Cardiologist-Directed Sedation Management in Patients Undergoing Transvenous Lead Extraction: A Single-Centre Retrospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Data Analysis
2.2. Transvenous Lead Extraction and Sedation Management
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Lead Characteristics and TLE Techniques
3.3. Medication Dosages during DS
3.4. Deep Sedation-Related Outcomes
3.5. Procedural TLE Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurtz, S.M.; Ochoa, J.A.; Lau, E.; Shkolnikov, Y.; Pavri, B.B.; Frisch, D.; Greenspon, A.J. Implantation trends and patient profiles for pacemakers and implantable cardioverter defibrillators in the United States: 1993–2006. Pacing Clin. Electrophysiol. 2010, 33, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Greenspon, A.J.; Patel, J.D.; Lau, E.; Ochoa, J.A.; Frisch, D.R.; Ho, R.T.; Pavri, B.B.; Kurtz, S.M. 16-Year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States: 1993 to 2008. J. Am. Coll. Cardiol. 2011, 58, 1001–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udo, E.O.; Zuithoff, N.P.A.; van Hemel, N.M.; de Cock, C.C.; Hendriks, T.; Doevendans, P.A.; Moons, K.G.M. Incidence and predictors of short- and long-term complications in pacemaker therapy: The FOLLOWPACE study. Heart Rhythm 2012, 9, 728–735. [Google Scholar] [CrossRef]
- Schwarzwald, S.N.; Kersten, D.J.; Shaikh, Z.A.; Needelman, B.S.; Feldman, A.M.; Germano, J.; Islam, S.; Cohen, T.J. Mechanisms of Lead Failure by Recall Status and Manufacturer: Results From the Pacemaker and Implantable Defibrillator Leads Survival Study (“PAIDLESS”). J. Invasive Cardiol. 2018, 30, 147–151. [Google Scholar]
- Zabek, A.; Malecka, B.; Haberka, K.; Boczar, K.; Pfitzner, R.; Debski, M.; Lelakowski, J. The analysis of indications and early results of transvenous lead extraction in patients with a pacemaker, ICD and CRT—Single-center experience. Acta Cardiol. 2015, 70, 685–692. [Google Scholar] [CrossRef]
- Bongiorni, M.G.; Burri, H.; Deharo, J.C.; Starck, C.; Kennergren, C.; Saghy, L.; Rao, A.; Tascini, C.; Lever, N.; Kutarski, A.; et al. 2018 EHRA expert consensus statement on lead extraction: Recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: Endorsed by APHRS/HRS/LAHRS. EP Eur. 2018, 20, 1217. [Google Scholar] [CrossRef]
- Wilkoff, B.L.; Love, C.J.; Byrd, C.L.; Bongiorni, M.G.; Carrillo, R.G.; Crossley, G.H.; Epstein, L.M.; Friedman, R.A.; Kennergren, C.E.H.; Mitkowski, P.; et al. Transvenous Lead Extraction: Heart Rhythm Society Expert Consensus on Facilities, Training, Indications, and Patient Management. Heart Rhythm 2009, 6, 1085–1104. [Google Scholar] [CrossRef]
- Epstein, L.M.; Maytin, M. Strategies for Transvenous Lead Extraction Procedures. J. Innov. Card. Rhythm Manag. 2017, 8, 2702–2716. [Google Scholar] [CrossRef] [Green Version]
- Bongiorni, M.G.; Kennergren, C.; Butter, C.; Deharo, J.C.; Kutarski, A.; Rinaldi, C.A.; Romano, S.L.; Maggioni, A.P.; Andarala, M.; Auricchio, A.; et al. The European Lead Extraction ConTRolled (ELECTRa) study: A European Heart Rhythm Association (EHRA) Registry of Transvenous Lead Extraction Outcomes. Eur. Heart J. 2017, 38, 2995–3005. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology 2002, 96, 1004–1017. [Google Scholar] [CrossRef]
- Lachlan, T.; He, H.; Aggour, H.; Sahota, P.; Harvey, S.; Patel, K.; Foster, W.; Yusuf, S.; Panikker, S.; Dhanjal, T.; et al. Safety and feasibility of trans-venous cardiac device extraction using conscious sedation alone-Implications for the post-COVID-19 era. J. Arrhythmia 2021, 37, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Kottkamp, H.; Hindricks, G.; Eitel, C.; Müller, K.; Siedziako, A.; Koch, J.; Anastasiou-Nana, M.; Varounis, C.; Arya, A.; Sommer, P.; et al. Deep sedation for catheter ablation of atrial fibrillation: A prospective study in 650 consecutive patients. J. Cardiovasc. Electrophysiol. 2011, 22, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.J.; Davidson, N.C.; Royle, M.; Bennett, D.H.; Clarke, B.; Garratt, C.J.; Hall, M.C.S.; Zaidi, A.M.; Patterson, K.; Fitzpatrick, A.P. Safety and acceptability of implantation of internal cardioverter-defibrillators under local anesthetic and conscious sedation. Pacing Clin. Electrophysiol. 2007, 30, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Stronati, G.; Capucci, A. Sedation in cardiac arrhythmias management. Expert Rev. Cardiovasc. Ther. 2018, 16, 163–173. [Google Scholar] [CrossRef]
- Deharo, J.C.; Bongiorni, M.G.; Rozkovec, A.; Bracke, F.; Defaye, P.; Fernandez-Lozano, I.; Golzio, P.G.; Hansky, B.; Kennergren, C.; Manolis, A.S.; et al. Pathways for training and accreditation for transvenous lead extraction: A European Heart Rhythm Association position paper. Europace 2012, 14, 124–134. [Google Scholar] [CrossRef]
- Lü, F.; Lin, J.; Benditt, D.G. Conscious sedation and anesthesia in the cardiac electrophysiology laboratory. J. Cardiovasc. Electrophysiol. 2013, 24, 237–245. [Google Scholar] [CrossRef]
- Furniss, S.S.; Sneyd, J.R. Safe sedation in modern cardiological practice. Heart 2015, 101, 1526–1530. [Google Scholar] [CrossRef]
- O’Connor, M.; Schmidt, P.; Knoll, K.; Schaarschmidt, C.; Bock, M.; Bahlke, F.; Georgi, M.; Fröhlich, R.; Sonne, C.; Kottmaier, M.; et al. Patient Characteristics, Procedural Characteristics, and Outcomes in Patients Having Lead Extraction in a High-Volume Center. Am. J. Cardiol. 2022, 176, 51–57. [Google Scholar] [CrossRef]
- Hyman, M.C.; Vemulapalli, S.; Szeto, W.Y.; Stebbins, A.; Patel, P.A.; Matsouaka, R.A.; Herrmann, H.C.; Anwaruddin, S.; Kobayashi, T.; Desai, N.D.; et al. Conscious Sedation Versus General Anesthesia for Transcatheter Aortic Valve Replacement: Insights from the National Cardiovascular Data Registry Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation 2017, 136, 2132–2140. [Google Scholar] [CrossRef]
- Patzelt, J.; Ulrich, M.; Magunia, H.; Sauter, R.; Droppa, M.; Jorbenadze, R.; Becker, A.S.; Walker, T.; von Bardeleben, R.S.; Grasshoff, C.; et al. Comparison of Deep Sedation with General Anesthesia in Patients Undergoing Percutaneous Mitral Valve Repair. J. Am. Heart Assoc. 2017, 6, e007485. [Google Scholar] [CrossRef] [Green Version]
- Notarstefano, P.; Pratola, C.; Toselli, T.; Baldo, E.; Ferrari, R. Sedation with midazolam for electrical cardioversion. Pacing Clin. Electrophysiol. 2007, 30, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.J. Sympathetic and hemodynamic effects of moderate and deep sedation with propofol in humans. Anesthesiology 2005, 103, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Bode, K.; Whittaker, P.; Lucas, J.; Müssigbrodt, A.; Hindricks, G.; Richter, S.; Doering, M. Deep sedation for transvenous lead extraction: A large single-centre experience. EP Eur. 2019, 21, 1246–1253. [Google Scholar] [CrossRef]
- Perel, A. Non-anaesthesiologists should not be allowed to administer propofol for procedural sedation: A Consensus Statement of 21 European National Societies of Anaesthesia. Eur. J. Anaesthesiol. 2011, 28, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Kezerashvili, A.; Fisher, J.D.; DeLaney, J.; Mushiyev, S.; Monahan, E.; Taylor, V.; Kim, S.G.; Ferrick, K.J.; Gross, J.N.; Palma, E.C.; et al. Intravenous sedation for cardiac procedures can be administered safely and cost-effectively by non-anesthesia personnel. J. Interv. Card. Electrophysiol. 2008, 21, 43–51. [Google Scholar] [CrossRef]
- Morani, G.; Borio, G.; Bolzan, B.; Ribichini, F.L. Safety and efficacy of a cardiologist-only approach to deep sedation for electrical cardioversion. J. Cardiovasc. Med. 2019, 20, 16–22. [Google Scholar] [CrossRef]
- Coll-Vinent, B.; Sala, X.; Fernández, C.; Bragulat, E.; Espinosa, G.; Miró, O.; Millá, J.; Sánchez, M. Sedation for cardioversion in the emergency department: Analysis of effectiveness in four protocols. Ann. Emerg. Med. 2003, 42, 767–772. [Google Scholar] [CrossRef]
- Kaye, P.; Govier, M. Procedural sedation with propofol for emergency DC cardioversion. Emerg. Med. J. 2014, 31, 904–908. [Google Scholar] [CrossRef] [Green Version]
- Protopapas, A.A.; Stournaras, E.; Neokosmidis, G.; Stogiannou, D.; Filippidis, A.; Protopapas, A.N. Endoscopic sedation practices of Greek gastroenterologists: A nationwide survey. Ann. Gastroenterol. 2020, 33, 366–373. [Google Scholar] [CrossRef]
- Foerschner, L.; Harfoush, N.; Thoma, M.; Spitzbauer, L.; Popa, M.; Bourier, F.; Reents, T.; Kantenwein, V.; Telishevska, M.; Wimbauer, K.; et al. Deep sedation with propofol in patients undergoing left atrial ablation procedures—Is it safe? Heart Rhythm O2 2022, 3, 288–294. [Google Scholar] [CrossRef]
- Thomas, S.P.; Thakkar, J.; Kovoor, P.; Thiagalingam, A.; Ross, D.L. Sedation for electrophysiological procedures. Pacing Clin. Electrophysiol. 2014, 37, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Gong, J.; He, X.; Wu, Z.; Shen, J.; Shang, J. Body mass index and pharmacodynamics of target-controlled infusion of propofol: A prospective non-randomized controlled study. J. Clin. Pharm. Ther. 2022, 47, 662–667. [Google Scholar] [CrossRef] [PubMed]

| Mean ± SD or Count (%) | p-Value | |||
|---|---|---|---|---|
| Total | No Complications (n = 256) | Complications (n = 72) | ||
| Baseline characteristics | ||||
| Age (years) | 65.4 ± 17.1 | 64.7 ± 17.5 | 68.0 ± 15.6 | 0.1 |
| Male (n, %) | 232 (70.7%) | 181 (70.7%) | 51 (70.8%) | 0.9 |
| BMI (kg/m2) | 27.1 ± 5.2 | 27.3 ± 5.3 | 26.3 ± 4.7 | 0.2 |
| Heart function (LV-EF in %) | 42.6 ± 14.4 | 43.8 ± 14.1 | 38.7 ± 15.0 | 0.01 |
| Kidney function (GFR in ml/min) | 68.8 ± 27.0 | 70.9 ± 27.3 | 61.1 ± 24.5 | 0.01 |
| Comorbidities | ||||
| Ischemic Cardiomyopathy (n, %) | 128 | 96 (37.5%) | 32 (44.4%) | 0.3 |
| Dilatative Cardiomyopathy (n, %) | 56 | 41 (16.0%) | 15 (20.8%) | 0.4 |
| Channelopathy (n, %) | 11 | 9 (3.5%) | 2 (2.8%) | 0.6 |
| Structural heart disease (n, %) | 37 | 30 (11.7%) | 7 (9.7%) | 0.8 |
| Congenital heart disease (n, %) | 18 | 16 (6.3%) | 2 (2.8%) | 0.4 |
| Other heart disease (n, %) | 78 | 64 (25.0%) | 14 (19.4%) | 0.4 |
| ASA classification | ||||
| ASA 1 (n, %) | 7 | 7 (2.7%) | 0 (0.0%) | 0.2 |
| ASA 2 (n, %) | 30 | 29 (11.3%) | 1 (1.4%) | 0.01 |
| ASA 3 (n, %) | 272 | 209 (81.6%) | 63 (87.5%) | 0.3 |
| ASA 4 (n, %) | 17 | 9 (3.5%) | 8 (11.1%) | 0.01 |
| TLE indications | ||||
| Pocket infection (n, %) | 47 | 36 (14.1%) | 11 (15.3%) | 0.8 |
| Lead endocarditis (n, %) | 48 | 34 (13.3%) | 14 (19.4%) | 0.2 |
| Lead failure (n, %) | 148 | 124 (48.4%) | 24 (33.3%) | 0.02 |
| Lead dislocation (n, %) | 24 | 20 (7.8%) | 4 (5.6%) | 0.6 |
| Chronic pain (n, %) | 1 | 1 (0.4%) | 0 (0.0%) | 0.8 |
| Lead perforation (n, %) | 33 | 21 (8.2%) | 12 (16.7%) | 0.04 |
| Vascular complication (n, %) | 18 | 11 (4.3%) | 7 (9.7%) | 0.1 |
| Patients demand (n, %) | 8 | 8 (3.1%) | 0 (0.0%) | 0.2 |
| System relocation (n, %) | 1 | 1 (0.4%) | 0 (0.0%) | 0.8 |
| Procedural data | ||||
| Duration (h) | 1.7 ± 0.8 | 1.6 ± 0.7 | 2.0 ± 0.8 | <0.01 |
| Midazolam (mg/kg) | 42.9 ± 26.5 | 43.2 ± 26.5 | 42.1 ± 26.5 | 0.8 |
| Propofol (mg/kg/h) | 3.5 ± 1.2 | 3.6 ± 1.2 | 3.3 ± 1.1 | 0.1 |
| Fentanyl (µg/kg) | 0.4 ± 0.6 | 0.3 ± 0.6 | 0.4 ± 0.8 | 0.3 |
| Mepivacaine 1% (mL) | 38.0 ± 19.0 | 38.0 ± 18.5 | 38.1 ± 20.6 | 0.9 |
| Propofol change rates (n) | 2.1 ± 2.1 | 2.1 ± 2.2 | 2.4 ± 2.0 | 0.2 |
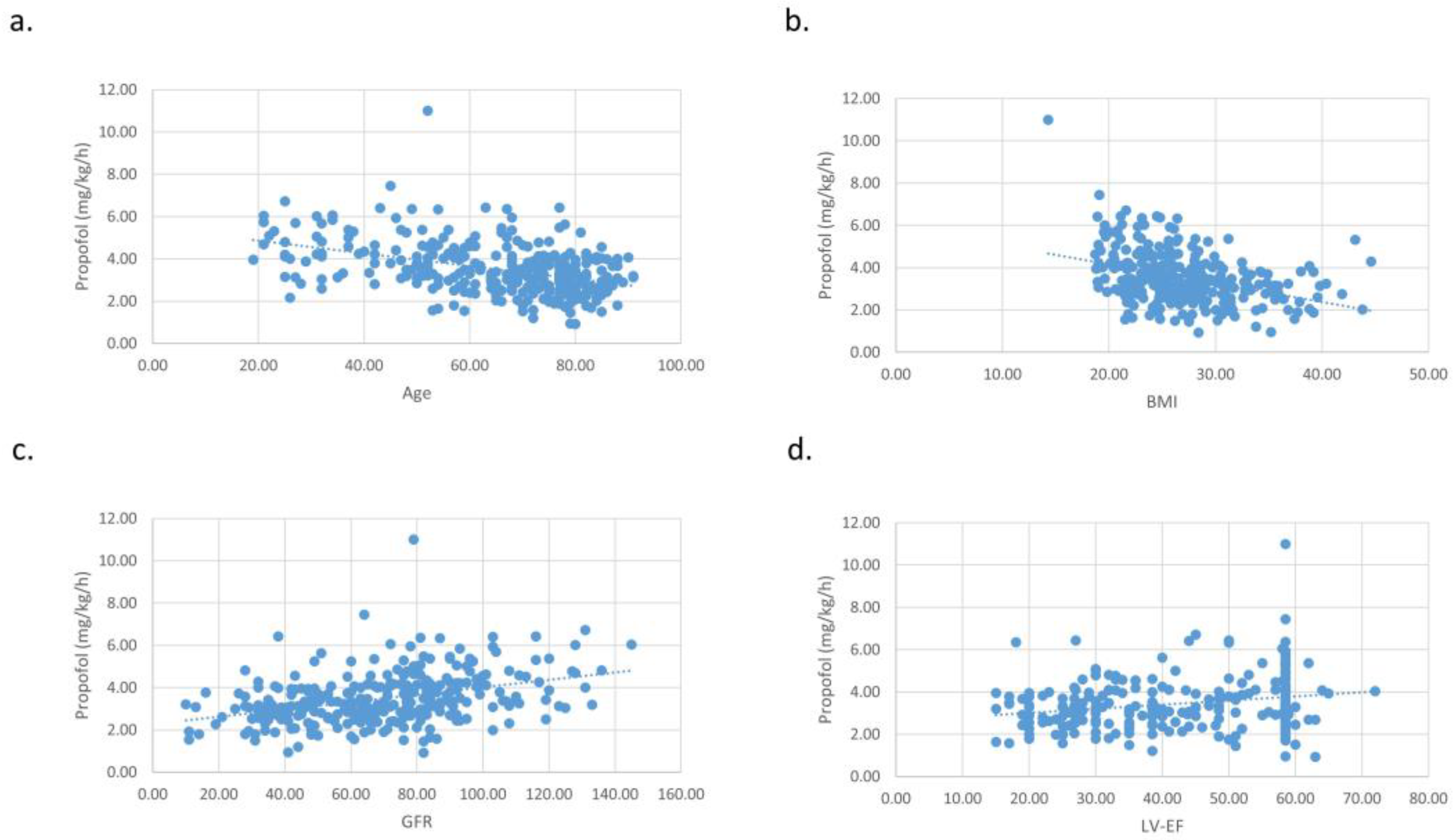
| Midazolam (µg/kg) | Fentanyl (µg/kg) | Propofol (mg/kg/h) | Propofol Rate Changes | |
|---|---|---|---|---|
| Age | ||||
| Pearson’s r | −0.215 | −0.236 | −0.428 | −0.156 |
| p-value | <0.001 | <0.001 | <0.001 | 0.005 |
| BMI | ||||
| Pearson’s r | −0.227 | −0.108 | −0.382 | 0.079 |
| p-value | <0.001 | 0.059 | <0.001 | 0.173 |
| Heart function (LV-EF) | ||||
| Pearson’s r | 0.078 | 0.165 | 0.229 | −0.063 |
| p-value | 0.204 | 0.007 | <0.001 | 0.300 |
| Kidney function (GFR) | ||||
| Pearson’s r | 0.173 | 0.188 | 0.384 | 0.067 |
| p-value | 0.003 | 0.001 | <0.001 | 0.264 |
| Intraprocedural Complication | TLE Indication: Lead Endocarditis | p-Value | |
| No Lead Endocarditis (n = 58) | Lead Endocarditis (n = 14) | ||
| Hypotension, requiring arterenol (n, %) | 41 (70.6%) | 13 (92.9%) | 0.01 |
| Bradycardia requiring atropine (n, %) | 8 (13.8%) | 1 (0.7%) | 0.8 |
| Hypotension (n, %) | 6 (10.4%) | 0 (0.0%) | 0.03 |
| Emergency intubation (n, %) | 1 (1.7%) | 0 (0.0%) | 0.7 |
| Airway management (n, %) | 2 (3.4%) | 0 (0.0%) | 0.6 |
| Intraprocedural Complication | TLE Indication: Lead Perforation | p-Value | |
| No Lead Perforation (n = 60) | Lead Perforation (n = 12) | ||
| Hypotension requiring arterenol (n, %) | 44 (73.3%) | 10 (83.3%) | 0.05 |
| Bradycardia requiring atropine (n, %) | 8 (13.3%) | 1 (8.3%) | 0.9 |
| Hypotension (n, %) | 5 (8.3%) | 1 (8.3%) | 0.8 |
| Emergency intubation (n, %) | 1 (1.7%) | 0 (0.0%) | 0.7 |
| Airway management (n, %) | 2 (3.3%) | 0 (0.0%) | 0.6 |
| Intraprocedural Complication | TLE Indication: Pocket Infection | p-Value | |
| No Pocket Infection (n = 61) | Pocket Infection (n = 11) | ||
| Hypotension requiring arterenol(n, %) | 45 (73.8%) | 9 (81.8%) | 0.5 |
| Bradycardia requiring atropine (n, %) | 7 (11.5%) | 2 (18.1%) | 0.5 |
| Hypotension (n, %) | 6 (9.8%) | 0 (0.0%) | 0.6 |
| Emergency intubation (n, %) | 1 (1.6%) | 0 (0.0%) | 0.7 |
| Airway management (n, %) | 2 (3.3%) | 0 (0.0%) | 0.6 |
| Intraprocedural Complication | TLE Indication: Lead Failure | p-Value | |
| No Lead Failure (n = 48) | Lead Failure (n = 24) | ||
| Hypotension requiring arterenol (n, %) | 37 (77.1%) | 17 (70.8%) | 0.03 |
| Bradycardia requiring atropine (n, %) | 4 (8.3%) | 5 (20.8%) | 0.5 |
| Hypotension (n, %) | 4 (8.3%) | 2 (8.3%) | 0.6 |
| Emergency intubation (n, %) | 1 (2.1%) | 0 (0.0%) | 0.4 |
| Airway management (n, %) | 2 (4.2%) | 0 (0.0%) | 0.2 |
| Intraprocedural Complication | TLE Indication: Vascular Complications | p-Value | |
| No Vascular Complications (n = 65) | Vascular Complications (n = 7) | ||
| Hypotension requiring arterenol (n, %) | 50 (76.9%) | 4 (57.1%) | 0.6 |
| Bradycardia requiring atropine (n, %) | 9 (13.8%) | 0 (0.0%) | 0.5 |
| Hypotension (n, %) | 4 (6.2%) | 2 (28.6%) | 0.01 |
| Emergency intubation (n, %) | 1 (1.5%) | 0 (0.0%) | 0.8 |
| Airway management (n, %) | 1 (1.5%) | 1 (14.3%) | 0.01 |
| Medication | Total | TLE Indication: Lead Endocarditis | p-Value | |
| No Lead Endocarditis | Lead Endocarditis | |||
| Midazolam (µg/kg) | 42.9 ± 26.5 | 43.1 ± 25.6 | 42.0 ± 31.6 | 0.8 |
| Fentanyl (µg/kg) | 0.4 ± 0.6 | 0.4 ± 0.6 | 0.2 ± 0.4 | 0.2 |
| Propofol (mg/kg/h) | 3.5 ± 1.2 | 3.6 ± 1.2 | 3.1 ± 1.3 | 0.02 |
| Propofol rate changes (n) | 2.1 ± 2.1 | 2.2 ± 2.2 | 1.8 ± 1.4 | 0.2 |
| Medication | Total | TLE Indication: Lead Perforation | p-Value | |
| No Lead Perforation | Lead Perforation | |||
| Midazolam (µg/kg) | 42.9 ± 26.5 | 42.8 ± 26.9 | 43.9 ± 22.9 | 0.8 |
| Fentanyl (µg/kg) | 0.4 ± 0.6 | 0.4 ± 0.6 | 0.2 ± 0.4 | 0.3 |
| Propofol (mg/kg/h) | 3.5 ± 1.2 | 3.5 ± 1.2 | 3.4 ± 1.3 | 0.6 |
| Propofol rate changes (n) | 2.1 ± 2.1 | 2.2 ± 2.1 | 1.9 ± 2.1 | 0.6 |
| Medication | Total | TLE Indication: Pocket Infection | p-Value | |
| No Pocket Infection | Pocket Infection | |||
| Midazolam (µg/kg) | 42.9 ± 26.5 | 43.2 ± 27.1 | 41.4 ± 21.9 | 0.7 |
| Fentanyl (µg/kg) | 0.4 ± 0.6 | 0.3 ± 0.6 | 0.5 ± 0.8 | 0.02 |
| Propofol (mg/kg/h) | 3.5 ± 1.2 | 3.5 ± 1.2 | 3.3 ± 1.1 | 0.2 |
| Propofol rate changes (n) | 2.1 ± 2.1 | 2.2 ± 2.2 | 1.8 ± 1.5 | 0.3 |
| Medication | Total | TLE Indication: Lead Failure | p-Value | |
| No Lead Failure | Lead Failure | |||
| Midazolam (µg/kg) | 42.9 ± 26.5 | 40.6 ± 24.2 | 45.6 ± 28.8 | 0.1 |
| Fentanyl (µg/kg) | 0.4 ± 0.6 | 0.3 ± 0.6 | 0.4 ± 0.7 | 0.7 |
| Propofol (mg/kg/h) | 3.5 ± 1.2 | 3.4 ± 1.2 | 3.7 ± 1.2 | 0.02 |
| Propofol rate changes (n) | 2.1 ± 2.1 | 1.9 ± 1.7 | 2.4 ± 2.5 | 0.02 |
| Medication | Total | TLE Indication: Vascular Complications | p-Value | |
| No Vascular Complications | Vascular Complications | |||
| Midazolam (µg/kg) | 42.9 ± 26.5 | 43.3 ± 26.6 | 37.2 ± 23.7 | 0.3 |
| Fentanyl (µg/kg) | 0.4 ± 0.6 | 0.4 ± 0.6 | 0.4 ± 0.5 | 0.9 |
| Propofol (mg/kg/h) | 3.5 ± 1.2 | 3.5 ± 1.2 | 3.6 ± 1.0 | 0.8 |
| Propofol rate changes (n) | 2.1 ± 2.1 | 2.1 ± 2.1 | 1.7 ± 2.1 | 0.4 |
| Patients Requiring Arterenol (n = 54) | Patients without Arterenol Application (n = 274) | p-Value | |
|---|---|---|---|
| Midazolam (µg/kg) | 44.3 ± 25.7 | 42.6 ± 26.7 | 0.7 |
| Fentanyl (µg/kg) | 0.4 ± 0.7 | 0.3 ± 0.6 | 0.3 |
| Propofol (mg/kg/h) | 3.4 ± 1.1 | 3.5 ± 1.2 | 0.5 |
| Propofol rate changes (n) | 2.6 ± 2.1 | 2.0 ± 2.1 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bock, M.; O’Connor, M.; Chouchane, A.; Schmidt, P.; Schaarschmidt, C.; Knoll, K.; Bahlke, F.; Englert, F.; Storz, T.; Kottmaier, M.; et al. Cardiologist-Directed Sedation Management in Patients Undergoing Transvenous Lead Extraction: A Single-Centre Retrospective Analysis. J. Clin. Med. 2023, 12, 4900. https://doi.org/10.3390/jcm12154900
Bock M, O’Connor M, Chouchane A, Schmidt P, Schaarschmidt C, Knoll K, Bahlke F, Englert F, Storz T, Kottmaier M, et al. Cardiologist-Directed Sedation Management in Patients Undergoing Transvenous Lead Extraction: A Single-Centre Retrospective Analysis. Journal of Clinical Medicine. 2023; 12(15):4900. https://doi.org/10.3390/jcm12154900
Chicago/Turabian StyleBock, Matthias, Matthew O’Connor, Amir Chouchane, Philip Schmidt, Claudia Schaarschmidt, Katharina Knoll, Fabian Bahlke, Florian Englert, Theresa Storz, Marc Kottmaier, and et al. 2023. "Cardiologist-Directed Sedation Management in Patients Undergoing Transvenous Lead Extraction: A Single-Centre Retrospective Analysis" Journal of Clinical Medicine 12, no. 15: 4900. https://doi.org/10.3390/jcm12154900





