All-Inside Anterior Cruciate Ligament Reconstruction Technique: Tips and Tricks
Abstract
:1. Introduction
2. Surgical Technique
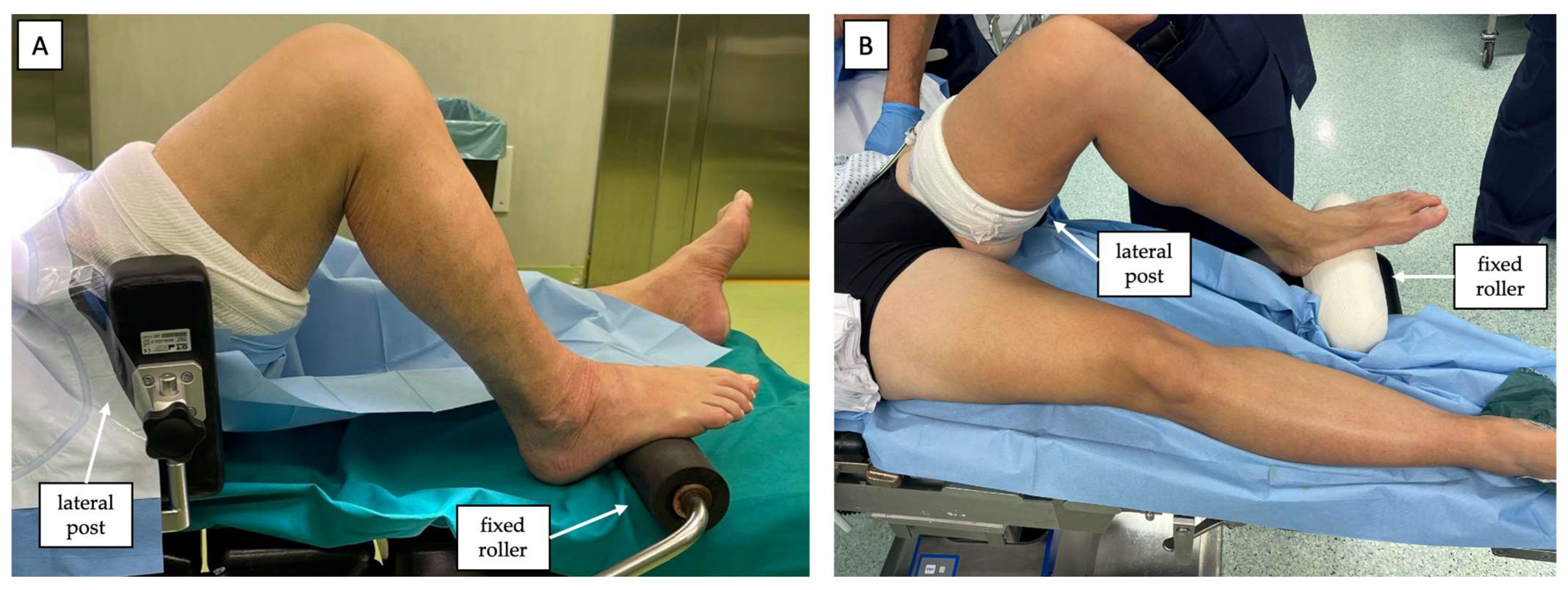
2.1. Patient Positioning and Preoperative Setting
2.2. Graft Harvesting
2.3. Graft Preparation
2.4. Internal Brace
2.5. Starting Arthroscopic Setting
2.6. Femoral Socket
2.7. Tibial Socket
2.8. Shuttle Sutures
2.9. Femoral Socket Graft Passage
2.10. Tibial Socket Graft Passage and Tensioning
- (1)
- Check the amount of graft in the tibial socket according to the position of the annular tag at a predetermined distance from both ends of the graft and the annular tag in the middle of the graft.
- (2)
- Tighten the femoral side further by approximately 5 mm.
- (3)
- Perform additional cycling of the knee.
- (4)
- Remove the graft from the tibial socket and repeat the retrograde reaming by increasing the drilling by 5 mm, put the shuttle suture back in place, retrieve it without soft tissue bridge interposition, and proceed with graft passage and tensioning.
3. Discussion
3.1. Patient-Reported Outcome Measures (PROMs)
3.2. Advantages of the All-Inside ACLR Technique
3.3. Internal Brace Augmentation
3.4. The All-Inside ACLR Technique’s Complications and Drawbacks
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, M.M. Optimizing outcomes of ACL surgery-Is autograft reconstruction the only reasonable option? J. Orthop. Res. 2021, 39, 1843–1850. [Google Scholar] [CrossRef]
- Bosco, F.; Giustra, F.; Crivellaro, M.; Giai Via, R.; Lavia, A.D.; Capella, M.; Sabatini, L.; Risitano, S.; Rovere, G.; Massè, A.; et al. Is augmentation the best solution in partial anterior cruciate ligament tears? A literature systematic review and meta-analysis. J. Orthop. 2022, 36, 11–17. [Google Scholar] [CrossRef]
- Pavan, D.; Morello, F.; Monachino, F.; Rovere, G.; Camarda, L.; Pitarresi, G. Similar biomechanical properties of four tripled tendon graft models for ACL reconstruction. Arch. Orthop. Trauma Surg. 2022, 142, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Pautasso, A.; Capella, M.; Barberis, L.; Drocco, L.; Giai Via, R.; Bistolfi, A.; Massè, A.; Sabatini, L. All-inside technique in ACL reconstruction: Mid-term clinical outcomes and comparison with AM technique (Hamstrings and BpTB grafts). Eur. J. Orthop. Surg. Traumatol. 2021, 31, 465–472. [Google Scholar] [CrossRef]
- Connaughton, A.J.; Geeslin, A.G.; Uggen, C.W. All-inside ACL reconstruction: How does it compare to standard ACL reconstruction techniques? J. Orthop. 2017, 14, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.D.; Kalmam, V.R.; Grawl, D.M. Isometry testing for anterior cruciate ligament reconstruction revisited. Arthroscopy 1995, 11, 647–659. [Google Scholar] [CrossRef]
- Lubowitz, J.H.; Ahmad, C.S.; Anderson, K. All-inside anterior cruciate ligament graft-link technique: Second-generation, no-incision anterior cruciate ligament reconstruction. Arthroscopy 2011, 27, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Nuelle, C.W.; Balldin, B.C.; Slone, H.S. All-Inside Anterior Cruciate Ligament Reconstruction. Arthroscopy 2022, 38, 2368–2369. [Google Scholar] [CrossRef]
- Placella, G.; Bartoli, M.; Peruzzi, M.; Speziali, A.; Pace, V.; Cerulli, G. Return to sport activity after anterior cruciate ligament reconstruction in skeletally immature athletes with manual drilling original all inside reconstruction at 8 years follow-up. Acta Orthop. Traumatol. Turc. 2016, 50, 635–638. [Google Scholar] [CrossRef]
- Schurz, M.; Tiefenboeck, T.M.; Winnisch, M.; Syre, S.; Plachel, F.; Steiner, G.; Hajdu, S.; Hofbauer, M. Clinical and Functional Outcome of All-Inside Anterior Cruciate Ligament Reconstruction at a Minimum of 2 Years’ Follow-up. Arthroscopy 2016, 32, 332–337. [Google Scholar] [CrossRef]
- Lubowitz, J.H.; Schwartzberg, R.; Smith, P. Randomized controlled trial comparing all-inside anterior cruciate ligament reconstruction technique with anterior cruciate ligament reconstruction with a full tibial tunnel. Arthroscopy 2013, 29, 1195–1200. [Google Scholar] [CrossRef]
- Galan, H.; Escalante, M.; Della Vedova, F.; Slullitel, D. All inside full thickness quadriceps tendon ACL reconstruction: Long term follow up results. J. Exp. Orthop. 2020, 7, 13. [Google Scholar] [CrossRef]
- Sokal, P.A.; Norris, R.; Maddox, T.W.; Oldershaw, R.A. The diagnostic accuracy of clinical tests for anterior cruciate ligament tears are comparable but the Lachman test has been previously overestimated: A systematic review and meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 3287–3303. [Google Scholar] [CrossRef]
- Kern, M.; Love, D.; Cotter, E.J.; Postma, W. Quadruple-Bundle Semitendinosus-Gracilis Graft Technique for All-Inside Anterior Cruciate Ligament Reconstruction. Arthrosc. Tech. 2016, 5, e1317–e1320. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.J.; Hyatt, A.E.; Gatt, C.J., Jr.; Dhawan, A. Hamstring autograft size can be predicted and is a potential risk factor for anterior cruciate ligament reconstruction failure. Arthroscopy 2014, 30, 882–890. [Google Scholar] [CrossRef]
- Waly, A.H.; ElShafie, H.I.; Morsy, M.G.; ElShafie, M.H.; Galal, M.A.; Mohamed Ayman, E.H.; Gawish, H.M. All-Inside Anterior Cruciate Ligament Reconstruction with Suture Tape Augmentation: Button Tie-Over Technique (BTOT). Arthrosc. Tech. 2021, 10, e2559–e2570. [Google Scholar] [CrossRef]
- Nyland, J.; Caborn, D.N.; Jakob, R. Knee arthroscopy: The science of art. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2457–2458. [Google Scholar] [CrossRef]
- Bhimani, R.; Shahriarirad, R.; Ranjbar, K.; Erfani, A.; Ashkani-Esfahani, S. Transportal versus all-inside techniques of anterior cruciate ligament reconstruction: A systematic review. J. Orthop. Surg. Res. 2021, 16, 734. [Google Scholar] [CrossRef]
- Lowenstein, N.A.; Haber, D.B.; Ostergaard, P.J.; Collins, J.E.; Matzkin, E.G. All-inside anterior cruciate ligament reconstruction using quadrupled semitendinosus: Comparable 2-year outcomes in male and female patients. Arthroscopy 2021, 37, 3140–3148. [Google Scholar] [CrossRef]
- Brzezinski, A.; Nasra, M.; Pfaff, W.; Imbergamo, C.; Simon, M.; Tarapore, R.; Xavier, J.; Ghodbane, S.; Gatt, C. Five-strand hamstring grafts are biomechanically comparable to four-strand grafts and offer greater diameter for anterior cruciate ligament reconstruction. Arthrosc. Sports Med. Rehabil. 2022, 4, E1731–E1738. [Google Scholar] [CrossRef]
- Buranapuntaruk, T.; Kongrukgreatiyos, K.; Itthipanichpong, T. All-Inside Arthroscopic Anterior Cruciate Ligament Reconstruction and Internal Brace with Recycling Suture. Arthrosc. Tech. 2021, 10, e2429–e2434. [Google Scholar] [CrossRef]
- Yosmaoglu, H.B.; Baltaci, G.; Ozer, H.; Atay, A. Effects of additional gracilis tendon harvest on muscle torque, motor coordination, and knee laxity in ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1287–1292. [Google Scholar] [CrossRef]
- Kouloumentas, P.; Kavroudakis, E.; Charalampidis, E.; Kavroudakis, D.; Triantafyllopoulos, G.K. Superior knee flexor strength at 2 years with all-inside short-graft anterior cruciate ligament reconstruction vs a conventional hamstring technique. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 3592–3598. [Google Scholar] [CrossRef]
- Monaco, E.; Redler, A.; Fabbri, M.; Proietti, L.; Gaj, E.; Daggett, M.; Ferretti, A. Isokinetic flexion strength recovery after ACL reconstruction: A comparison between all inside graft-link technique and full tibial tunnel technique. Phys. Sportsmed. 2019, 47, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Salaha, Z.F.M.; Ammarullah, M.I.; Abdullah, N.N.A.A.; Aziz, A.U.A.; Gan, H.-S.; Abdullah, A.H.; Abdul Kadir, M.R.; Ramlee, M.H. Biomechanical Effects of the Porous Structure of Gyroid and Voronoi Hip Implants: A Finite Element Analysis Using an Experimentally Validated Model. Materials 2023, 16, 3298. [Google Scholar] [CrossRef]
- Kim, S.G.; Kurosawa, H.; Sakuraba, K.; Ikeda, H.; Takazawa, S.; Takazawa, Y. Development and application of an inside-to-out drill bit for anterior cruciate ligament reconstruction. Arthroscopy 2005, 21, 1012. [Google Scholar] [CrossRef] [PubMed]
- Osti, M.; Krawinkel, A.; Hoffelner, T.; Benedetto, K.P. Quantification of tibial bone loss in antegrade versus retrograde tunnel placement for anterior cruciate ligament reconstruction. Int. Orthop. 2015, 39, 1611–1614. [Google Scholar] [CrossRef]
- Lowenstein, N.A.; Altwies, M.E.; Hoang, V.; Anthony, T.; Matzkin, E.G. Technique for All-Inside Anterior Cruciate Ligament Reconstruction Using Quadrupled Semitendinosus and Gracilis Autograft. Arthrosc. Tech. 2023, 12, e1083–e1089. [Google Scholar] [CrossRef]
- Mackay, G.M.; Blyth, M.J.; Anthony, I.; Hopper, G.P.; Ribbans, W.J. A review of ligament augmentation with the InternalBrace™: The surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg. Technol. Int. 2015, 26, 239–255. [Google Scholar]
- Smith, P.A.; Bley, J.A. Allograft Anterior Cruciate Ligament Reconstruction Utilizing Internal Brace Augmentation. Arthrosc. Tech. 2016, 5, e1143–e1147. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Cao, Y.; Song, G.; Li, Y.; Zhang, Z.; Feng, Z.; Zhang, H. Suture tape augmentation, a novel application of synthetic materials in anterior cruciate ligament reconstruction: A systematic review. Front. Bioeng. Biotechnol. 2023, 10, 1065314. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, M.; Zhao, T.; Wang, L.; Dong, S.; Tan, H. All-inside versus complete tibial tunnel techniques in anterior cruciate ligament reconstruction: A systematic review and meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2023, 18, 127. [Google Scholar] [CrossRef] [PubMed]
- Lubowitz, J.H. All-inside ACL: Retroconstruction controversies. Sports Med. Arthrosc. Rev. 2010, 18, 20–26. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosco, F.; Giustra, F.; Ghirri, A.; Cacciola, G.; Massè, A.; Capella, M. All-Inside Anterior Cruciate Ligament Reconstruction Technique: Tips and Tricks. J. Clin. Med. 2023, 12, 5793. https://doi.org/10.3390/jcm12185793
Bosco F, Giustra F, Ghirri A, Cacciola G, Massè A, Capella M. All-Inside Anterior Cruciate Ligament Reconstruction Technique: Tips and Tricks. Journal of Clinical Medicine. 2023; 12(18):5793. https://doi.org/10.3390/jcm12185793
Chicago/Turabian StyleBosco, Francesco, Fortunato Giustra, Alessandro Ghirri, Giorgio Cacciola, Alessandro Massè, and Marcello Capella. 2023. "All-Inside Anterior Cruciate Ligament Reconstruction Technique: Tips and Tricks" Journal of Clinical Medicine 12, no. 18: 5793. https://doi.org/10.3390/jcm12185793
APA StyleBosco, F., Giustra, F., Ghirri, A., Cacciola, G., Massè, A., & Capella, M. (2023). All-Inside Anterior Cruciate Ligament Reconstruction Technique: Tips and Tricks. Journal of Clinical Medicine, 12(18), 5793. https://doi.org/10.3390/jcm12185793





