How to Choose the Optimal Starting Dose of Clomiphene Citrate (50 or 100 mg per Day) for a First Cycle of Ovulation Induction in Anovulatory PCOS Women?
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Therapeutic
2.3. Cycle Outcome
2.4. Response to CC
2.5. Statistical Analysis
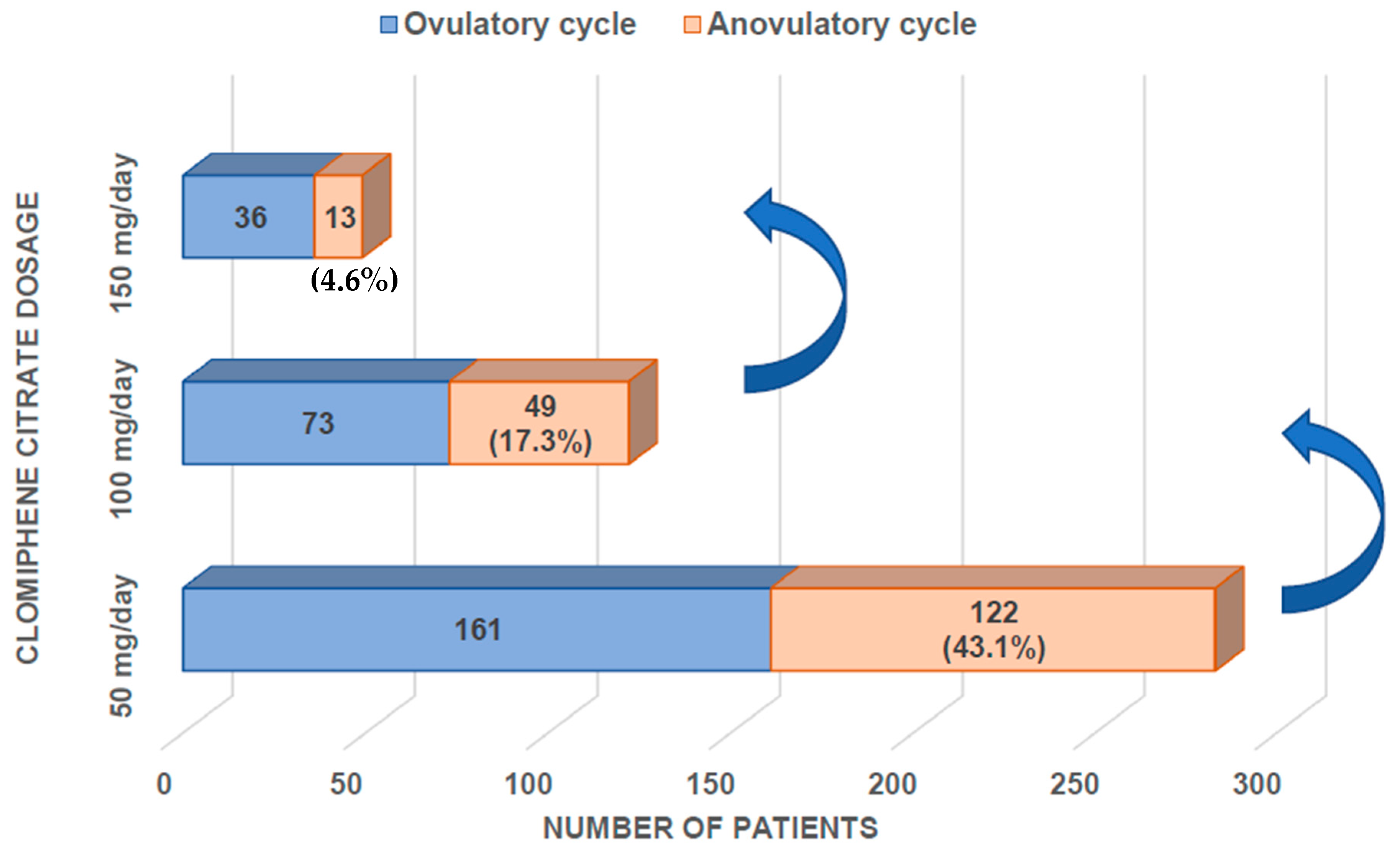
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef]
- Jayasena, C.N.; Franks, S. The management of patients with polycystic ovary syndrome. Nat. Rev. Endocrinol. 2014, 10, 624–636. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- The Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Hum. Reprod. 2008, 23, 462–477. [Google Scholar] [CrossRef]
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Stener-Victorin, E.; Fauser, B.C.J.M.; Norman, R.J.; Teede, H. The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update 2016, 22, 687–708. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef]
- Franik, S.; Le, Q.K.; Kremer, J.A.; Kiesel, L.; Farquhar, C. Aromatase inhibitors (letrozole) for ovulation induction in infertile women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2022, 9, CD010287. [Google Scholar] [CrossRef]
- Hughes, E.; Collins, J.; Vandekerckhove, P. Clomiphene citrate for ovulation induction in women with oligo-amenorrhoea. Cochrane Database Syst. Rev 1996, 22, CD000056. [Google Scholar] [CrossRef]
- Homburg, R. Clomiphene citrate—End of an era? A mini-review. Hum. Reprod. 2005, 20, 2043–2051. [Google Scholar] [CrossRef]
- Homburg, R. Polycystic ovary syndrome. Best. Pract. Res. Clin. Obs. Gynaecol. 2008, 22, 261–274. [Google Scholar] [CrossRef]
- Beck, J.I.; Boothroyd, C.; Proctor, M.; Farquhar, C.; Hughes, E. Oral anti-oestrogens and medical adjuncts for subfertility associated with anovulation. Cochrane Database Syst. Rev. 2005, 25, CD002249. [Google Scholar] [CrossRef]
- Dewailly, D.; Hieronimus, S.; Mirakian, P.; Hugues, J.-N. Polycystic ovary syndrome (PCOS). Ann. Endocrinol. 2010, 71, 8–13. [Google Scholar] [CrossRef]
- Balen, A.H. Ovulation induction in the management of anovulatory polycystic ovary syndrome. Mol. Cell Endocrinol. 2013, 373, 77–82. [Google Scholar] [CrossRef]
- Brown, J.; Farquhar, C.; Beck, J.; Boothroyd, C.; Hughes, E. Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Syst. Rev. 2009, 7, CD002249. [Google Scholar] [CrossRef]
- Melo, A.S.; Ferriani, R.A.; Navarro, P.A. Treatment of infertility in women with polycystic ovary syndrome: Approach to clinical practice. Clinics 2015, 70, 765–769. [Google Scholar] [CrossRef]
- Wang, L.; Qi, H.; Baker, P.N.; Zhen, Q.; Zeng, Q.; Shi, R.; Tong, C.; Ge, Q. Altered Circulating Inflammatory Cytokines Are Associated with Anovulatory Polycystic Ovary Syndrome (PCOS) Women Resistant to Clomiphene Citrate Treatment. Med. Sci. Monit. 2017, 23, 1083–1089. [Google Scholar] [CrossRef]
- Ellakwa, H.E.; Sanad, Z.F.; Hamza, H.A.; Emara, M.A.; Elsayed, M.A. Predictors of patient responses to ovulation induction with clomiphene citrate in patients with polycystic ovary syndrome experiencing infertility. Int. J. Gynecol. Obstet. 2016, 133, 59–63. [Google Scholar] [CrossRef]
- Imani, B.; Eijkemans, M.J.; te Velde, E.R.; Habbema, J.D.; Fauser, B.C. Predictors of patients remaining anovulatory during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J. Clin. Endocrinol. Metab. 1998, 83, 2361–2365. [Google Scholar] [CrossRef][Green Version]
- Imani, B.; Eijkemans, M.J.; te Velde, E.R.; Habbema, J.D.; Fauser, B.C. Predictors of chances to conceive in ovulatory patients during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J. Clin. Endocrinol. Metab. 1999, 84, 1617–1622. [Google Scholar] [CrossRef]
- Overbeek, A.; Kuijper, E.A.M.; Hendriks, M.L.; Blankenstein, M.A.; Ketel, I.J.G.; Twisk, J.W.R.; Hompes, P.G.A.; Homburg, R.; Lambalk, C.B. Clomiphene citrate resistance in relation to follicle-stimulating hormone receptor Ser680Ser-polymorphism in polycystic ovary syndrome. Hum. Reprod. 2009, 24, 2007–2013. [Google Scholar] [CrossRef]
- Xi, W.; Yang, Y.; Mao, H.; Zhao, X.; Liu, M.; Fu, S. Circulating anti-mullerian hormone as predictor of ovarian response to clomiphene citrate in women with polycystic ovary syndrome. J. Ovarian Res. 2016, 9, 3. [Google Scholar] [CrossRef]
- Mercorio, A.; Della Corte, L.; De Angelis, M.C.; Buonfantino, C.; Ronsini, C.; Bifulco, G.; Giampaolino, P. Ovarian Drilling: Back to the Future. Medicina 2022, 58, 1002. [Google Scholar] [CrossRef]
- Practice Committees of the American Society for Reproductive Medicine and the Society for Reproductive Endocrinology and Infertility. Diagnosis and treatment of luteal phase deficiency: A committee opinion. Fertil. Steril. 2021, 115, 1416–1423. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Carmina, E.; Dewailly, D.; Gambineri, A.; Kelestimur, F.; Moghetti, P.; Pugeat, M.; Qiao, J.; Wijeyaratne, C.N.; Witchel, S.F.; et al. Epidemiology, diagnosis and management of hirsutism: A consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum. Reprod. Update 2012, 18, 146–170. [Google Scholar] [CrossRef]
- Jonard, S.; Robert, Y.; Dewailly, D. Revisiting the ovarian volume as a diagnostic criterion for polycystic ovaries. Hum. Reprod. 2005, 20, 2893–2898. [Google Scholar] [CrossRef]
- Dewailly, D.; Gronier, H.; Poncelet, E.; Robin, G.; Leroy, M.; Pigny, P.; Duhamel, A.; Catteau-Jonard, S. Diagnosis of polycystic ovary syndrome (PCOS): Revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum. Reprod. 2011, 26, 3123–3129. [Google Scholar] [CrossRef]
- Balen, A.H.; Laven, J.S.E.; Tan, S.-L.; Dewailly, D. Ultrasound assessment of the polycystic ovary: International consensus definitions. Hum. Reprod. Update 2003, 9, 505–514. [Google Scholar] [CrossRef]
- Dewailly, D.; Lujan, M.E.; Carmina, E.; Cedars, M.I.; Laven, J.; Norman, R.J.; Escobar-Morreale, H.F. Definition and significance of polycystic ovarian morphology: A task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum. Reprod. Update 2014, 20, 334–352. [Google Scholar] [CrossRef]
- Robin, G.; Gallo, C.; Catteau-Jonard, S.; Lefebvre-Maunoury, C.; Pigny, P.; Duhamel, A.; Dewailly, D. Polycystic Ovary-Like Abnormalities (PCO-L) in women with functional hypothalamic amenorrhea. J. Clin. Endocrinol. Metab. 2012, 97, 4236–4243. [Google Scholar] [CrossRef]
- Fraissinet, A.; Robin, G.; Pigny, P.; Lefebvre, T.; Catteau-Jonard, S.; Dewailly, D. Use of the serum anti-Müllerian hormone assay as a surrogate for polycystic ovarian morphology: Impact on diagnosis and phenotypic classification of polycystic ovary syndrome. Hum. Reprod. 2017, 32, 1716–1722. [Google Scholar] [CrossRef]
- Jonard, S.; Robert, Y.; Cortet-Rudelli, C.; Pigny, P.; Decanter, C.; Dewailly, D. Ultrasound examination of polycystic ovaries: Is it worth counting the follicles? Hum. Reprod. 2003, 18, 598–603. [Google Scholar] [CrossRef]
- Dewailly, D.; Pigny, P.; Soudan, B.; Catteau-Jonard, S.; Decanter, C.; Poncelet, E.; Duhamel, A. Reconciling the definitions of polycystic ovary syndrome: The ovarian follicle number and serum anti-Müllerian hormone concentrations aggregate with the markers of hyperandrogenism. J. Clin. Endocrinol. Metab. 2010, 95, 4399–4405. [Google Scholar] [CrossRef]
- Pigny, P.; Jonard, S.; Robert, Y.; Dewailly, D. Serum anti-Mullerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 941–945. [Google Scholar] [CrossRef]
- Pigny, P.; Merlen, E.; Robert, Y.; Cortet-Rudelli, C.; Decanter, C.; Jonard, S.; Dewailly, D. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: Relationship to the ovarian follicle excess and to the follicular arrest. J. Clin. Endocrinol. Metab. 2003, 88, 5957–5962. [Google Scholar] [CrossRef]
- Pigny, P.; Gorisse, E.; Ghulam, A.; Robin, G.; Catteau-Jonard, S.; Duhamel, A.; Dewailly, D. Comparative assessment of five serum antimüllerian hormone assays for the diagnosis of polycystic ovary syndrome. Fertil. Steril. 2016, 105, 1063–1069.e3. [Google Scholar] [CrossRef]
- Coelho Neto, M.A.; Ludwin, A.; Borrell, A.; Benacerraf, B.; Dewailly, D.; da Silva Costa, F.; Condous, G.; Alcazar, J.L.; Jokubkiene, L.; Guerriero, S.; et al. Counting ovarian antral follicles by ultrasound: A practical guide. Ultrasound Obstet. Gynecol. 2018, 51, 10–20. [Google Scholar] [CrossRef]
- Gadalla, M.A.; Huang, S.; Wang, R.; Norman, R.J.; Abdullah, S.A.; El Saman, A.M.; Ismail, A.M.; van Wely, M.; Mol, B.W.J. Effect of clomiphene citrate on endometrial thickness, ovulation, pregnancy and live birth in anovulatory women: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 64–76. [Google Scholar] [CrossRef]
- Harrell, F.E.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Buuren, S.v.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Segalas, C.; Leyrat, C.; Carpenter, J.R.; Williamson, E. Propensity score matching after multiple imputation when a confounder has missing data. Stat. Med. 2023, 42, 1082–1095. [Google Scholar] [CrossRef]
- Mahran, A.; Abdelmeged, A.; El-Adawy, A.R.; Eissa, M.K.; Shaw, R.W.; Amer, S.A. The predictive value of circulating anti-Müllerian hormone in women with polycystic ovarian syndrome receiving clomiphene citrate: A prospective observational study. J. Clin. Endocrinol. Metab. 2013, 98, 4170–4175. [Google Scholar] [CrossRef]
- Dewailly, D.; Andersen, C.Y.; Balen, A.; Broekmans, F.; Dilaver, N.; Fanchin, R.; Griesinger, G.; Kelsey, T.W.; La Marca, A.; Lambalk, C.; et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum. Reprod. Update 2014, 20, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Dumont, A.; Robin, G.; Catteau-Jonard, S.; Dewailly, D. Role of Anti-Müllerian Hormone in pathophysiology, diagnosis and treatment of Polycystic Ovary Syndrome: A review. Reprod. Biol. Endocrinol. 2015, 13, 137. [Google Scholar] [CrossRef]
- Pellatt, L.; Rice, S.; Dilaver, N.; Heshri, A.; Galea, R.; Brincat, M.; Brown, K.; Simpson, E.R.; Mason, H.D. Anti-Müllerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil. Steril. 2011, 96, 1246–1251.e1. [Google Scholar] [CrossRef] [PubMed]
- Köninger, A.; Sauter, L.; Edimiris, P.; Kasimir-Bauer, S.; Kimmig, R.; Strowitzki, T.; Schmidt, B. Predictive markers for the FSH sensitivity of women with polycystic ovarian syndrome. Hum. Reprod. 2014, 29, 518–524. [Google Scholar] [CrossRef]
- Amer, S.A.; Li, T.C.; Ledger, W.L. The value of measuring anti-Mullerian hormone in women with anovulatory polycystic ovary syndrome undergoing laparoscopic ovarian diathermy. Hum. Reprod. 2009, 24, 2760–2766. [Google Scholar] [CrossRef]
- Gülşen, M.S.; Ulu, İ.; Köpük, Y.Ş.; Kıran, G. The role of anti-Müllerian hormone in predicting clomiphene citrate resistance in women with polycystic ovarian syndrome. Gynecol. Endocrinol. 2019, 35, 86–89. [Google Scholar] [CrossRef]
- Hestiantoro, A.; Negoro, Y.S.; Afrita, Y.; Wiweko, B.; Sumapradja, K.; Natadisastra, M. Anti-Müllerian hormone as a predictor of polycystic ovary syndrome treated with clomiphene citrate. Clin. Exp. Reprod. Med. 2016, 43, 207–214. [Google Scholar] [CrossRef]
- Vaiarelli, A.; Drakopoulos, P.; Blockeel, C.; De Vos, M.; van de Vijver, A.; Camus, M.; Cosyns, S.; Tournaye, H.; Polyzos, N.P. Limited ability of circulating anti-Müllerian hormone to predict dominant follicular recruitment in PCOS women treated with clomiphene citrate: A comparison of two different assays. Gynecol. Endocrinol. 2016, 32, 227–230. [Google Scholar] [CrossRef]
- Giampaolino, P.; Della Corte, L.; De Rosa, N.; Mercorio, A.; Bruzzese, D.; Bifulco, G. Ovarian volume and PCOS: A controversial issue. Gynecol. Endocrinol. 2018, 34, 229–232. [Google Scholar] [CrossRef]
- Zhu, J.-L.; Chen, Z.; Feng, W.-J.; Long, S.-L.; Mo, Z.-C. Sex hormone-binding globulin and polycystic ovary syndrome. Clin. Chim. Acta 2019, 499, 142–148. [Google Scholar] [CrossRef]
- Calzada, M.; López, N.; Noguera, J.A.; Mendiola, J.; Hernández, A.I.; Corbalán, S.; Sanchez, M.; Torres, A.M. AMH in combination with SHBG for the diagnosis of polycystic ovary syndrome. J. Obstet. Gynaecol. 2019, 39, 1130–1136. [Google Scholar] [CrossRef]
- Simó, R.; Sáez-López, C.; Barbosa-Desongles, A.; Hernández, C.; Selva, D.M. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol. Metab. 2015, 26, 376–383. [Google Scholar] [CrossRef]
- Deswal, R.; Yadav, A.; Dang, A.S. Sex hormone binding globulin—An important biomarker for predicting PCOS risk: A systematic review and meta-analysis. Syst. Biol. Reprod. Med. 2018, 64, 12–24. [Google Scholar] [CrossRef]
- Sachdeva, G.; Gainder, S.; Suri, V.; Sachdeva, N.; Chopra, S. Comparison of Clinical, Metabolic, Hormonal, and Ultrasound Parameters among the Clomiphene Citrate-Resistant and Clomiphene Citrate-Sensitive Polycystic Ovary Syndrome Women. J. Hum. Reprod. Sci. 2019, 12, 216–223. [Google Scholar] [CrossRef]
- Ghobadi, C.; Amer, S.; Lashen, H.; Lennard, M.S.; Ledger, W.L.; Rostami-Hodjegan, A. Evaluation of the relationship between plasma concentrations of en- and zuclomiphene and induction of ovulation in anovulatory women being treated with clomiphene citrate. Fertil. Steril. 2009, 91, 1135–1140. [Google Scholar] [CrossRef]
- Mürdter, T.E.; Kerb, R.; Turpeinen, M.; Schroth, W.; Ganchev, B.; Böhmer, G.M.; Igel, S.; Schaeffeler, E.; Zanger, U.; Brauch, H.; et al. Genetic polymorphism of cytochrome P450 2D6 determines oestrogen receptor activity of the major infertility drug clomiphene via its active metabolites. Hum. Mol. Genet. 2012, 21, 1145–1154. [Google Scholar] [CrossRef]
- Ghobadi, C.; Gregory, A.; Crewe, H.K.; Rostami-Hodjegan, A.; Lennard, M.S. CYP2D6 is primarily responsible for the metabolism of clomiphene. Drug Metab. Pharmacokinet. 2008, 23, 101–105. [Google Scholar] [CrossRef]
- Zhou, S.-F. Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part II. Clin. Pharmacokinet. 2009, 48, 761–804. [Google Scholar] [CrossRef]
- Robin, C.; Hennart, B.; Broly, F.; Gruchala, P.; Robin, G.; Catteau-Jonard, S. Could Cytochrome P450 2D6, 3A4 and 3A5 Polymorphisms Explain the Variability in Clinical Response to Clomiphene Citrate of Anovulatory PCOS Women? Front. Endocrinol. 2021, 12, 718917. [Google Scholar] [CrossRef]
- Ji, M.; Kim, K.-R.; Lee, W.; Choe, W.; Chun, S.; Min, W.-K. Genetic Polymorphism of CYP2D6 and Clomiphene Concentrations in Infertile Patients with Ovulatory Dysfunction Treated with Clomiphene Citrate. J. Korean Med. Sci. 2016, 31, 310–314. [Google Scholar] [CrossRef]


| Variables | Values * | ||
|---|---|---|---|
| Age (years) | 27.5 ± 3.7 | ||
| Menstrual Cycles (%) | Regular anovulatory cycles | 17 (5.9%) | |
| Oligomenorrhea | 185 (65.5%) | ||
| Amenorrhoea | 81 (28.6%) | ||
| BMI (kg/m2) | 25.8 ± 5.4 | ||
| Waist circumference (cm) | 85.2 ± 15.2 | ||
| Modified Ferriman and Gallwey Score | 5.0 (0 to 9.0) | ||
| Estradiol (pg/mL) | 37.0 (28.0 to 50.0) | ||
| FSH (IU/L) | 5.0 ± 1.3 | ||
| LH (IU/L) | 5.9 (4.0 to 9.1) | ||
| AMH (pmol/L) | 71.8 (53.6 to 107.4) | ||
| Total testosterone (ng/mL) | 0.4 ± 0.2 | ||
| Δ4-androstenedione (ng/mL) | 1.6 (1.2 to 2.2) | ||
| 17-OHP (ng/mL) | 0.6 (0.5 to 0.9) | ||
| SHBG (nmol/L) | 38.4 (24.0 to 49.8) | ||
| SDHEA (μmol/L) | 4.6 (3.4 to 6.4) | ||
| Fasting insulin (mUI/L) | 6.0 (3.1 to 9.8) | ||
| Mean ovarian surface (cm2) | 5.7 ± 1.6 | ||
| PCOM and/or elevated AMH | 280 (99%) | ||
| Phenotype PCOS (%) | A = OA + HA + PCOM | 225 (79.5%) | |
| B = OA + HA | 3 (1.0%) | ||
| D = OA + PCOM | 55 (19.5%) | ||
| Mean number of CC cycles | 3.2 ± 1.7 | ||
| Mean number of CC ovulatory cycles | 2.3 ± 1.5 | ||
| Sensitive to 50 mg (n = 179) | Resistant to 50 mg (n = 104) | p Value | |||
|---|---|---|---|---|---|
| Age (years) | 27.7 ± 3.8 | 27.0 ± 3.6 | 0.12 | ||
| Menstrual cycles (%) | Regular anovulatory cycles | 14 (7.8%) | 3 (2.9%) | 0.12 | |
| Oligomenorrhea | 119 (66.7%) | 66 (63.5%) | |||
| Amenorrhoea | 46 (25.5%) | 35 (33.6%) | |||
| BMI (kg/m2) | 24.9 ± 5.2 | 27.1 ± 5.4 | 0.001 | ||
| Waist circumference (cm) | 83.4 ± 15.8 | 88.3 ± 13.7 | 0.028 | ||
| Modified Ferriman and Gallwey Score | 5.0 (0 to 9.0) | 3.0 (0 to 9.0) | 0.90 | ||
| Estradiol (pg/mL) | 37.0 (28.0 to 49.5) | 39.0 (28.0 to 50.0) | 0.87 | ||
| FSH (IU/L) | 5.01 ± 1.4 | 4.9 ± 1.1 | 0.48 | ||
| LH (IU/L) | 5.7 (3.9 to 8.9) | 6.2 (4.2 to 9.2) | 0.33 | ||
| AMH (pmol/L) | 69.4 (51.6 to 101.2) | 89.5 (56.0 to 130.0) | 0.014 | ||
| Total testosterone (ng/mL) | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.046 | ||
| Δ4-androstenedione (ng/mL) | 1.5 (1.1 to 2.1) | 1.7 (1.3 to 2.3) | 0.023 | ||
| 17-OHP (ng/mL) | 0.6 (0.4 to 0.9) | 0.7 (0.5 to 0.9) | 0.046 | ||
| SHBG (nmol/L) | 39.6 (28.7 to 53.7) | 27.2 (18.0 to 40.5) | <0.001 | ||
| SDHEA (μmol/L) | 4.4 (3.0 to 6.4) | 4.8 (3.6 to 6.2) | 0.43 | ||
| Fasting insulin (mUI/L) | 4.5 (2.9 to 7.1) | 7.9 (3.9 to 11.6) | 0.002 | ||
| Mean ovarian surface (cm2) | 5.7 ± 1.7 | 5.9 ± 1.5 | 0.42 | ||
| PCOS anovulatory phenotypes (%) | A + B | 142 (79.3%) | 86 (82.7%) | 0.48 | |
| D | 37 (20.7%) | 18 (17.3%) | |||
| Parameters | OR (95% CI) | p |
|---|---|---|
| AMH (pmol/L) | 1.08 (1.03 to 1.14) * | 0.002 |
| SHBG (nmol/L) | 0.96 (0.94 to 0.99) | 0.002 |
| No Ovulation | Ovulation | ||
|---|---|---|---|
| AMH tested alone | AMH > 86.1 pmol/L | 46.12% | 53.88% |
| AMH < 86.1 pmol/L | 29.86% | 70.14% | |
| SHBG tested alone | SHBG < 28.3 nmol/L | 53,72% | 46.28% |
| SHBG > 28.3 nmol/L | 27.93% | 72.07% | |
| AMH and SHBG combined | AMH > 86.1 pmol/L SHBG < 28.3 nmol/L | 46.67% | 53.33% |
| AMH < 86.1 pmol/L SHBG > 28.3 nmol/L | 19.42% | 80.58% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huyghe, L.; Robin, C.; Dumont, A.; Decanter, C.; Kyheng, M.; Dewailly, D.; Catteau-Jonard, S.; Robin, G. How to Choose the Optimal Starting Dose of Clomiphene Citrate (50 or 100 mg per Day) for a First Cycle of Ovulation Induction in Anovulatory PCOS Women? J. Clin. Med. 2023, 12, 4943. https://doi.org/10.3390/jcm12154943
Huyghe L, Robin C, Dumont A, Decanter C, Kyheng M, Dewailly D, Catteau-Jonard S, Robin G. How to Choose the Optimal Starting Dose of Clomiphene Citrate (50 or 100 mg per Day) for a First Cycle of Ovulation Induction in Anovulatory PCOS Women? Journal of Clinical Medicine. 2023; 12(15):4943. https://doi.org/10.3390/jcm12154943
Chicago/Turabian StyleHuyghe, Lucie, Camille Robin, Agathe Dumont, Christine Decanter, Maeva Kyheng, Didier Dewailly, Sophie Catteau-Jonard, and Geoffroy Robin. 2023. "How to Choose the Optimal Starting Dose of Clomiphene Citrate (50 or 100 mg per Day) for a First Cycle of Ovulation Induction in Anovulatory PCOS Women?" Journal of Clinical Medicine 12, no. 15: 4943. https://doi.org/10.3390/jcm12154943
APA StyleHuyghe, L., Robin, C., Dumont, A., Decanter, C., Kyheng, M., Dewailly, D., Catteau-Jonard, S., & Robin, G. (2023). How to Choose the Optimal Starting Dose of Clomiphene Citrate (50 or 100 mg per Day) for a First Cycle of Ovulation Induction in Anovulatory PCOS Women? Journal of Clinical Medicine, 12(15), 4943. https://doi.org/10.3390/jcm12154943






