Hypotension Prediction Index and Incidence of Perioperative Hypotension: A Single-Center Propensity-Score-Matched Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bijker, J.B.; van Klei, W.A.; Kappen, T.H.; van Wolfswinkel, L.; Moons, K.G.M.; Kalkman, C.J. Incidence of Intraoperative Hypotension as a Function of the Chosen Definition. Anesthesiology 2007, 107, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Green, R.S.; Butler, M.B. Postintubation Hypotension in General Anesthesia. J. Intensive Care Med. 2015, 31, 667–675. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Pang, Q.-Y.; Liu, H. Association of Intra-operative Hypotension with Acute Kidney Injury, Myocardial Injury and Mortality in Non-cardiac Surgery: A Meta-analysis. Int. J. Clin. Pract. 2019, 73, e13394. [Google Scholar] [CrossRef]
- Wesselink, E.M.; Kappen, T.H.; Torn, H.M.; Slooter, A.J.C.; van Klei, W.A. Intraoperative Hypotension and the Risk of Postoperative Adverse Outcomes: A Systematic Review. Br. J. Anaesth. 2018, 121, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Mascha, E.J.; Yang, D.; Maheshwari, K.; Cohen, B.; Khanna, A.K.; Ruetzler, K.; Turan, A.; Sessler, D.I. Associations of Intraoperative Radial Arterial Systolic, Diastolic, Mean, and Pulse Pressures with Myocardial and Acute Kidney Injury after Noncardiac Surgery. Anesthesiology 2020, 132, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, V.; Maheshwari, K.; Yang, D.; Mascha, E.J.; Singh, A.; Sessler, D.I.; Kurz, A. Relationship between Intraoperative Hypotension, Defined by Either Reduction from Baseline or Absolute Thresholds, and Acute Kidney and Myocardial Injury after Noncardiac Surgery. Anesthesiology 2017, 126, 47–65. [Google Scholar] [CrossRef]
- Futier, E.; Lefrant, J.-Y.; Guinot, P.-G.; Godet, T.; Lorne, E.; Cuvillon, P.; Bertran, S.; Leone, M.; Pastene, B.; Piriou, V.; et al. Effect of Individualized vs Standard Blood Pressure Management Strategies on Postoperative Organ Dysfunction Among High-Risk Patients Undergoing Major Surgery: A Randomized Clinical Trial. JAMA 2017, 318, 1346. [Google Scholar] [CrossRef]
- Mathis, M.R.; Naik, B.I.; Freundlich, R.E.; Shanks, A.M.; Heung, M.; Kim, M.; Burns, M.L.; Colquhoun, D.A.; Rangrass, G.; Janda, A.; et al. Preoperative Risk and the Association between Hypotension and Postoperative Acute Kidney Injury. Anesthesiology 2020, 132, 461–475. [Google Scholar] [CrossRef]
- Hatib, F.; Jian, Z.; Buddi, S.; Lee, C.; Settels, J.; Sibert, K.; Rinehart, J.; Cannesson, M. Machine-Learning Algorithm to Predict Hypotension Based on High-Fidelity Arterial Pressure Waveform Analysis. Anesthesiology 2018, 129, 663–674. [Google Scholar] [CrossRef]
- Davies, S.J.; Vistisen, S.T.; Jian, Z.; Hatib, F.; Scheeren, T.W.L. Ability of an Arterial Waveform Analysis–Derived Hypotension Prediction Index to Predict Future Hypotensive Events in Surgical Patients. Anesth. Analg. 2020, 130, 352–359. [Google Scholar] [CrossRef]
- Grundmann, C.D.; Wischermann, J.M.; Fassbender, P.; Bischoff, P.; Frey, U.H. Hemodynamic Monitoring with Hypotension Prediction Index versus Arterial Waveform Analysis Alone and Incidence of Perioperative Hypotension. Acta Anaesthesiol. Scand. 2021, 65, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, K.; Shimada, T.; Yang, D.; Khanna, S.; Cywinski, J.B.; Irefin, S.A.; Ayad, S.; Turan, A.; Ruetzler, K.; Qiu, Y.; et al. Hypotension Prediction Index for Prevention of Hypotension during Moderate- to High-Risk Noncardiac Surgery. Anesthesiology 2020, 133, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. A Critical Appraisal of Propensity-Score Matching in the Medical Literature between 1996 and 2003. Stat. Med. 2008, 27, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Wijnberge, M.; Geerts, B.F.; Hol, L.; Lemmers, N.; Mulder, M.P.; Berge, P.; Schenk, J.; Terwindt, L.E.; Hollmann, M.W.; Vlaar, A.P.; et al. Effect of a Machine Learning–Derived Early Warning System for Intraoperative Hypotension vs Standard Care on Depth and Duration of Intraoperative Hypotension During Elective Noncardiac Surgery. JAMA 2020, 323, 1052–1060. [Google Scholar] [CrossRef]
- Schneck, E.; Schulte, D.; Habig, L.; Ruhrmann, S.; Edinger, F.; Markmann, M.; Habicher, M.; Rickert, M.; Koch, C.; Sander, M. Hypotension Prediction Index Based Protocolized Haemodynamic Management Reduces the Incidence and Duration of Intraoperative Hypotension in Primary Total Hip Arthroplasty: A Single Centre Feasibility Randomised Blinded Prospective Interventional Trial. J. Clin. Monit. Comput. 2020, 34, 1149–1158. [Google Scholar] [CrossRef]
- Kim, J.; Na, S.; Yoo, Y.C.; Koh, S.O. Implementing a Sepsis Resuscitation Bundle Improved Clinical Outcome: A Before-and-After Study. Korean J. Crit. Care Med. 2014, 29, 250–256. [Google Scholar] [CrossRef]
- Mukai, A.; Suehiro, K.; Watanabe, R.; Juri, T.; Hayashi, Y.; Tanaka, K.; Fujii, T.; Ohira, N.; Oda, Y.; Okutani, R.; et al. Impact of Intraoperative Goal-Directed Fluid Therapy on Major Morbidity and Mortality after Transthoracic Oesophagectomy: A Multicentre, Randomised Controlled Trial. Br. J. Anaesth. 2020, 125, 953–961. [Google Scholar] [CrossRef]
- Michard, F.; Giglio, M.T.; Brienza, N. Perioperative Goal-Directed Therapy with Uncalibrated Pulse Contour Methods: Impact on Fluid Management and Postoperative Outcome. Br. J. Anaesth. 2017, 119, 22–30. [Google Scholar] [CrossRef]
- McCambridge, J.; Witton, J.; Elbourne, D.R. Systematic Review of the Hawthorne Effect: New Concepts Are Needed to Study Research Participation Effects. J. Clin. Epidemiol. 2014, 67, 267–277. [Google Scholar] [CrossRef]
- Südfeld, S.; Brechnitz, S.; Wagner, J.Y.; Reese, P.C.; Pinnschmidt, H.O.; Reuter, D.A.; Saugel, B. Post-Induction Hypotension and Early Intraoperative Hypotension Associated with General Anaesthesia. Br. J. Anaesth. 2017, 119, 57–64. [Google Scholar] [CrossRef]
- Mulder, M.P.; Harmannij-Markusse, M.; Donker, D.W.; Fresiello, L.; Potters, J.-W. Is Continuous Intraoperative Monitoring of Mean Arterial Pressure as Good as the Hypotension Prediction Index Algorithm?: Research Letter. Anesthesiology 2023, 138, 657–658. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulou, T.; Tsoumpa, M.; Griva, P.; Galarioti, V.; Matsota, P. Prediction and Prevention of Intraoperative Hypotension with the Hypotension Prediction Index: A Narrative Review. J. Clin. Med. 2022, 11, 5551. [Google Scholar] [CrossRef] [PubMed]
- Solares, G.J.; Garcia, D.; Garcia, M.I.M.; Crespo, C.; Rabago, J.L.; Iglesias, F.; Larraz, E.; Zubizarreta, I.; Rabanal, J.M. Real-World Outcomes of the Hypotension Prediction Index in the Management of Intraoperative Hypotension during Non-Cardiac Surgery: A Retrospective Clinical Study. J. Clin. Monit. Comput. 2023, 37, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Tsoumpa, M.; Kyttari, A.; Matiatou, S.; Tzoufi, M.; Griva, P.; Pikoulis, E.; Riga, M.; Matsota, P.; Sidiropoulou, T. The Use of the Hypotension Prediction Index Integrated in an Algorithm of Goal Directed Hemodynamic Treatment during Moderate and High-Risk Surgery. J. Clin. Med. 2021, 10, 5884. [Google Scholar] [CrossRef] [PubMed]
- Murabito, P.; Astuto, M.; Sanfilippo, F.; Via, L.L.; Vasile, F.; Basile, F.; Cappellani, A.; Longhitano, L.; Distefano, A.; Volti, G.L. Proactive Management of Intraoperative Hypotension Reduces Biomarkers of Organ Injury and Oxidative Stress during Elective Non-Cardiac Surgery: A Pilot Randomized Controlled Trial. J. Clin. Med. 2022, 11, 392. [Google Scholar] [CrossRef]
- Kouz, K.; García, M.I.M.; Cerutti, E.; Lisanti, I.; Draisci, G.; Frassanito, L.; Sander, M.; Akbari, A.A.; Frey, U.H.; Grundmann, C.D.; et al. Intraoperative Hypotension When Using Hypotension Prediction Index Software during Major Noncardiac Surgery: A European Multicentre Prospective Observational Registry (EU HYPROTECT). BJA Open 2023, 6, 100140. [Google Scholar] [CrossRef]
- Maheshwari, K.; Turan, A.; Mao, G.; Yang, D.; Niazi, A.K.; Agarwal, D.; Sessler, D.I.; Kurz, A. The Association of Hypotension during Non-cardiac Surgery, before and after Skin Incision, with Postoperative Acute Kidney Injury: A Retrospective Cohort Analysis. Anaesthesia 2018, 73, 1223–1228. [Google Scholar] [CrossRef]
- Saugel, B.; Kouz, K.; Hoppe, P.; Maheshwari, K.; Scheeren, T.W.L. Predicting Hypotension in Perioperative and Intensive Care Medicine. Best Pract. Res. Clin. Anaesthesiol. 2019, 33, 189–197. [Google Scholar] [CrossRef]
- Edwards Lifesciences Corporation. Acumen Hypotension Prediction Index Software. Available online: http://www.edwards.com/pages/acumen-hpi (accessed on 10 July 2023).
- Edwards Lifesciences Corporation. FloTrac System. Available online: https://www.edwards.com/devices/hemodynamic-monitoring/FloTrac (accessed on 10 July 2023).
- Sponholz, C.; Schuwirth, C.; Koenig, L.; Hoyer, H.; Coldewey, S.M.; Schelenz, C.; Doenst, T.; Kortgen, A.; Bauer, M. Intraoperative Reduction of Vasopressors Using Processed Electroencephalographic Monitoring in Patients Undergoing Elective Cardiac Surgery: A Randomized Clinical Trial. J. Clin. Monit. Comput. 2020, 34, 71–80. [Google Scholar] [CrossRef]
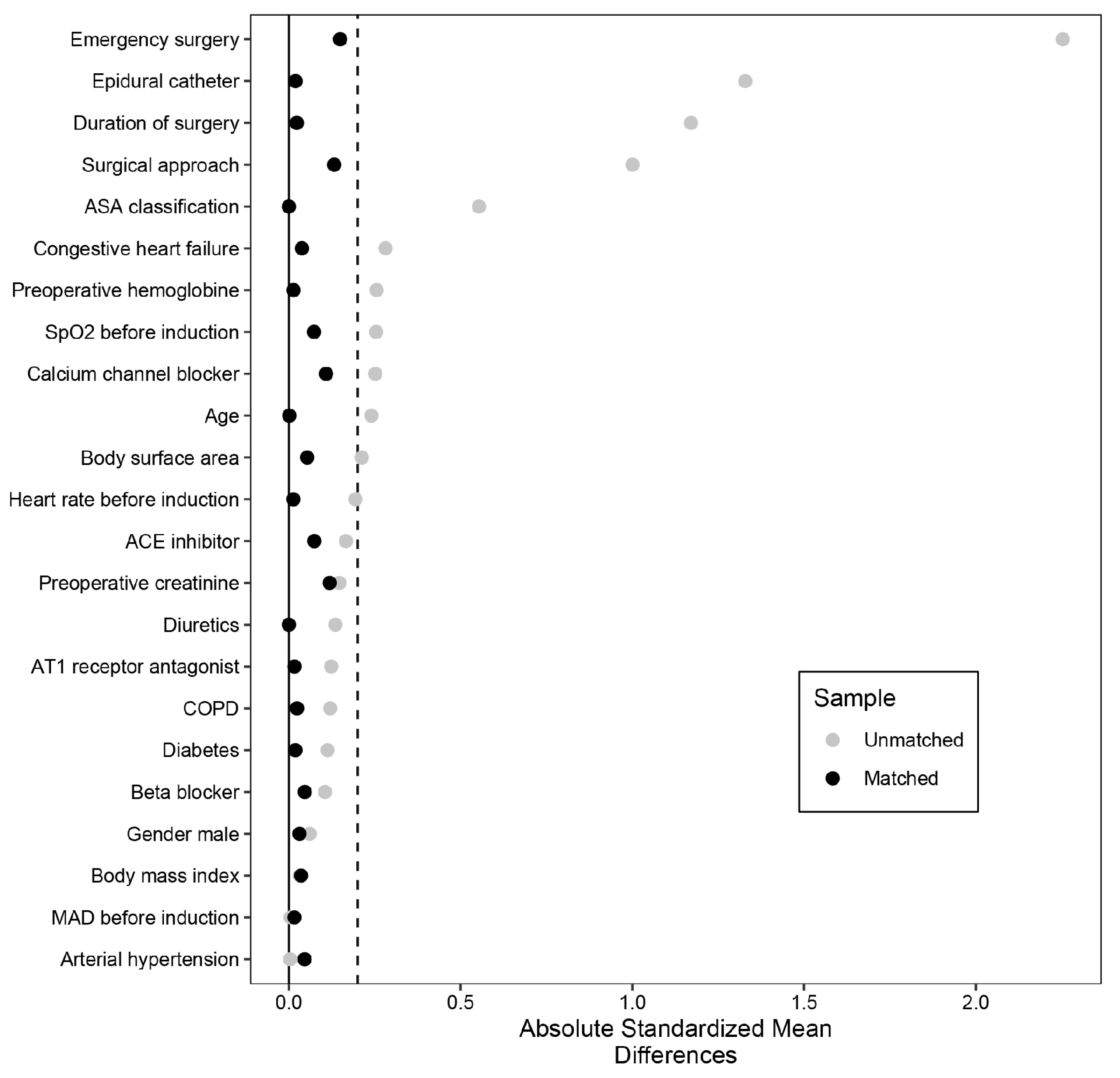
| Parameter | Non-HPI n = 565 | HPI n = 204 | p Value | SMD |
|---|---|---|---|---|
| Age in years, median (IQR) | 70.0 (61.0, 77.0) | 68.0 (60.0, 74.0) | 0.007 | 0.237 |
| Gender male, n (%) | 352 (62.3) | 133 (65.2) | 0.516 | 0.060 |
| Body surface area in m2, median (IQR) | 1.96 (1.80, 2.12) | 2.04 (1.88, 2.17) | 0.002 | 0.205 |
| Body mass index in kg/m2, median (IQR) | 27.0 (24.0, 30.5) | 27.8 (24.8, 30.4) | 0.161 | 0.028 |
| ASA classification, n (%) | <0.001 | 0.576 | ||
| I | 0 (0) | 0 (0) | ||
| II | 118 (20.9) | 94 (46.1) | ||
| III | 384 (68.0) | 101 (49.5) | ||
| IV | 63 (9.4) | 9 (4.4) | ||
| Emergency surgery, n (%) | 131 (23.2) | 2 (1.0) | <0.001 | 0.725 |
| Congestive heart failure, n (%) | 53 (9.4) | 8 (3.9) | 0.020 | 0.220 |
| Chronic obstructive pulmonary disease, n (%) | 82 (14.5) | 22 (10.8) | 0.224 | 0.112 |
| Diabetes, n (%) | 127 (22.5) | 37 (18.1) | 0.231 | 0.108 |
| Arterial hypertension, n (%) | 361 (63.9) | 130 (63.7) | 1.000 | 0.004 |
| ACE inhibitor, n (%) | 148 (26.2) | 40 (19.6) | 0.075 | 0.157 |
| Beta blocker, n (%) | 225 (39.8) | 71 (34.8) | 0.238 | 0.104 |
| Calcium channel blocker, n (%) | 123 (21.8) | 27 (13.2) | 0.011 | 0.226 |
| Diuretics, n (%) | 132 (32.4) | 37 (18.1) | 0.148 | 0.129 |
| AT1 receptor antagonist, n (%) | 124 (21.9) | 56 (27.5) | 0.135 | 0.128 |
| Preoperative hemoglobin concentration in g/dL, median (IQR) | 12.5 (10.1, 14.1) | 13.0 (11.4, 14.1) | 0.029 | 0.222 |
| Preoperative creatinine in mg/dL, median (IQR) | 1.00 (0.80, 1.20) | 0.90 (0.80, 1.10) | 0.066 | 0.139 |
| Parameter | Non-HPI n = 565 | HPI n = 204 | p Value | SMD |
|---|---|---|---|---|
| Surgical approach, n (%) | <0.001 | 0.839 | ||
| Laparoscopy | 97 (17.2) | 63 (30.9) | ||
| Laparotomy | 218 (38.6) | 119 (58.3) | ||
| Combined | 37 (6.5) | 8 (3.9) | ||
| Other | 213 (37.7) | 14 (6.9) | ||
| Duration of surgery in min, median (IQR) | 143 (99, 210) | 311 (228, 392) | <0.001 | 1.250 |
| Epidural catheter, n (%) | 185 (32.7) | 169 (82.8) | <0.001 | 1.177 |
| Mean arterial pressure at induction in mmHg, median (IQR) | 95.0 (86.0, 105.0) | 96.0 (87.8, 103.3) | 0.829 | 0.005 |
| Heart rate before induction in bpm, median (IQR) | 76.0 (68.0, 90.0) | 76.5 (68.0, 87.0) | 0.341 | 0.166 |
| Oxygen saturation before in induction in %, median (IQR) | 97.0 (95.0, 99.0) | 97.0 (96.0, 99.0) | 0.167 | 0.203 |
| Parameter | Non-HPI n = 565 | HPI n = 204 | p Value | SMD |
|---|---|---|---|---|
| Mean minimal alveolar concentration in balanced anesthesia, median (IQR) | 0.93 (0.84, 1.03) | 0.97 (0.90, 1.04) | 0.005 | 0.082 |
| Cumulative dose of Ropivacaine in epidural application in mg, median (IQR) | 142 (112, 187) | 225 (155, 262) | <0.001 | 0.905 |
| Cumulative dose of Norepinephrinein mg, median (IQR) | 0.72 (0.20, 1.93) | 2.24 (0.94, 3.96) | <0.001 | 0.393 |
| Cumulative dose of Dobutaminein mg, median (IQR) | 30.8 (11.1, 47.1) | 45.0 (22.7, 82.4) | 0.161 | 0.138 |
| Amount of crystalloid fluid in mL, median (IQR) | 3000 (2000, 4500) | 6000 (5000, 8000) | <0.001 | 0.982 |
| Amount of colloid fluid in mL, median (IQR) | 500 (500, 1000) | 750 (500, 1000) | 0.272 | 0.128 |
| Estimated blood loss in mL, median (IQR) | 455 (200, 700) | 600 (300, 900) | <0.001 | 0.298 |
| Primary Endpoints | Non-HPI n = 565 | HPI n = 204 | p Value | SMD |
|---|---|---|---|---|
| Time-weighted average (MAP < 65 mmHg) in mmHg, median (IQR) | 0.200 (0.040, 0.540) | 0.050 (0.010, 0.200) | <0.001 | 0.384 |
| Secondary Endpoints | ||||
| Number of hypotensive events (MAP < 65 mmHg) per patient, median (IQR) | 2.00 (1.00, 5.00) | 2.00 (0.00, 4.00) | 0.097 | 0.022 |
| Cumulative duration of hypotension (MAP < 65 mmHg) per patient in minutes, median (IQR) | 5.00 (1.00, 13.0) | 3.00 (1.00, 10.0) | 0.002 | 0.147 |
| Time-weighted average (MAP < 60 mmHg) in mmHg, median (IQR) | 0.040 (0.000, 0.160) | 0.010 (0.000, 0.050) | <0.001 | 0.278 |
| Time-weighted average (MAP < 55 mmHg) in mmHg, median (IQR) | 0.000 (0.000, 0.030) | 0.000 (0.000, 0.020) | 0.022 | 0.164 |
| Number of events HPI > 85, median (IQR) | NA | 8.00 (4.75, 14.3) | NA | NA |
| Parameter | Non-HPI n = 136 | HPI n = 136 | p Value | SMD |
|---|---|---|---|---|
| Age in years, median (IQR) | 67.0 (59.0, 75.0) | 68.00 (60.0, 75.0) | 0.725 | 0.001 |
| Gender male, n (%) | 85 (62.5) | 83 (61.0) | 0.901 | 0.031 |
| Body surface area in m2, median (IQR) | 2.00 (1.86, 2.10) | 1.99 (1.88, 2.11) | 0.671 | 0.053 |
| Body mass index in kg/m2, median (IQR) | 27.2 (23.9, 31.3) | 27.05 (24.8, 30.0) | 0.948 | 0.035 |
| ASA classification, n (%) | 0.206 | <0.001 | ||
| I | 0 (0) | 0 (0) | ||
| II | 51 (37.5) | 56 (41.2) | ||
| III | 82 (60.3) | 72 (52.9) | ||
| IV | 3 (2.2) | 8 (5.9) | ||
| Emergency surgery, n (%) | 4 (2.9) | 2 (1.5) | 0.680 | 0.149 |
| Congestive heart failure, n (%) | 5 (3.7) | 6 (4.4) | 1.000 | 0.038 |
| Chronic obstructive pulmonary disease, n (%) | 14 (10.3) | 13 (9.6) | 1.000 | 0.024 |
| Diabetes, n (%) | 21 (15.4) | 22 (16.2) | 1.000 | 0.019 |
| Arterial hypertension, n (%) | 87 (64.0) | 84 (61.8) | 0.802 | 0.046 |
| ACE inhibitor, n (%) | 24 (17.6) | 28 (20.6) | 0.644 | 0.074 |
| Beta blocker, n (%) | 49 (36.0) | 46 (33.8) | 0.799 | 0.046 |
| Calcium channel blocker, n (%) | 22 (16.2) | 17 (12.5) | 0.489 | 0.109 |
| Diuretics, n (%) | 25 (18.4) | 25 (18.4) | 1.000 | <0.001 |
| AT1 receptor antagonist, n (%) | 38 (27.9) | 37 (27.2) | 1.000 | 0.017 |
| Preoperative hemoglobin concentration in g/dL, median (IQR) | 12.9 (11.3, 14.4) | 13.00 (11.3, 14.3) | 0.907 | 0.014 |
| Preoperative creatinine in mg/dL, median (IQR) | 0.900 (0.800, 1.10) | 0.900 (0.800, 1.10) | 0.893 | 0.119 |
| Parameter | Non-HPI n = 136 | HPI n = 136 | p Value | SMD |
|---|---|---|---|---|
| Surgical approach, n (%) | 0.065 | 0.132 | ||
| Laparoscopy | 35 (25.7) | 32 (23.5) | ||
| Laparotomy | 67 (49.3) | 84 (61.8) | ||
| Combined | 17 (12.5) | 6 (4.4) | ||
| Other | 17 (12.5) | 14 (10.3) | ||
| Duration of surgery in min, median (IQR) | 255 (166, 342) | 266 (174, 332) | 0.767 | 0.023 |
| Epidural catheter, n (%) | 102 (75.0) | 101 (74.3) | 1.000 | 0.020 |
| Mean arterial pressure at induction in mmHg, median (IQR) | 94.0 (86.0, 106) | 95.5 (87.5, 104) | 0.945 | 0.016 |
| Heart rate before induction in bpm, median (IQR) | 75.0 (66.0, 85.0) | 76.5 (68.0, 87.0) | 0.688 | 0.013 |
| Oxygen saturation before in induction in %, median (IQR) | 98.0 (96.0, 99.0) | 98.0 (96.0, 99.0) | 0.584 | 0.073 |
| Parameter | Non-HPI n = 136 | HPI n = 136 | p Value | SMD |
|---|---|---|---|---|
| Mean minimal alveolar concentration in balanced anesthesia, median (IQR) | 0.960 (0.880, 1.07) | 0.970 (0.870, 1.03) | 0.813 | 0.090 |
| Cumulative dose of Ropivacaine in epidural application in mg, median (IQR) | 187 (121, 225) | 187 (150, 255) | 0.128 | 0.188 |
| Cumulative dose of Norepinephrine in mg, median (IQR) | 1.76 (0.69, 3.18) | 1.99 (0.87, 3.53) | 0.429 | 0.033 |
| Cumulative dose of Dobutamine in mg, median (IQR) | 43.6 (29.8, 58.4) | 48.2 (27.4, 74.8) | 0.741 | 0.050 |
| Amount of crystalloid fluid in mL, median (IQR) | 4000 (3000, 7000) | 6000 (4000, 7500) | 0.001 | 0.281 |
| Amount of colloid fluid in mL, median (IQR) | 1000 (500, 1000) | 500.0 (500, 100) | 0.709 | 0.104 |
| Estimated blood loss in mL, median (IQR) | 480 (200, 900) | 550 (300, 900) | 0.115 | 0.045 |
| Primary Endpoints | Non-HPI n = 136 | HPI n = 136 | p Value | SMD |
|---|---|---|---|---|
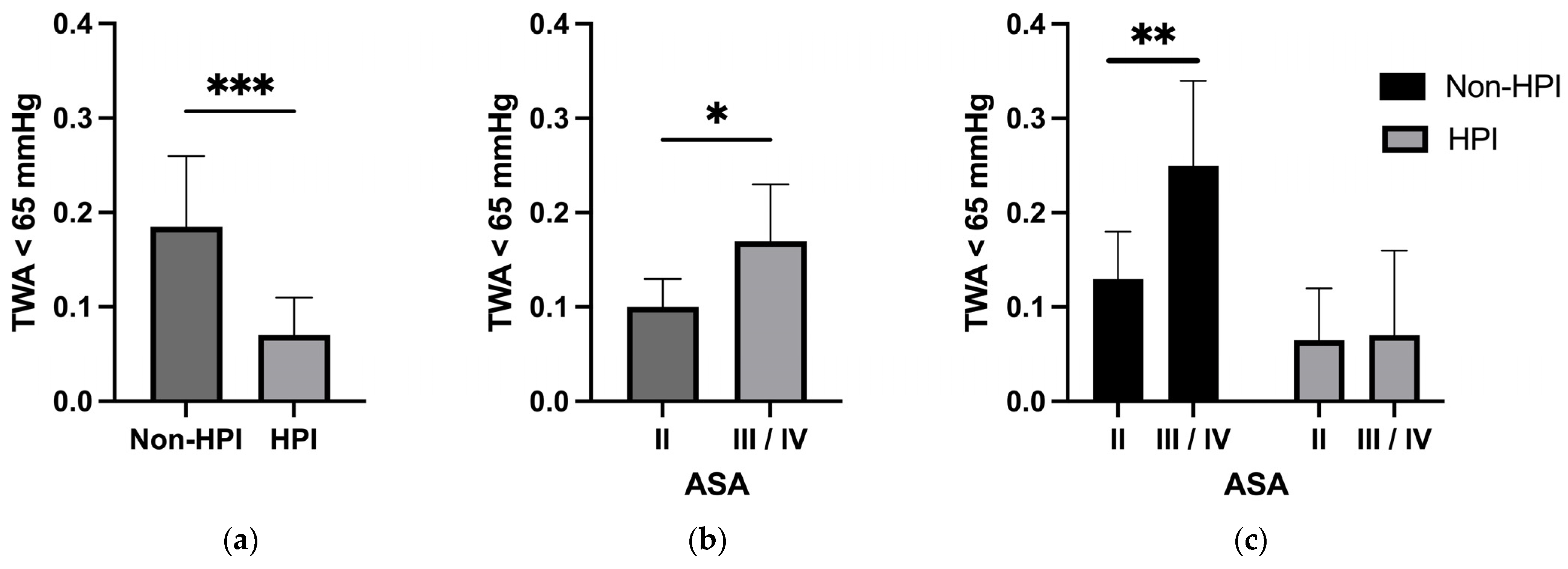
| Time-weighted average (MAD < 65 mmHg) in mmHg, median (IQR) | 0.180 (0.060, 0.410) | 0.070 (0.020, 0.240) | <0.001 | 0.243 |
| Secondary endpoints | ||||
| Number of hypotensive events (MAD < 65 mmHg) per patient, median (IQR) | 3.00 (1.00, 5.25) | 2.00 (1.00, 4.00) | 0.002 | 0.338 |
| Cumulative duration of hypotension (MAD < 65 mmHg) per patient in minutes, median (IQR) | 7.00 (2.00, 15.3) | 3.00 (1.00, 10.0) | 0.001 | 0.279 |
| Time-weighted average (MAD < 60 mmHg) in mmHg, median (IQR) | 0.040 (0.000, 0.120) | 0.010 (0.000, 0.060) | 0.005 | 0.153 |
| Time-weighted average (MAD < 55 mmHg) in mmHg, median (IQR) | 0.010 (0.000, 0.030) | 0.000 (0.000, 0.020) | 0.046 | 0.053 |
| Number of HPI alarms > 85, median (IQR) | NA | 8.00 (4.00, 12.3) | NA | NA |
| Parameter | Non-HPI n = 136 | HPI n = 136 | p Value | SMD |
|---|---|---|---|---|
| Postoperative hemoglobin in g/dL, median (IQR) | 10.2 (8.70, 11.7) | 9.70 (8.60, 11.2) | 0.118 | 0.186 |
| ICU submissions, n (%) | 91 (66.9) | 113 (83.1) | 0.003 | 0.380 |
| Length of stay in ICU in hours, median (IQR) | 70.0 (25.0, 129.5) | 91.0 (47.0, 113.0) | 0.161 | 0.065 |
| Death in ICU, n (%) | 6 (6.5) | 5 (4.4) | 0.728 | 0.091 |
| Acute renal failure, n (%) | 20 (14.7) | 11 (8.1) | 0.127 | 0.209 |
| Postoperative maximum creatinine, median (IQR) | 1.00 (0.80, 1.30) | 1.00 (0.80, 1.30) | 0.795 | 0.030 |
| Need for renal replacement therapy in ICU, n (%) | 3 (3.2) | 4 (3.5) | 1.000 | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Runge, J.; Graw, J.; Grundmann, C.D.; Komanek, T.; Wischermann, J.M.; Frey, U.H. Hypotension Prediction Index and Incidence of Perioperative Hypotension: A Single-Center Propensity-Score-Matched Analysis. J. Clin. Med. 2023, 12, 5479. https://doi.org/10.3390/jcm12175479
Runge J, Graw J, Grundmann CD, Komanek T, Wischermann JM, Frey UH. Hypotension Prediction Index and Incidence of Perioperative Hypotension: A Single-Center Propensity-Score-Matched Analysis. Journal of Clinical Medicine. 2023; 12(17):5479. https://doi.org/10.3390/jcm12175479
Chicago/Turabian StyleRunge, Julian, Jessica Graw, Carla D. Grundmann, Thomas Komanek, Jan M. Wischermann, and Ulrich H. Frey. 2023. "Hypotension Prediction Index and Incidence of Perioperative Hypotension: A Single-Center Propensity-Score-Matched Analysis" Journal of Clinical Medicine 12, no. 17: 5479. https://doi.org/10.3390/jcm12175479





