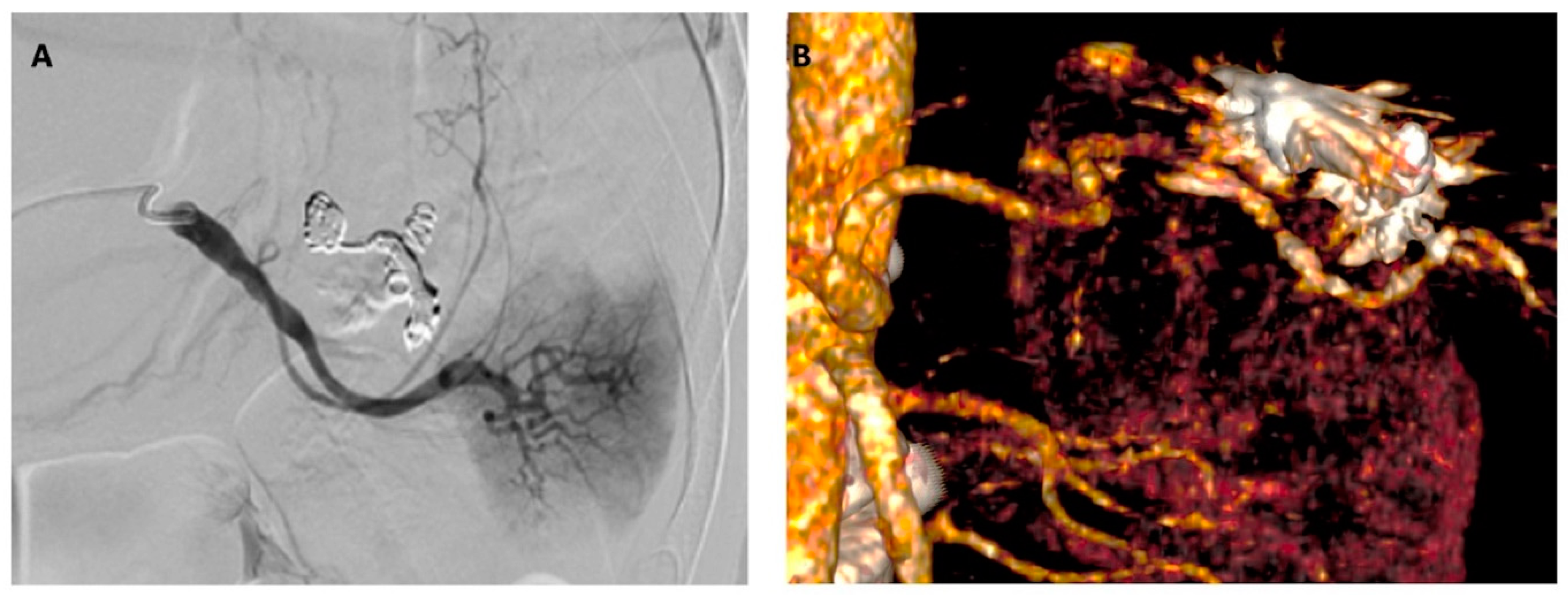
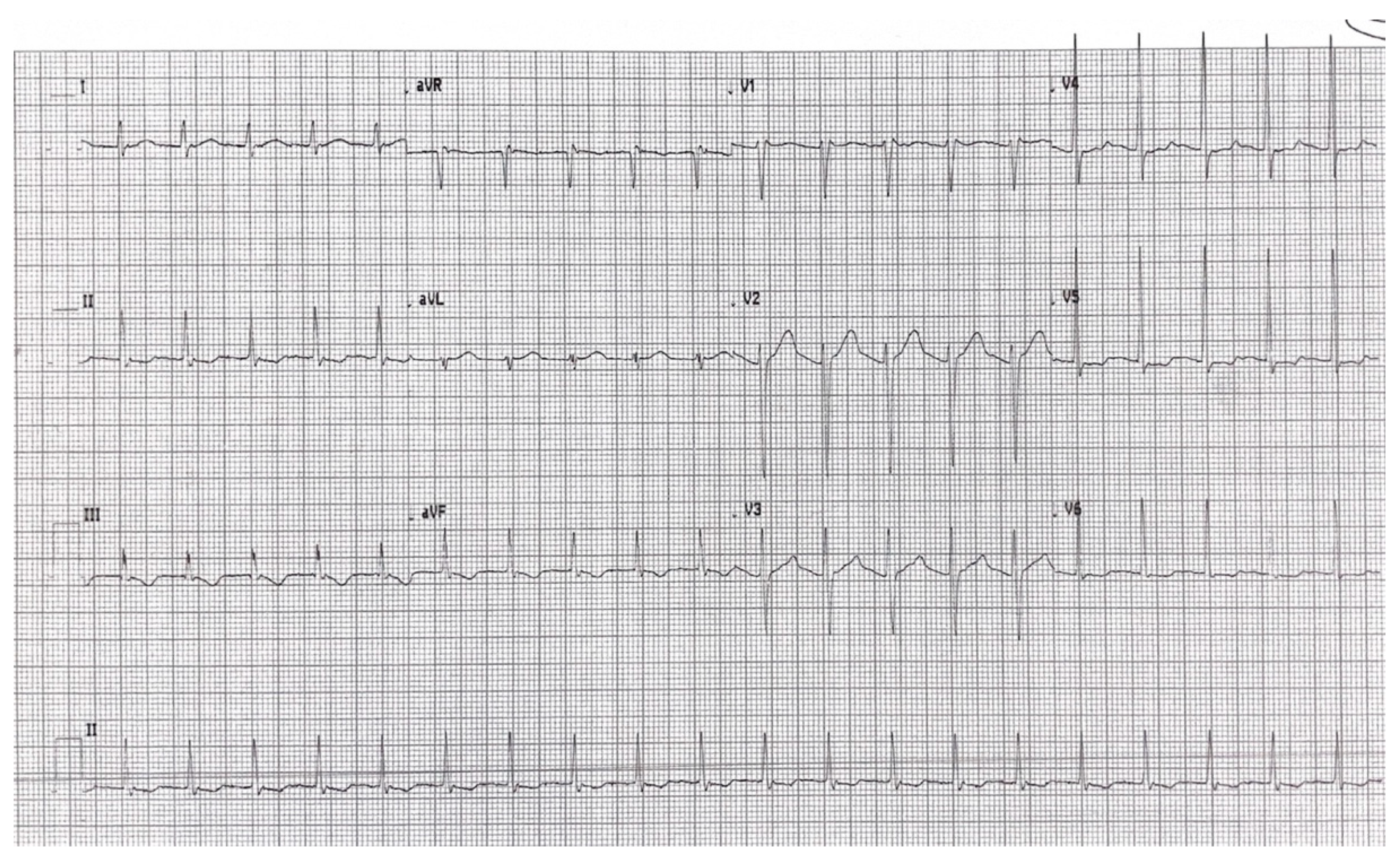
2. Detailed Case Description
3. Discussion
- The aorta is an elastic artery, in which the media is composed of SMCs lying between layers of elastic fibers. In contrast, medium and small arteries are muscular arteries, in which the SMCs are attached to each other and not the layers of elastic fibers [22]. Moreover, elastin has an established role in increasing SMC differentiation and decreasing SMC proliferation, and the lack of SMC and elastic fiber interaction may increase proliferation in muscular arteries [23].
- The aorta (especially its thoracic portion) bears most of the pulsatile blood forces, whereas other arteries experience significantly less stress. Those physiologically different forces may activate different pathways in the mutant SMCs, leading to different vascular disease presentation [24].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blanchard, A.; Ohanian, V.; Critchley, D. The structure and function of alpha-actinin. J. Muscle Res. Cell Motil. 1989, 10, 280–289. [Google Scholar] [CrossRef]
- Young, P.; Ferguson, C.; Bañuelos, S.; Gautel, M. Molecular structure of the sarcomeric Z-disk: Two types of titin interactions lead to an asymmetrical sorting of alpha-actinin. EMBO J. 1998, 17, 1614–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, P.; Gautel, M. The interaction of titin and alpha-actinin is controlled by a phospholipid-regulated intramolecular pseudoligand mechanism. EMBO J. 2000, 19, 6331–6340. [Google Scholar] [CrossRef]
- Eilertsen, K.J.; Kazmierski, S.T.; Keller, T.C., 3rd. Interaction of alpha-actinin with cellular titin. Eur. J. Cell Biol. 1997, 74, 361–364. [Google Scholar] [PubMed]
- Salmikangas, P.; van der Ven, P.F.M.; Lalowski, M.; Taivainen, A.; Zhao, F.; Suila, H.; Schröder, R.; Lappalainen, P.; Fürst, D.O.; Carpén, O. Myotilin, the limb-girdle muscular dystrophy 1A (LGMD1A) protein, cross-links actin filaments and controls sarcomere assembly. Hum. Mol. Genet. 2003, 12, 189–203. [Google Scholar] [CrossRef]
- Bang, M.L.; Mudry, R.E.; McElhinny, A.S.; Trombitás, K.; Geach, A.J.; Yamasaki, R.; Sorimachi, H.; Granzier, H.; Gregorio, C.C.; Labeit, S. Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J. Cell Biol. 2001, 153, 413–427. [Google Scholar] [CrossRef]
- Sjöblom, B.; Salmazo, A.; Djinović-Carugo, K. α-Actinin structure and regulation. Cell. Mol. Life Sci. 2008, 65, 2688. [Google Scholar] [CrossRef]
- Ranta-Aho, J.; Olive, M.; Vandroux, M.; Roticiani, G.; Dominguez, C.; Johari, M.; Torella, A.; Böhm, J.; Turon, J.; Nigro, V.; et al. Mutation update for the ACTN2 gene. Hum. Mutat. 2022, 43, 1745–1756. [Google Scholar] [CrossRef]
- Guo, D.C.; Papke, C.L.; Tran-Fadulu, V.; Regalado, E.S.; Avidan, N.; Johnson, R.J.; Kim, D.H.; Pannu, H.; Willing, M.C.; Sparks, E.; et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am. J. Hum. Genet. 2009, 84, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Vandekerckhove, J.; Weber, K. At least six different actins are expressed in a higher mammal: An analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J. Mol. Biol. 1978, 126, 783–802. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.M.; Crawford, K.; Lessard, J.L. A comprehensive analysis of the developmental and tissue-specific expression of the isoactin multigene family in the rat. Dev. Biol. 1991, 148, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Fatigati, V.; Murphy, R.A. Actin and tropomyosin variants in smooth muscles. Dependence on tissue type. J. Biol. Chem. 1984, 259, 14383–14388. [Google Scholar] [CrossRef]
- Zhu, L.; Vranckx, R.; Khau Van Kien, P.; Lalande, A.; Boisset, N.; Mathieu, F.; Wegman, M.; Glancy, L.; Gasc, J.-M.; Brunotte, F.; et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat. Genet. 2006, 38, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Pannu, H.; Tran-Fadulu, V.; Papke, C.L.; Scherer, S.; Liu, Y.; Presley, C.; Guo, D.; Estrera, A.L.; Safi, H.J.; Brasier, A.R.; et al. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum. Mol. Genet. 2007, 16, 2453–2462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, D.C.; Pannu, H.; Tran-Fadulu, V. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat. Genet. 2007, 39, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Ziane, R.; Huang, H.; Moghadaszadeh, B.; Beggs, A.H.; Levesque, G.; Chahine, M. Cell membrane expression of cardiac sodium channel Nav1.5 is modulated by α-actinin-2 interaction. Biochemistry 2010, 49, 166–178. [Google Scholar] [CrossRef] [Green Version]
- Girolami, F.; Iascone, M.; Tomberli, B.; Bardi, S.; Benelli, M.; Marseglia, G.; Pescucci, C.; Pezzoli, L.; Sana, M.E.; Basso, C.; et al. Novel α-actinin 2 variant associated with familial hypertrophic cardiomyopathy and juvenile atrial arrhythmias: A massively parallel sequencing study. Circ. Cardiovasc. Genet. 2014, 7, 741–750. [Google Scholar] [CrossRef]
- Chiu, C.; Bagnall, R.D.; Ingles, J.; Yeates, L.; Kennerson, M.; Donald, J.A.; Jormakka, M.; Lind, J.M.; Semsarian, C. Mutations in alpha-actinin-2 cause hypertrophic cardiomyopathy: A genome-wide analysis. J. Am. Coll. Cardiol. 2010, 55, 1127–1135. [Google Scholar] [CrossRef] [Green Version]
- Coll, M.; Pérez-Serra, A.; Mates, J.; Del Olmo, B.; Puigmulé, M.; Fernandez-Falgueras, A.; Iglesias, A.; Pico, F.; Lopez, L.; Brugada, R.; et al. Incomplete Penetrance and Variable Expressivity: Hallmarks in Channelopathies Associated with Sudden Cardiac Death. Biology 2017, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.L.; Huang, H.; Jin, J.Y.; Li, J.J.; Chen, Y.Q.; Xiang, R. Whole-exome sequencing identifies a novel mutation (p.L320R) of alpha-actinin 2 in a Chinese family with dilated cardiomyopathy and ventricular tachycardia. Cytogenet. Genome Res. 2019, 157, 148–152. [Google Scholar] [CrossRef]
- Murphy, A.C.; Young, P.W. The actinin family of actin cross-linking proteins—A genetic perspective. Cell Biosci. 2015, 5, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphrey, J.D. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs; Springer: New York, NY, USA, 2022; pp. 249–264. [Google Scholar]
- Brooke, B.S.; Bayes-Genis, A.; Li, D.Y. New insights into elastin and vascular disease. Trends Cardiovasc. Med. 2003, 13, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Topouzis, S.; Majesky, M.W. Smooth muscle lineage diversity in the chick embryo. Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-beta 3. Dev. Biol. 1996, 178, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Angwin, C.; Ghali, N.; van Dijk, F.S. Case report: Two individuals with AEBP1-related classical-like EDS: Further clinical characterisation and description of novel AEBP1 variants. Front. Genet. 2023, 14, 1148224. [Google Scholar] [CrossRef] [PubMed]
- Law, N.L.; Villada, F.A.; Kruse, M.J. Rupture of splenic artery aneurysm in a man with polycythemia vera and acquired von Willebrand syndrome. BMJ Case Rep. 2021, 14, e243316. [Google Scholar] [CrossRef]
- Sellier, J.; Karam, C.; Beauchet, A.; Dallongeville, A.; Binsse, S.; Blivet, S.; Bourgault-Villada, I.; Charron, P.; Chinet, T.; Eyries, M.; et al. Higher prevalence of splenic artery aneurysms in hereditary hemorrhagic telangiectasia: Vascular implications and risk factors. PLoS ONE 2020, 15, e0226681. [Google Scholar] [CrossRef] [Green Version]
- Serratrice, C.; Cox, T.M.; Leguy-Seguin, V.; Morris, E.; Yousfi, K.; Monnet, O.; Sibert, A.; Allaham, W.; Belmatoug, N. Splenic Artery Aneurysms, A Rare Complication of Type 1 Gaucher Disease: Report of Five Cases. J. Clin. Med. 2019, 8, 219. [Google Scholar] [CrossRef] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palughi, M.; Sirignano, P.; Stella, N.; Rossi, M.; Fiorani, L.; Taurino, M. Rupture of Splenic Artery Aneurysm in Patient with ACTN2 Mutation. J. Clin. Med. 2023, 12, 4729. https://doi.org/10.3390/jcm12144729
Palughi M, Sirignano P, Stella N, Rossi M, Fiorani L, Taurino M. Rupture of Splenic Artery Aneurysm in Patient with ACTN2 Mutation. Journal of Clinical Medicine. 2023; 12(14):4729. https://doi.org/10.3390/jcm12144729
Chicago/Turabian StylePalughi, Martina, Pasqualino Sirignano, Nazzareno Stella, Michele Rossi, Laura Fiorani, and Maurizio Taurino. 2023. "Rupture of Splenic Artery Aneurysm in Patient with ACTN2 Mutation" Journal of Clinical Medicine 12, no. 14: 4729. https://doi.org/10.3390/jcm12144729




