One-Stage Immediate Alloplastic Breast Reconstruction in Large and Ptotic Breasts: An Institutional Algorithm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
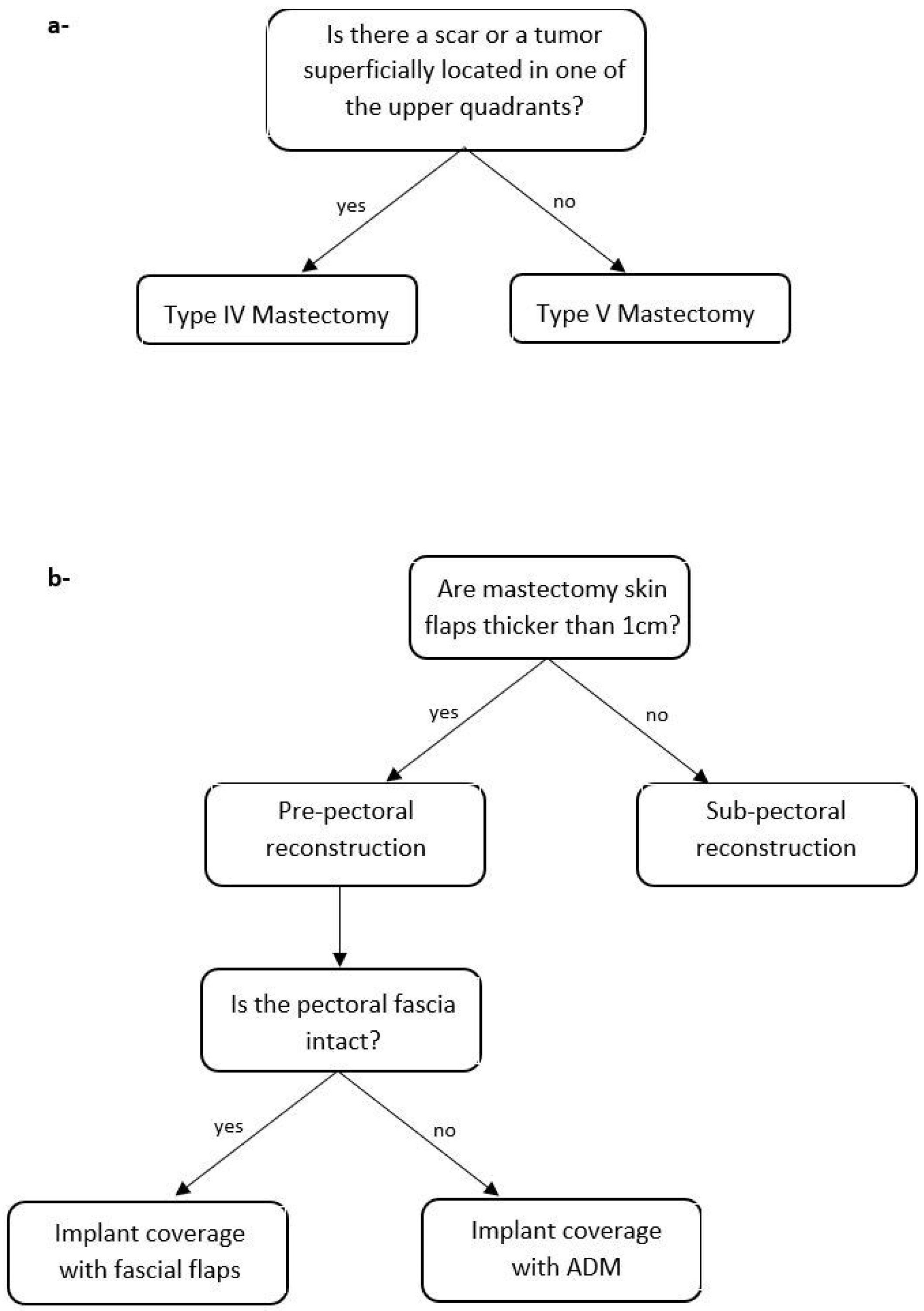
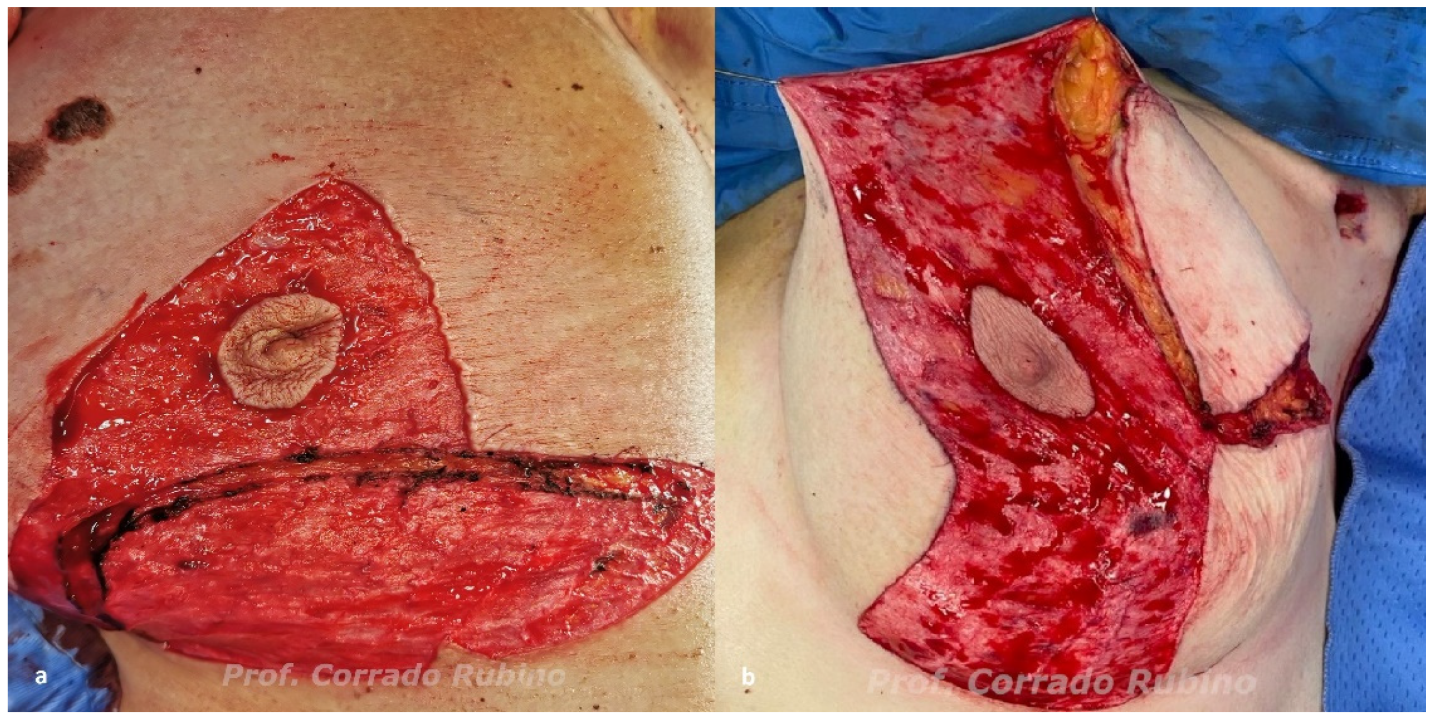
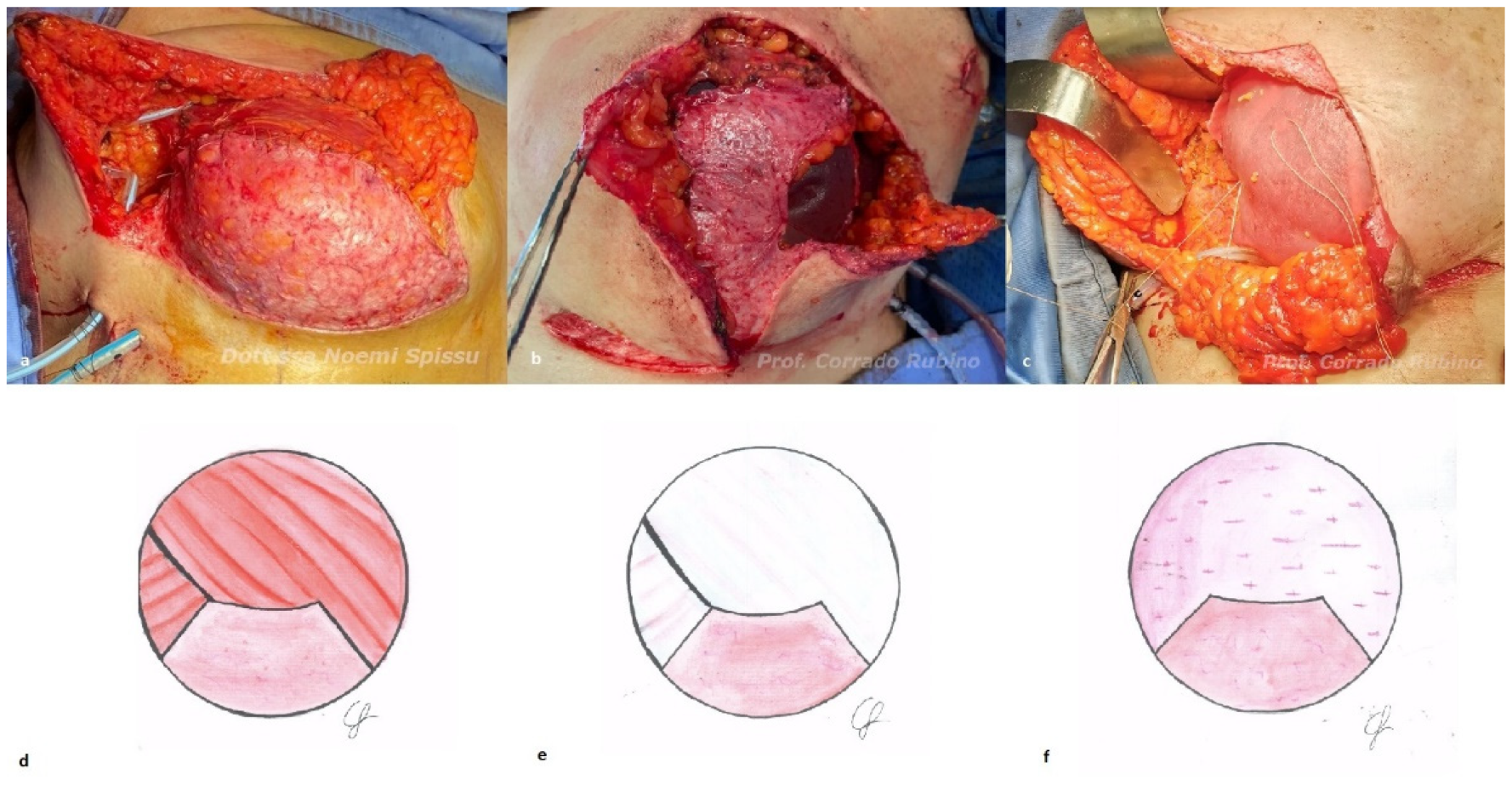
2.2. Algorithm
2.3. Ethical Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heneghan, H.M.; Prichard, R.S.; Lyons, R.; Regan, P.J.; Kelly, J.L.; Malone, C.; McLaughlin, R.; Sweeney, K.J.; Kerin, M.J. Quality of Life after Immediate Breast Reconstruction and Skin-Sparing Mastectomy–A Comparison with Patients Undergoing Breast Conserving Surgery. Eur. J. Surg. Oncol. 2011, 37, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Elder, E.E.; Brandberg, Y.; Björklund, T.; Rylander, R.; Lagergren, J.; Jurell, G.; Wickman, M.; Sandelin, K. Quality of Life and Patient Satisfaction in Breast Cancer Patients after Immediate Breast Reconstruction: A Prospective Study. Breast 2005, 14, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Dimovska, E.O.F.; Chen, C.; Chou, H.; Lin, Y.; Cheng, M. Outcomes and Quality of Life in Immediate One-stage versus Two-stage Breast Reconstructions without an Acellular Dermal Matrix: 17- Years of Experience. J. Surg. Oncol. 2021, 124, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.J. A preliminary report on a method of planning the mammaplasty. Plast. Reconstr. Surg. 1956, 17, 367–375. [Google Scholar] [CrossRef]
- Toth, B.A.; Lappert, P. Modified Skin Incisions for Mastectomy: The Need for Plastic Surgical Input in Preoperative Planning. Plast. Reconstr. Surg. 1991, 87, 1048–1053. [Google Scholar] [CrossRef]
- Di Candia, M.; Lie, K.H.; Forouhi, P.; Malata, C.M. Experience with the Wise Mammaplasty Skin Resection Pattern in Skin-Sparing Mastectomy and Immediate Breast Reconstruction for Large Breast Volumes. Int. J. Surg. 2011, 9, 41–45. [Google Scholar] [CrossRef]
- Kilgo, M.S.; Kaufman, G.J.; Shen, A.E.; Korsh, J.; Baranchuk, N.V.; Douglas, B.K.; Brewer, B.W. A Comparison of Elliptical Mastectomy to Inverted-T Pattern Mastectomy in Two-Stage Prosthetic Breast Reconstruction. Plast. Reconstr. Surg. 2015, 136, 426e–433e. [Google Scholar] [CrossRef]
- Gunn, J.; Dortch, J.; TerKonda, S.; Schilling, K.; Li, Z.; Diehl, N.; Gibson, T.; Bagaria, S.; Perdikis, G.; McLaughlin, S. Comparing Morbidity Rates between Wise Pattern and Standard Horizontal Elliptical Mastectomy Incisions in Patients Undergoing Immediate Breast Reconstruction. Breast J. 2019, 25, 20–25. [Google Scholar] [CrossRef]
- Bostwick, J. Implant Reconstruction with Breast Skin and Volume Reduction Using an Inverted T-Incision. In Plastic and Reconstructive Breast Surgery 2; Quality Medical Publishing: St. Louis, MO, USA, 1990; pp. 1369–1373. [Google Scholar]
- Chopra, S.; Al-Ishaq, Z.; Vidya, R. The Journey of Prepectoral Breast Reconstruction through Time. World J. Plast Surg. 2021, 10, 3–13. [Google Scholar] [CrossRef]
- du Plessis, M.I.; Cottler, P.S.; Campbell, C.A. Acellular Dermal Matrix Favorably Modulates the Healing Response after Surgery. Plast. Reconstr. Surg. 2022, 150, 290e–299e. [Google Scholar] [CrossRef]
- Burke, J.F.; Yannas, I.V.; Quinby, W.C.; Bondoc, C.C.; Jung, W.K. Successful Use of a Physiologically Acceptable Artificial Skin in the Treatment of Extensive Burn Injury. Ann. Surg. 1981, 194, 413–428. [Google Scholar] [CrossRef]
- Gierek, M.; Łabuś, W.; Kitala, D.; Lorek, A.; Ochała-Gierek, G.; Zagórska, K.M.; Waniczek, D.; Szyluk, K.; Niemiec, P. Human Acellular Dermal Matrix in Reconstructive Surgery—A Review. Biomedicines 2022, 10, 2870. [Google Scholar] [CrossRef]
- Hallberg, H.; Rafnsdottir, S.; Selvaggi, G.; Strandell, A.; Samuelsson, O.; Stadig, I.; Svanberg, T.; Hansson, E.; Lewin, R. Benefits and Risks with Acellular Dermal Matrix (ADM) and Mesh Support in Immediate Breast Reconstruction: A Systematic Review and Meta-Analysis. J. Plast. Surg. Hand Surg. 2018, 52, 130–147. [Google Scholar] [CrossRef]
- Margulies, I.G.; Salzberg, C.A. The Use of Acellular Dermal Matrix in Breast Reconstruction: Evolution of Techniques over 2 Decades. Gland Surg. 2019, 8, 3–10. [Google Scholar] [CrossRef]
- Marcasciano, M.; Mazzocchi, M.; Kaciulyte, J.; Spissu, N.; Casella, D.; Ribuffo, D.; Dessy, L.A. Skin Cancers and Dermal Substitutes: Is It Safe? Review of the Literature and Presentation of a 2-Stage Surgical Protocol for the Treatment of Non-Melanoma Skin Cancers of the Head in Fragile Patients. Int. Wound J. 2018, 15, 756–768. [Google Scholar] [CrossRef]
- Ward, R.M.; Sung, V.W.; Clemons, J.L.; Myers, D.L. Vaginal Paravaginal Repair with an AlloDerm Graft: Long-Term Outcomes. Am. J. Obstet. Gynecol. 2007, 197, 670.e1–670.e5. [Google Scholar] [CrossRef]
- Gierek, M.; Łabuś, W.; Słaboń, A.; Ziółkowska, K.; Ochała-Gierek, G.; Kitala, D.; Szyluk, K.; Niemiec, P. Co-Graft of Acellular Dermal Matrix and Split Thickness Skin Graft—A New Reconstructive Surgical Method in the Treatment of Hidradenitis Suppurativa. Bioengineering 2022, 9, 389. [Google Scholar] [CrossRef]
- Guo, X.; Mu, D.; Gao, F. Efficacy and Safety of Acellular Dermal Matrix in Diabetic Foot Ulcer Treatment: A Systematic Review and Meta-Analysis. Int. J. Surg. 2017, 40, 1–7. [Google Scholar] [CrossRef]
- Neumayer, L.; Giobbie-Hurder, A.; Jonasson, O.; Fitzgibbons, R.; Dunlop, D.; Gibbs, J.; Reda, D.; Henderson, W. Veterans Affairs Cooperative Studies Program 456 Investigators Open Mesh versus Laparoscopic Mesh Repair of Inguinal Hernia. N. Engl. J. Med. 2004, 350, 1819–1827. [Google Scholar] [CrossRef]
- Todros, S.; Pavan, P.G.; Natali, A.N. Biomechanical Properties of Synthetic Surgical Meshes for Pelvic Prolapse Repair. J. Mech. Behav. Biomed. Mater. 2015, 55, 271–285. [Google Scholar] [CrossRef]
- Logan Ellis, H.; Asaolu, O.; Nebo, V.; Kasem, A. Biological and Synthetic Mesh Use in Breast Reconstructive Surgery: A Literature Review. World J. Surg. Oncol. 2016, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Derderian, C.A.; Karp, N.S.; Choi, M. Wise-Pattern Breast Reconstruction: Modification Using Alloderm and a Vascularized Dermal-Subcutaneous Pedicle. Ann. Plast. Surg. 2009, 62, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Rathinaezhil, R.; Ugolini, F.; Osman, H. Early Experience with Implant Based Breast Reconstruction for Early Breast Cancer in Ptotic Breasts with Non Biological Mesh and Lower Pole Dermal Sling. Ann. Surg. Innov. Res. 2015, 9, 3. [Google Scholar] [CrossRef]
- Kankam, H.; Hourston, G.; Forouhi, P.; Di Candia, M.; Wishart, G.; Malata, C. Combination of Acellular Dermal Matrix with a De-Epithelialised Dermal Flap during Skin-Reducing Mastectomy and Immediate Breast Reconstruction. Ann. R. Coll. Surg. Engl. 2018, 100, e197–e202. [Google Scholar] [CrossRef] [PubMed]
- Maruccia, M.; Elia, R.; Gurrado, A.; Moschetta, M.; Nacchiero, E.; Bolletta, A.; Testini, M.; Giudice, G. Skin-Reducing Mastectomy and Pre-Pectoral Breast Reconstruction in Large Ptotic Breasts. Aesthetic Plast. Surg. 2020, 44, 664–672. [Google Scholar] [CrossRef]
- Vrekoussis, T.; Perabo, M.; Himsl, I.; Günthner-Biller, M.; Dian, D. Bilateral Prophylactic Skin-Reducing Nipple-Sparing Mastectomy with Immediate Breast Reconstruction Using Only a Vascularized Dermal–Subcutaneous Pedicle: Technique and Possible Advantages. Arch. Gynecol. Obstet. 2013, 287, 749–753. [Google Scholar] [CrossRef]
- Lewin, R.; Jepsen, C.; Hallberg, H.; Hansson, E. Immediate Breast Reconstruction with a Wise Pattern Mastectomy and NAC-Sparing McKissock Vertical Bipedicle Dermal Flap. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 1432–1439. [Google Scholar] [CrossRef]
- Santanelli, F.; Paolini, G.; Campanale, A.; Longo, B.; Amanti, C. The “Type V” Skin-Sparing Mastectomy for Upper QuadrantSkin Resections. Ann. Plast. Surg. 2010, 65, 135–139. [Google Scholar] [CrossRef]
- Marongiu, F.; Bertozzi, N.; Sibilio, A.; Curcio, A. The First Use of Autologous Dermal Patch in DTI Pre-Pectoral Breast Reconstruction After Skin Reducing Mastectomy: A New Useful and Cheap Reconstruction Option. Aesthetic Plast. Surg. 2022, 46, 590–592. [Google Scholar] [CrossRef]
- Carlson, G.W.; Bostwick, J.; Styblo, T.M.; Moore, B.; Bried, J.T.; Murray, D.R.; Wood, W.C. Skin-Sparing Mastectomy: Oncologic and Reconstructive Considerations. Ann. Surg. 1997, 225, 570–578. [Google Scholar] [CrossRef]
- Lauritzen, E.; Damsgaard, T.E. Use of Indocyanine Green Angiography Decreases the Risk of Complications in Autologous- and Implant-Based Breast Reconstruction: A Systematic Review and Meta-Analysis. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 1703–1717. [Google Scholar] [CrossRef]
- Rubino, C.; Rampazzo, S.; Rodio, M.; Spissu, N. Autologous Coverage for Direct-to-Implant Pre-Pectoral Reconstruction in Large and Ptotic Breasts: A New Technique. Plast. Surg. Unit Univ. Hosp. Trust Sassari Sassari Italy 2023. submitted. [Google Scholar]
- Newman, M.K. Reconstruction of the Ptotic Breast Using Wise Pattern Skin Deepithelialization. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1077. [Google Scholar] [CrossRef]
- Friedman, H.I.; Talebagha, S.; Gilstrap, J.; Mujadzic, M.; Chen, E. Wise Pattern Direct Implant Breast Reconstruction: A Review and Improved Outcomes Using Dermal Matrix. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2439. [Google Scholar] [CrossRef]
- Nava, M.B.; Cortinovis, U.; Ottolenghi, J.; Riggio, E.; Pennati, A.; Catanuto, G.; Greco, M.; Rovere, G.Q. della Skin-Reducing Mastectomy. Plast. Reconstr. Surg. 2006, 118, 603–610. [Google Scholar] [CrossRef]
- Ross, G.L. One Stage Breast Reconstruction Following Prophylactic Mastectomy for Ptotic Breasts: The Inferior Dermal Flap and Implant. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 1204–1208. [Google Scholar] [CrossRef]
- Pagliara, D.; Montella, R.A.; Garganese, G.; Bove, S.; Costantini, M.; Rinaldi, P.M.; Pino, V.; Grieco, F.; Rubino, C.; Salgarello, M. Improving Decision-Making in Prepectoral Direct-to-Implant Reconstruction After Nipple Sparing Mastectomy: The Key Role of Flap Thickness Ratio. Clin. Breast Cancer 2022, 23, e37–e44. [Google Scholar] [CrossRef]
- Salgarello, M.; Pagliara, D.; Barone Adesi, L.; Visconti, G.; Wild, J.B.; Matey, P. Direct to Implant Breast Reconstruction With Prepectoral Micropolyurethane Foam-Coated Implant: Analysis of Patient Satisfaction. Clin. Breast Cancer 2021, 21, e454–e461. [Google Scholar] [CrossRef]
- Frey, J.D.; Salibian, A.A.; Choi, M.; Karp, N.S. Mastectomy Flap Thickness and Complications in Nipple-Sparing Mastectomy: Objective Evaluation Using Magnetic Resonance Imaging. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1439. [Google Scholar] [CrossRef]
- Vidya, R.; Iqbal, F.M.; Becker, H.; Zhadan, O. Rippling Associated with Pre-Pectoral Implant Based Breast Reconstruction: A New Grading System. World J. Plast. Surg. 2019, 8, 311–315. [Google Scholar] [CrossRef]
- Cattelani, L.; Polotto, S.; Arcuri, M.F.; Pedrazzi, G.; Linguadoca, C.; Bonati, E. One-Step Prepectoral Breast Reconstruction With Dermal Matrix–Covered Implant Compared to Submuscular Implantation: Functional and Cost Evaluation. Clin. Breast Cancer 2018, 18, e703–e711. [Google Scholar] [CrossRef] [PubMed]
- Ribuffo, D.; Berna, G.; De Vita, R.; Di Benedetto, G.; Cigna, E.; Greco, M.; Valdatta, L.; Onesti, M.G.; Lo Torto, F.; Marcasciano, M.; et al. Dual-Plane Retro-Pectoral Versus Pre-Pectoral DTI Breast Reconstruction: An Italian Multicenter Experience. Aesthetic Plast. Surg. 2021, 45, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.S.; Winocour, S.; Jacobson, S.R. Red Breast Syndrome: A Review of Available Literature. Aesthetic Plast. Surg. 2015, 39, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Ganske, I.; Hoyler, M.; Fox, S.E.; Morris, D.J.; Lin, S.J.; Slavin, S.A. Delayed Hypersensitivity Reaction to Acellular Dermal Matrix in Breast Reconstruction: The Red Breast Syndrome? Ann. Plast. Surg. 2014, 73, S139–S143. [Google Scholar] [CrossRef] [PubMed]
- Suijker, J.; Blok, Y.L.; de Vries, R.; van den Tol, M.P.; Krekel, N.M.A. Pectoral Fascia Preservation in Oncological Mastectomy to Reduce Complications and Improve Reconstructions: A Systematic Review. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2700. [Google Scholar] [CrossRef]
- Liu, J.; Hou, J.; Li, Z.; Wang, B.; Sun, J. Efficacy of Acellular Dermal Matrix in Capsular Contracture of Implant-Based Breast Reconstruction: A Single-Arm Meta-Analysis. Aesthetic Plast. Surg. 2020, 44, 735–742. [Google Scholar] [CrossRef]
- Salgarello, M.; Gasperoni, C.; Gasperoni, P. A Personal Technique: Mammaplasty with J Scar. Ann. Plast. Surg. 2002, 48, 124–130. [Google Scholar]
- Laporta, R.; Longo, B.; Sorotos, M.; Pagnoni, M.; Santanelli Di Pompeo, F. One-Stage DIEP Flap Breast Reconstruction: Algorithm for Immediate Contralateral Symmetrization: Symmetrization Algorithm. Microsurgery 2016, 36, 7–19. [Google Scholar] [CrossRef]
- Huang, J.-J.; Wu, C.-W.; Leon Lam, W.; Lin, C.-Y.; Nguyen, D.H.; Cheng, M.-H. Simultaneous Contralateral Breast Reduction/Mastopexy With Unilateral Breast Reconstruction Using Free Abdominal Flaps. Ann. Plast. Surg. 2011, 67, 336–342. [Google Scholar] [CrossRef]
- Panayi, A.; Agha, R.; Sieber, B.; Orgill, D. Impact of Obesity on Outcomes in Breast Reconstruction: A Systematic Review and Meta-Analysis. J. Reconstr. Microsurg. 2018, 34, 363–375. [Google Scholar] [CrossRef]
- Kim, J.Y.S.; Mlodinow, A.S.; Khavanin, N.; Hume, K.M.; Simmons, C.J.; Weiss, M.J.; Murphy, R.X.; Gutowski, K.A. Individualized Risk of Surgical Complications: An Application of the Breast Reconstruction Risk Assessment Score. Plast. Reconstr. Surg. Glob. Open 2015, 3, e405. [Google Scholar] [CrossRef]
- Negenborn, V.L.; Dikmans, R.E.G.; Bouman, M.B.; Winters, H.A.H.; Twisk, J.W.R.; Ruhé, P.Q.; Mureau, M.A.M.; Smit, J.M.; Tuinder, S.; Hommes, J.; et al. Predictors of Complications after Direct-to-Implant Breast Reconstruction with an Acellular Dermal Matrix from a Multicentre Randomized Clinical Trial. Br. J. Surg. 2018, 105, 1305–1312. [Google Scholar] [CrossRef] [Green Version]
- Pusic, A.L.; Klassen, A.F.; Scott, A.M.; Klok, J.A.; Cordeiro, P.G.; Cano, S.J. Development of a New Patient-Reported Outcome Measure for Breast Surgery: The BREAST-Q. Plast. Reconstr. Surg. 2009, 124, 345–353. [Google Scholar] [CrossRef]
- Duraes, E.F.R.; Durand, P.; Morisada, M.; Scomacao, I.; Duraes, L.C.; de Sousa, J.B.; Abedi, N.; Djohan, R.S.; Bernard, S.; Moreira, A.; et al. A Novel Validated Breast Aesthetic Scale. Plast. Reconstr. Surg. 2022, 149, 1297–1308. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rampazzo, S.; Spissu, N.; Pinna, M.; Sini, G.A.M.; Trignano, E.; Nonnis, R.; Sanna, C.; Rodio, M.; Tettamanzi, M.; Rubino, C. One-Stage Immediate Alloplastic Breast Reconstruction in Large and Ptotic Breasts: An Institutional Algorithm. J. Clin. Med. 2023, 12, 1170. https://doi.org/10.3390/jcm12031170
Rampazzo S, Spissu N, Pinna M, Sini GAM, Trignano E, Nonnis R, Sanna C, Rodio M, Tettamanzi M, Rubino C. One-Stage Immediate Alloplastic Breast Reconstruction in Large and Ptotic Breasts: An Institutional Algorithm. Journal of Clinical Medicine. 2023; 12(3):1170. https://doi.org/10.3390/jcm12031170
Chicago/Turabian StyleRampazzo, Silvia, Noemi Spissu, Michela Pinna, Germana A. M. Sini, Emilio Trignano, Rita Nonnis, Claudia Sanna, Manuela Rodio, Matilde Tettamanzi, and Corrado Rubino. 2023. "One-Stage Immediate Alloplastic Breast Reconstruction in Large and Ptotic Breasts: An Institutional Algorithm" Journal of Clinical Medicine 12, no. 3: 1170. https://doi.org/10.3390/jcm12031170





