CHECGAIT: A Functional Electrical Stimulation Clinical Pathway to Reduce Foot Drop during Walking in Adult Patients with Upper Motor Neuron Lesions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
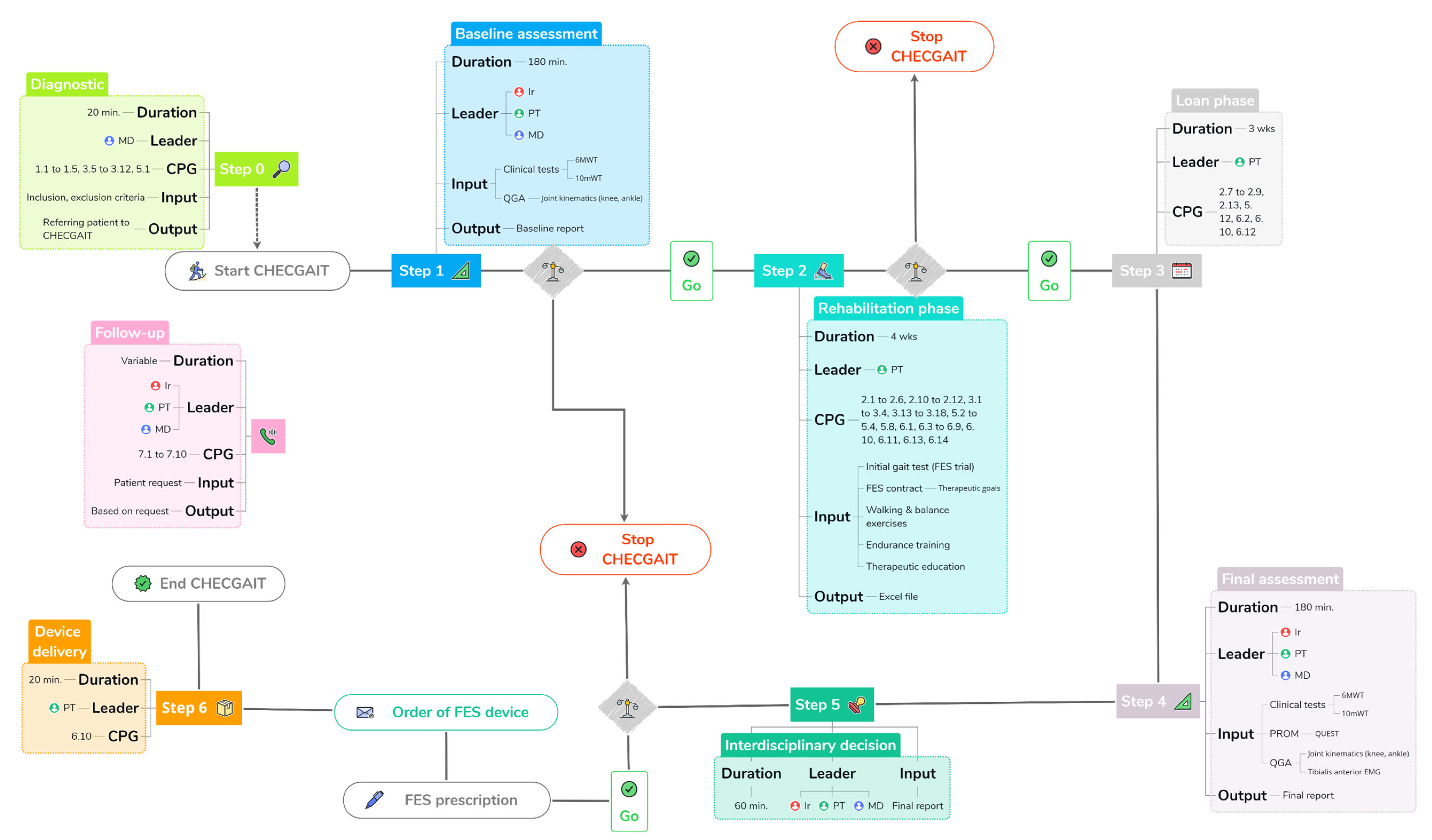
2.2. Structure and Steps of CHECGAIT
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Step 0: Diagnostic
3.2. Step 1: Baseline Assessment
3.3. Steps 2 and 3: Rehabilitation and Loan Phases
3.4. Step 4: Final Assessment
3.5. Step 5: Interdisciplinary Decision
3.6. Step 6: Device Delivery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gracies, J.-M. Pathophysiology of Spastic Paresis. I: Paresis and Soft Tissue Changes. Muscle Nerve 2005, 31, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.-M. Pathophysiology of Spastic Paresis. II: Emergence of Muscle Overactivity. Muscle Nerve 2005, 31, 552–571. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, A.; Malouin, F.; Richards, C.L.; Dumas, F. Mechanisms of Disturbed Motor Control in Ankle Weakness during Gait after Stroke. Gait Posture 2002, 15, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Kottink, A.I.R.; Oostendorp, L.J.M.; Buurke, J.H.; Nene, A.V.; Hermens, H.J.; IJzerman, M.J. The Orthotic Effect of Functional Electrical Stimulation on the Improvement of Walking in Stroke Patients with a Dropped Foot: A Systematic Review. Artif. Organs 2004, 28, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Liberson, W.T.; Holmquest, H.J.; Scot, D.; Dow, M. Functional Electrotherapy: Stimulation of the Peroneal Nerve Synchronized with the Swing Phase of the Gait of Hemiplegic Patients. Arch. Phys. Med. Rehabil. 1961, 42, 101–105. [Google Scholar]
- Lyons, G.M.; Sinkjaer, T.; Burridge, J.H.; Wilcox, D.J. A Review of Portable FES-Based Neural Orthoses for the Correction of Drop Foot. IEEE Trans. Neural Syst. Rehabil. Eng. 2002, 10, 260–279. [Google Scholar] [CrossRef]
- Bulley, C.; Meagher, C.; Street, T.; Adonis, A.; Peace, C.; Singleton, C.; Burridge, J. Development of Clinical Guidelines for Service Provision of Functional Electrical Stimulation to Support Walking: Mixed Method Exploration of Stakeholder Views. BMC Neurol. 2021, 21, 263. [Google Scholar] [CrossRef]
- Bulley, C.; Adonis, A.; Burridge, J.; Joiner, S.; Street, T.; Singleton, C.; Taylor, P.; Van Der Linden, M. Evidence Based Clinical Practice Guidelines for the Use of Functional Electric Stimulation to Improve Mobility in Adults with Lower Limb Impairment Due to an Upper Motor Neuron Lesion; Queen Margaret University: Musselburgh, UK, 2022. [Google Scholar] [CrossRef]
- Taylor, P.; Burridge, J.; Dunkerley, A.; Wood, D.; Norton, J.; Singleton, C.; Swain, I. Clinical Audit of 5 Years Provision of the Odstock Dropped Foot Stimulator. Artif. Organs 1999, 23, 440–442. [Google Scholar] [CrossRef]
- Taylor, P.; Humphreys, L.; Swain, I. The Long-Term Cost-Effectiveness of the Use of Functional Electrical Stimulation for the Correction of Dropped Foot Due to Upper Motor Neuron Lesion. J. Rehabil. Med. 2013, 45, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Sackett, D.L.; Rosenberg, W.M.; Gray, J.A.; Haynes, R.B.; Richardson, W.S. Evidence Based Medicine: What It Is and What It Isn’t. BMJ 1996, 312, 71–72. [Google Scholar] [CrossRef] [Green Version]
- Enright, P.L. The Six-Minute Walk Test. Respir. Care 2003, 48, 783–785. [Google Scholar]
- Flansbjer, U.-B.; Holmbäck, A.M.; Downham, D.; Patten, C.; Lexell, J. Reliability of Gait Performance Tests in Men and Women with Hemiparesis After Stroke. J. Rehabil. Med. 2005, 37, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Leardini, A.; Sawacha, Z.; Paolini, G.; Ingrosso, S.; Nativo, R.; Benedetti, M.G. A New Anatomically Based Protocol for Gait Analysis in Children. Gait Posture 2007, 26, 560–571. [Google Scholar] [CrossRef]
- Medical Research Council. Aids to the Investigation of the Peripheral Nervous System, Memorandum No. 45; Pendragon House: London, OH, USA, 1976; ISBN 0-11-450033-9. [Google Scholar]
- Bohannon, R.W.; Smith, M.B. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Chantraine, F.; Filipetti, P.; Schreiber, C.; Remacle, A.; Kolanowski, E.; Moissenet, F. Proposition of a Classification of Adult Patients with Hemiparesis in Chronic Phase. PLoS ONE 2016, 11, e0156726. [Google Scholar] [CrossRef] [Green Version]
- Turner-Stokes, L. Goal Attainment Scaling (GAS) in Rehabilitation: A Practical Guide. Clin. Rehabil. 2009, 23, 362–370. [Google Scholar] [CrossRef]
- Demers, L.; Weiss-Lambrou, R.; Ska, B. Development of the Quebec User Evaluation of Satisfaction with Assistive Technology (QUEST). Assist. Technol. 1996, 8, 3–13. [Google Scholar] [CrossRef]
- Begg, R.K.; Tirosh, O.; Said, C.M.; Sparrow, W.A.; Steinberg, N.; Levinger, P.; Galea, M.P. Gait Training with Real-Time Augmented Toe-Ground Clearance Information Decreases Tripping Risk in Older Adults and a Person with Chronic Stroke. Front. Hum. Neurosci. 2014, 8, 243. [Google Scholar] [CrossRef]
- Schreiber, C.; Moissenet, F. A Multimodal Dataset of Human Gait at Different Walking Speeds Established on Injury-Free Adult Participants. Sci. Data 2019, 6, 111. [Google Scholar] [CrossRef] [Green Version]
- Badger, J.; Taylor, P.; Swain, I. The Safety of Electrical Stimulation in Patients with Pacemakers and Implantable Cardioverter Defibrillators: A Systematic Review. J. Rehabil. Assist. Technol. Eng. 2017, 4, 205566831774549. [Google Scholar] [CrossRef] [Green Version]
- Johnston, T.E.; Keller, S.; Denzer-Weiler, C.; Brown, L. A Clinical Practice Guideline for the Use of Ankle-Foot Orthoses and Functional Electrical Stimulation Post-Stroke. J. Neurol. Phys. Ther. 2021, 45, 112–196. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Garrett, M.; Gronley, J.K.; Mulroy, S.J. Classification of Walking Handicap in the Stroke Population. Stroke 1995, 26, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Demers, L.; Monette, M.; Descent, M.; Jutai, J.; Wolfson, C. The Psychosocial Impact of Assistive Devices Scale (PIADS): Translation and Preliminary Psychometric Evaluation of a Canadian–French Version. Qual. Life Res. 2002, 11, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Newell, A.M.; VanSwearingen, J.M.; Hile, E.; Brach, J.S. The Modified Gait Efficacy Scale: Establishing the Psychometric Properties in Older Adults. Phys. Ther. 2012, 92, 318–328. [Google Scholar] [CrossRef]
| Inclusion | Exclusion |
|---|---|
| Foot drop during gait secondary to UMN lesion (stroke, MS, CP, traumatic brain injury, Parkinson’s disease, dystonia, partial SCI) | Fixed contractures of ankle joint in plantarflexion |
| Poor condition of skin at site of FES | |
| Chronic oedema at site of FES | |
| At chronic stage of the disease (>6 months since the onset) | Diagnosis of deep vein thrombosis |
| Able to walk minimum of 10 m without physical assistance (can be with an assistive device) | Receptive dysphasia (unable to understand instructions) |
| Severe anxiety | |
| Ability to understand instructions for FES device use or external support available | Important peripheral nerve damage (according to EMG) |
| Metal implants in region of FES |
| Disease | n | CHECGAIT Step | Reason |
|---|---|---|---|
| Stroke | 21 | Baseline assessment (n = 9) | Not needed (n = 2), surgery (1 cranioplasty, 1 SPLATT, 1 knee), walking speed too slow (<0.4 m s−1) (n = 2), botulinum toxin required (n = 1), medical instability (n = 1) |
| Rehabilitation and Loan (n = 7) | No effect on gait (n = 2), FES not tolerated (n = 1), fall (n = 1), prefers AFO (n = 1), not contacted by FES service (n = 1), second stroke (n = 1) | ||
| Final assessment (n = 5) | Therapeutic reorientation (botulinum toxin) (n = 1), walking speed too slow (<0.4 m s−1) (n = 1), no effect on gait (n = 1), prefers Dictus Band orthosis (n = 1), not present at QGA (n = 1) | ||
| MS | 9 | Rehabilitation (n = 6) | Does not want to use FES (n = 2), did not come to appointment (n = 1), COVID-19 (n = 1), no effect on gait (n = 1), not contacted by FES service (n = 1) |
| Final assessment (n = 3) | Walking speed too slow (<0.4 m s−1) (n = 1), does not want to use FES (n = 1), no effect on gait (n = 1) | ||
| SCI | 1 | Loan phase | Lost FES device (n = 1) |
| Parkinson | 1 | Final assessment | No effect on gait (n = 1) |
| TBI | 1 | Baseline assessment | Skin problems (n = 1) |
| Variables | Step 1—without FES | Step 4—with FES | Significant Differences: p Values | ||
|---|---|---|---|---|---|
| NP | P | NP | P | ||
| n = 14 | n = 57 | n = 14 | n = 57 | ||
| (1) | (2) | (3) | (4) | ||
| 6 MWT (m) | 301 ± 116 | 359 ± 103 | 377 ± 110 | 449 ± 112 | (1) vs. (3): <0.001, (2) vs. (4): <0.001 |
| 10 mWT (s) | 10.9 ± 3.1 | 9.0 ± 2.4 | 9.4 ± 2.3 | 7.4 ± 1.9 | (1) vs. (3): 0.005, (2) vs. (4): <0.001, (3) vs. (4): 0.001 |
| Variables | Step 4—without FES | Step 4—with FES | Significant Differences: p Values | ||
|---|---|---|---|---|---|
| NP | P | NP | P | ||
| n = 14 | n = 58 | n = 14 | n = 58 | ||
| (1) | (2) | (3) | (4) | ||
| Step width (m) | 0.17 ± 0.06 | 0.15 ± 0.05 | 0.16 ± 0.06 | 0.14 ± 0.04 | (2) vs. (4): <0.001 |
| Step frequency (s) | 86.0 ± 18.0 | 99.0 ± 16.4 | 86.9 ± 21.6 | 99.8 ± 15.8 | |
| Mean velocity (m s−1) | 0.65 ± 0.27 | 0.89 ± 0.28 | 0.68 ± 0.31 | 0.93 ± 0.27 | (1) vs. (2): 0.006, (3) vs. (4): 0.003, (2) vs. (4): <0.001 |
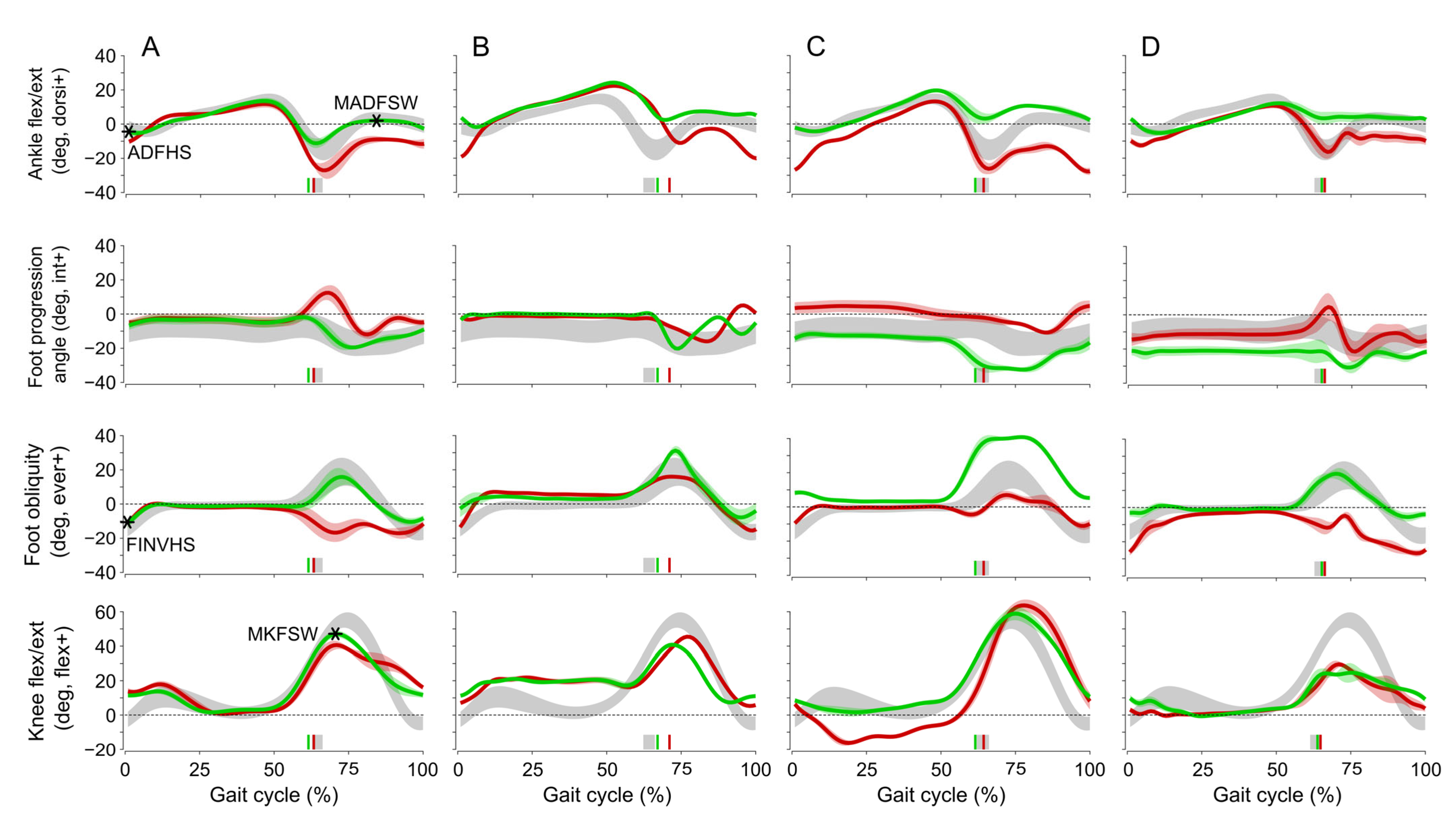
| Maximum knee flexion in swing (°) | 35.3 ± 13.1 | 37.5 ± 10.0 | 33.5 ± 13.0 | 36.9 ± 10.1 | |
| Maximum ankle dorsiflexion in swing (°) | −6.4 ± 8.7 | −2.3 ± 7.8 | −1.9 ± 8.7 | 3.0 ± 6.0 | (2) vs. (4): <0.001 |
| Ankle dorsiflexion at heel strike (°) | −13.7 ± 9.3 | −8.6 ± 7.1 | −5.8 ± 10.1 | −1.2 ± 6.4 | (1) vs. (3): <0.005, (2) vs. (4): <0.001 |
| Ankle inversion/eversion at heel strike (°) | 3.7 ± 8.8 | 10.5 ± 7.7 | 8.7 ± 6.4 | 14.0 ± 8.1 | (1) vs. (2): 0.005, (1) vs. (3): <0.003, (2) vs. (4): <0.001 |
| Minimum toe clearance (m) | 0.044 ± 0.022 | 0.058 ± 0.028 | 0.049 ± 0.016 | 0.065 ± 0.025 | (2) vs. (4): <0.001 |
| Stroke | MS | Others | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| without FES | with FES | without FES | with FES | without FES | with FES | |||||||
| NP n = 9 | P n = 24 | NP n = 9 | P n = 24 | NP n = 2 | P n = 17 | NP n = 2 | P n = 17 | NP n = 3 | P n = 17 | NP n = 3 | P n = 17 | |
| Step width (m) | 0.20 ± 0.05 | 0.17 ± 0.05 | 0.18 ± 0.05 | 0.16 ± 0.04 | 0.09 ± 0.04 | 0.13 ± 0.04 | 0.09 ± 0.01 | 0.12 ± 0.03 | 0.13 ± 0.01 | 0.14 ± 0.05 | 0.12 ± 0.03 | 0.13 ± 0.05 |
| Step frequency (s) | 82.5 ± 14.6 | 94.4 ± 16.6 | 83.5 ± 20.7 | 96.4 ± 16.6 | 81.2 ± 29.1 | 104.7 ± 13.0 | 83.6 ± 31.5 | 105.4 ± 13.1 | 99.6 ± 21.5 | 99.8 ± 18.0 | 99.4 ± 23.2 | 98.8 ± 16.5 |
| Mean velocity (m s−1) | 0.61 ± 0.26 | 0.80 ± 0.31 | 0.65 ± 0.32 | 0.85 ± 0.29 | 0.65 ± 0.52 | 0.96 ± 0.24 | 0.67 ± 0.55 | 1.01 ± 0.22 | 0.77 ± 0.18 | 0.93 ± 0.27 | 0.79 ± 0.18 | 0.96 ± 0.26 |
| Maximum knee flexion in swing (°) | 36.9 ± 9.9 | 36.3 ± 11.2 | 34.6 ± 9.4 | 35.4 ± 12.6 | 33.3 ± 18.4 | 38.7 ± 12.0 | 28.7 ± 20.6 | 38.5 ± 9.9 | 31.8 ± 22.8 | 38.1 ± 5.0 | 33.5 ± 22.3 | 37.3 ± 5.5 |
| Maximum ankle dorsiflexion in swing (°) | −7.6 ± 7.2 | −3.8 ± 9.7 | −4.3 ± 9.4 | 3.5 ± 6.7 | −6.3 ± 18.4 | −1.9 ± 6.2 | 6.3 ± 3.5 | 2.1 ± 4.1 | −2.6 ± 9.0 | −0.6 ± 5.9 | 0.1 ± 5.3 | 3.1 ± 6.6 |
| Ankle dorsiflexion at heel strike (°) | −16.5 ± 7.5 | −11.3 ± 6.9 | −8.5 ± 11.2 | −1.7 ± 7.8 | −10.5 ± 19.0 | −6.5 ± 5.4 | 4.5 ± 3.2 | −1.2 ± 4.3 | −7.4 ± 7.2 | −6.9 ± 7.8 | −4.4 ± 4.5 | −0.7 ± 6.4 |
| Ankle inversion/eversion at heel strike (°) | 1.0 ± 6.6 | 10.2 ± 7.6 | 7.4 ± 4.8 | 13.6 ± 9.4 | 5.5 ± 16.7 | 13.5 ± 7.6 | 12.1 ± 8.8 | 17.7 ± 6.1 | 10.6 ± 9.3 | 7.9 ± 7.1 | 10.2 ± 10.6 | 10.9 ± 6.8 |
| Minimum toe clearance (m) | 0.051 ± 0.022 | 0.055 ± 0.028 | 0.052 ± 0.018 | 0.064 ± 0.025 | 0.030 ± 0.010 | 0.067 ± 0.033 | 0.043 ± 0.003 | 0.072 ± 0.030 | 0.031 ± 0.013 | 0.052 ± 0.021 | 0.042 ± 0.016 | 0.060 ± 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Areno, G.; Chantraine, F.; Schreiber, C.; Masson, X.; Classen, T.; Pereira, J.A.C.; Dierick, F. CHECGAIT: A Functional Electrical Stimulation Clinical Pathway to Reduce Foot Drop during Walking in Adult Patients with Upper Motor Neuron Lesions. J. Clin. Med. 2023, 12, 5112. https://doi.org/10.3390/jcm12155112
Areno G, Chantraine F, Schreiber C, Masson X, Classen T, Pereira JAC, Dierick F. CHECGAIT: A Functional Electrical Stimulation Clinical Pathway to Reduce Foot Drop during Walking in Adult Patients with Upper Motor Neuron Lesions. Journal of Clinical Medicine. 2023; 12(15):5112. https://doi.org/10.3390/jcm12155112
Chicago/Turabian StyleAreno, Gilles, Frédéric Chantraine, Céline Schreiber, Xavier Masson, Tanja Classen, José Alexandre Carvalho Pereira, and Frédéric Dierick. 2023. "CHECGAIT: A Functional Electrical Stimulation Clinical Pathway to Reduce Foot Drop during Walking in Adult Patients with Upper Motor Neuron Lesions" Journal of Clinical Medicine 12, no. 15: 5112. https://doi.org/10.3390/jcm12155112







