Gamma Knife Radiosurgery for Cushing’s Disease: Evaluation of Biological Effective Dose from a Single-Center Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Baseline and Follow-Up Data
2.3. Radiosurgical Technique
2.4. Biological Effective Dose Calculation
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics and GKRS Parameters
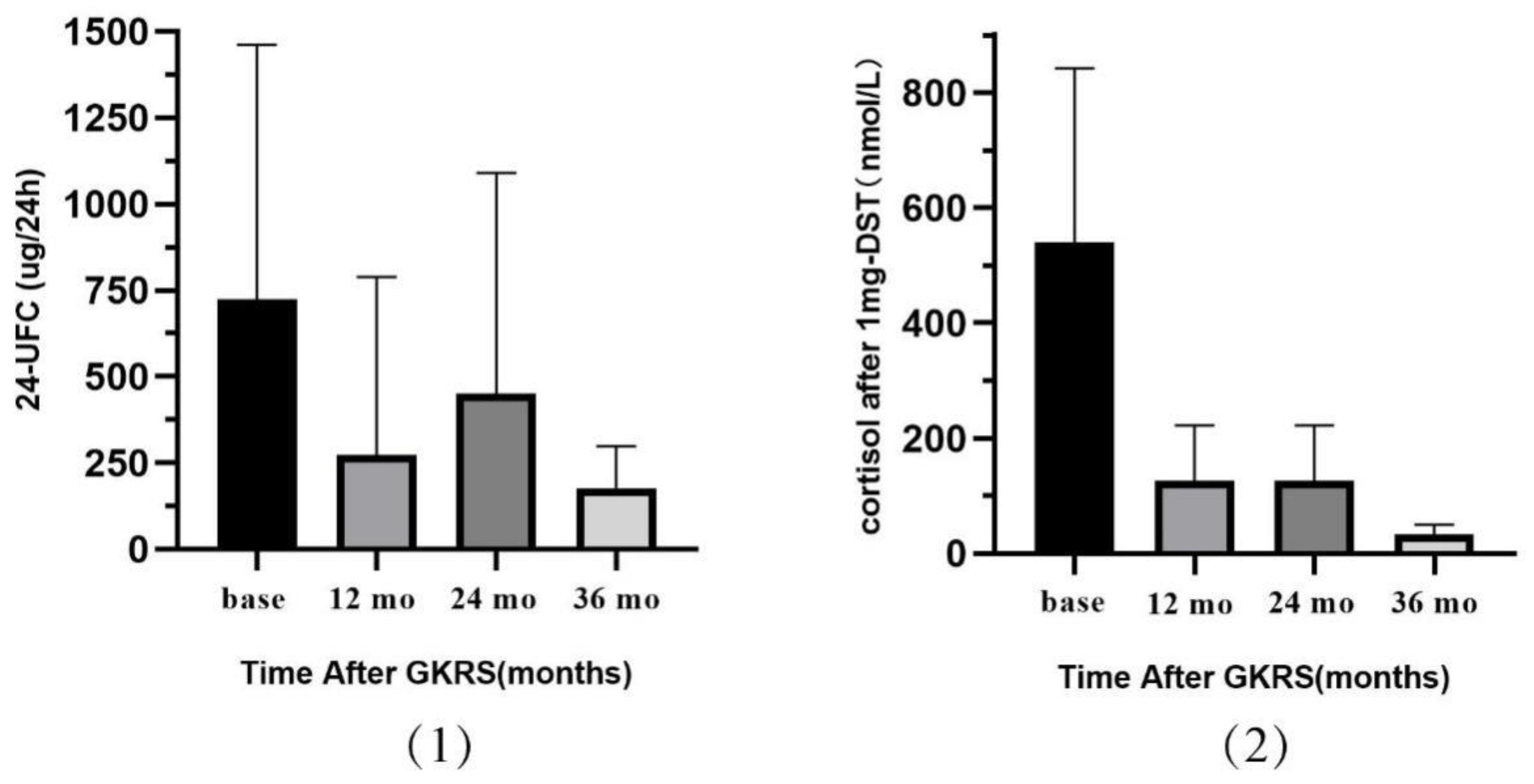
3.2. Endocrine and Imaging Outcomes
3.3. GKRS-Related Complications
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lonser, R.R.; Nieman, L.; Oldfield, E.H. Cushing’s disease: Pathobiology, diagnosis, and management. J. Neurosurg. 2017, 126, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Tritos, N.A.; Biller, B.M.K. Current management of Cushing’s disease. J. Intern. Med. 2019, 286, 526–541. [Google Scholar] [CrossRef]
- Broersen, L.H.A.; Biermasz, N.R.; van Furth, W.R.; de Vries, F.; Verstegen, M.J.T.; Dekkers, O.M.; Pereira, A.M. Endoscopic vs. microscopic transsphenoidal surgery for Cushing’s disease: A systematic review and meta-analysis. Pituitary 2018, 21, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Broersen, L.H.A.; Jha, M.; Biermasz, N.R.; Pereira, A.M.; Dekkers, O.M. Effectiveness of medical treatment for Cushing’s syndrome: A systematic review and meta-analysis. Pituitary 2018, 21, 631–641. [Google Scholar] [CrossRef]
- Bunevicius, A.; Sheehan, D.; Lee Vance, M.; Schlesinger, D.; Sheehan, J.P. Outcomes of Cushing’s disease following Gamma Knife radiosurgery: Effect of a center’s growing experience and era of treatment. J. Neurosurg. 2020, 134, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.R.; Oldfield, E.H.; Stratakis, C.A.; Nieman, L.K. The postoperative basal cortisol and CRH tests for prediction of long-term remission from Cushing’s disease after transsphenoidal surgery. J. Clin. Endocrinol. Metab. 2011, 96, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Graffeo, C.S.; Perry, A.; Link, M.J.; Brown, P.D.; Young, W.F.; Pollock, B.E. Biological effective dose as a predictor of hypopituitarism after single-fraction pituitary adenoma radiosurgery: Dosimetric analysis and cohort study of patients treated using contemporary techniques. Neurosurgery 2021, 88, E330–E335. [Google Scholar] [CrossRef]
- Millar, W.T.; Hopewell, J.W.; Paddick, I.; Lindquist, C.; Nordströn, H.; Lidberg, P.; Gårding, J. The role of the concept of biologically effective dose (BED) in treatment planning in radiosurgery. Phys. Med. 2015, 31, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Hopewell, J.W. Modelling the influence of treatment time on the biological effectiveness of single radiosurgery treatments: Derivation of “protective” dose modification factors. Br. J. Radiol. 2019, 92, 20180111. [Google Scholar] [CrossRef]
- Tritos, N.A. Cure of Cushing’s disease: Still an elusive goal? J. Clin. Endocrinol. Metab. 2021, 106, e367–e369. [Google Scholar] [CrossRef]
- Nieman, L.K.; Biller, B.M.; Findling, J.W.; Murad, M.H.; Newell-Price, J.; Savage, M.O.; Tabarin, A. Treatment of Cushing’s syndrome: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 2807–2831. [Google Scholar] [CrossRef]
- Fleseriu, M.; Auchus, R.; Bancos, I.; Ben-Shlomo, A.; Bertherat, J.; Biermasz, N.R.; Boguszewski, C.L.; Bronstein, M.D.; Buchfelder, M.; Carmichael, J.D.; et al. Consensus on diagnosis and management of Cushing’s disease: A guideline update. Lancet Diabetes Endocrinol. 2021, 9, 847–875. [Google Scholar] [CrossRef] [PubMed]
- Biller, B.M.; Grossman, A.B.; Stewart, P.M.; Melmed, S.; Bertagna, X.; Bertherat, J.; Buchfelder, M.; Colao, A.; Hermus, A.R.; Hofland, L.J.; et al. Treatment of adrenocorticotropin-dependent Cushing’s syndrome: A consensus statement. J. Clin. Endocrinol. Metab. 2008, 93, 2454–2462. [Google Scholar] [CrossRef]
- Carrasco, C.A.; Coste, J.; Guignat, L.; Groussin, L.; Dugué, M.A.; Gaillard, S.; Bertagna, X.; Bertherat, J. Midnight salivary cortisol determination for assessing the outcome of transsphenoidal surgery in Cushing’s disease. J. Clin. Endocrinol. Metab. 2008, 93, 4728–4734. [Google Scholar] [CrossRef] [PubMed]
- Balossier, A.; Tuleasca, C.; Cortet-Rudelli, C.; Soto-Ares, G.; Levivier, M.; Assaker, R.; Reyns, N. Gamma Knife surgery for recurrent or persistent Cushing disease: Long-term results and evaluation of biological effective dose in a series of 26 patients. Swiss Med. Wkly. 2021, 151, w20520. [Google Scholar] [CrossRef]
- Hughes, J.D.; Young, W.F.; Chang, A.Y.; Link, M.J.; Garces, Y.I.; Laack, N.N.; Thompson, G.B.; Pollock, B.E. Radiosurgical management of patients with persistent or recurrent Cushing disease after prior transsphenoidal surgery: A management algorithm based on a 25-year experience. Neurosurgery 2020, 86, 557–564. [Google Scholar] [CrossRef]
- Mehta, G.U.; Ding, D.; Patibandla, M.R.; Kano, H.; Sisterson, N.; Su, Y.H.; Krsek, M.; Nabeel, A.M.; El-Shehaby, A.; Kareem, K.A.; et al. Stereotactic radiosurgery for Cushing disease: Results of an international, multicenter study. J. Clin. Endocrinol. Metab. 2017, 102, 4284–4291. [Google Scholar] [CrossRef] [PubMed]
- Castinetti, F.; Nagai, M.; Dufour, H.; Kuhn, J.M.; Morange, I.; Jaquet, P.; Conte-Devolx, B.; Regis, J.; Brue, T. Gamma Knife radiosurgery is a successful adjunctive treatment in Cushing’s disease. Eur. J. Endocrinol. 2007, 156, 91–98. [Google Scholar] [CrossRef]
- Wan, H.; Chihiro, O.; Yuan, S. MASEP Gamma Knife radiosurgery for secretory pituitary adenomas: Experience in 347 consecutive cases. J. Exp. Clin. Cancer Res 2009, 28, 36. [Google Scholar] [CrossRef] [PubMed]
- Apaydin, T.; Ozkaya, H.M.; Durmaz, S.M.; Meral, R.; Kadioglu, P. Efficacy and safety of stereotactic radiotherapy in Cushing’s disease: A single center experience. Exp. Clin. Endocrinol. Diabetes 2021, 129, 482–491. [Google Scholar] [CrossRef]
- Tinnel, B.A.; Henderson, M.A.; Witt, T.C.; Fakiris, A.J.; Worth, R.M.; Des Rosiers, P.M.; Edmondson, J.W.; Timmerman, R.D.; Lo, S.S. Endocrine response after Gamma Knife-based stereotactic radiosurgery for secretory pituitary adenoma. Stereotact. Funct. Neurosurg. 2008, 86, 292–296. [Google Scholar] [CrossRef]
- Balossier, A.; Tuleasca, C.; Cortet-Rudelli, C.; Soto-Ares, G.; Levivier, M.; Assaker, R.; Reyns, N. Gamma Knife radiosurgery for acromegaly: Evaluating the role of the biological effective dose associated with endocrine remission in a series of 42 consecutive cases. Clin. Endocrinol. 2021, 94, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Inbar, O.; Ramesh, A.; Xu, Z.; Vance, M.L.; Schlesinger, D.; Sheehan, J.P. Gamma knife radiosurgery in patients with persistent acromegaly or Cushing’s disease: Long-term risk of hypopituitarism. Clin. Endocrinol. 2016, 84, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, C.L.; Attanasio, R.; Verrua, E.; Cozzi, R.; Ferrante, E.; Loli, P.; Montefusco, L.; Motti, E.; Ferrari, D.I.; Giugni, E.; et al. Efficacy and tolerability of Gamma Knife radiosurgery in acromegaly: A 10-year follow-up study. Clin. Endocrinol. 2009, 71, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Sims-Williams, H.P.; Rajapaksa, K.; Sinha, S.; Radatz, M.; Walton, L.; Yianni, J.; Newell-Price, J. Radiosurgery as primary management for acromegaly. Clin. Endocrinol. 2019, 90, 114–121. [Google Scholar] [CrossRef]
- Gupta, A.; Xu, Z.; Kano, H.; Sisterson, N.; Su, Y.H.; Krsek, M.; Nabeel, A.M.; El-Shehaby, A.; Karim, K.A.; Martínez-Moreno, N.; et al. Upfront Gamma Knife radiosurgery for Cushing’s disease and acromegaly: A multicenter, international study. J. Neurosurg. 2018, 131, 532–538. [Google Scholar] [CrossRef]
- Lee, C.C.; Chen, C.J.; Yen, C.P.; Xu, Z.; Schlesinger, D.; Fezeu, F.; Sheehan, J.P. Whole-sellar stereotactic radiosurgery for functioning pituitary adenomas. Neurosurgery 2014, 75, 227–237, discussion 237. [Google Scholar] [CrossRef] [PubMed]
- Marek, J.; Jezková, J.; Hána, V.; Krsek, M.; Bandúrová, L.; Pecen, L.; Vladyka, V.; Liščák, R. Is it possible to avoid hypopituitarism after irradiation of pituitary adenomas by the Leksell gamma knife? Eur. J. Endocrinol. 2011, 164, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Bunevicius, A.; Lavezzo, K.; Smith, P.W.; Vance, M.L.; Sheehan, J. Stereotactic radiosurgery before bilateral adrenalectomy is associated with lowered risk of Nelson’s syndrome in refractory Cushing’s disease patients. Acta Neurochir. 2021, 163, 1949–1956. [Google Scholar] [CrossRef]

| Variable | Value |
|---|---|
| Age at treatment (years), median (range) | 36 (20–71) |
| Sex (M:F) | 7:24 |
| Coexisting disease | |
| Cardiac dysfunction | 6/31 (19.4%) |
| Diabetes insipidus | 18/31 (58.1%) |
| Hypertension | 23/31 (74.2%) |
| Hyperlipidemia | 8/31 (25.8%) |
| Bone rarefaction | 5/31 (16.1%) |
| Radiosurgery indication | |
| Primary treatment | 21/31 (67.7%) |
| Residual | 7/31 (22.6%) |
| Recurrence | 3/31 (9.7%) |
| No visible tumor pre-GKRS | 2/31 (6.5%) |
| Cavernous sinus targeted | 6/31 (19.4%) |
| Suprasellar component targeted | 1/31 (3.2%) |
| Intrasellar | 18/31 (58.1%) |
| Pre-GKRS tumor volume (median (IQR), mm3) | 608.0 (462–978) |
| Pre-GKRS ACTH (median (IQR), ng/L) | 61.63 (47.47–108.87) |
| Pre-GKRS cortisol after 1 mg DST (median (IQR), nmol/L) | 478.0 (348.8–798.0) |
| Pre-GKRS 24 UFC (median (IQR), ug/24 h) | 388.2 (257.9–998.9) |
| Endocrine follow-up duration (median (IQR), months) | 22.0 (14–34) |
| Radiological follow-up duration (median (IQR), months) | 18.0 (9–37) |
| Margin dose (median (IQR), Gy) | 28.0 (26–31) |
| Maximum dose (median (IQR), Gy) | 56.0 (51.8–62) |
| Isodose line (median (IQR), %) | 50.0 (47–50) |
| Isocenters (median, IQR) | 4 (3–6) |
| Coverage percentage (median (IQR), %) | 0.967 (0.956–0.976) |
| Maximum optic apparatus point dose (median (IQR), Gy) | 6.8 (5.0–8.0) |
| Maximum dose received by the pituitary stalk (median (IQR), Gy) | 11.8 (8.8–17.8) |
| BED (median (IQR), Gy2.47) | 221.5 (192.2–259.6) |
| TDR, (median (IQR), Gy/m) | 1.8 (1.5–2.8) |
| Beam-on time (mean ± SD [range], hours) | 1.5 ± 0.5 (0.5–2.6) |
| Variable | Value |
|---|---|
| Endocrine remission rate | 14/31 (45.2%) |
| Duration to remission (median (IQR) months) | 20.0 (14.3–29.8) |
| Imaging regression rate | 17/31 (81.0%) |
| Imaging progression rate | 1/31 (4.8%) |
| Imaging stationary rate | 3/31 (14.2%) |
| Last ACTH (median (IQR), ng/L) | 47.0 (32.9–76.6) |
| Last cortisol after 1 mg DST (median (IQR), nmol/L) | 45.1 (33.4–173.1) |
| Last 24 UFC (median (IQR), ug/24 h) | 219.4 (145.0–595.3) |
| Total complications | 8/31 (25.8%) |
| New visual disturbance | 0/31 (0.0%) |
| Total new hypopituitary | 8/31 (25.8%) |
| Hypothyroid | 6/31 (19.4%) |
| Hypogonadism | 0/31 (0.0%) |
| Hypocortisolemia | 0/31 (0.0%) |
| Growth hormone deficiency | 2/31 (6.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Wang, M.; Wu, Y.; Deng, H.; Xu, Y.; Ren, Y.; Wang, C.; Wang, W. Gamma Knife Radiosurgery for Cushing’s Disease: Evaluation of Biological Effective Dose from a Single-Center Experience. J. Clin. Med. 2023, 12, 1288. https://doi.org/10.3390/jcm12041288
Gao Y, Wang M, Wu Y, Deng H, Xu Y, Ren Y, Wang C, Wang W. Gamma Knife Radiosurgery for Cushing’s Disease: Evaluation of Biological Effective Dose from a Single-Center Experience. Journal of Clinical Medicine. 2023; 12(4):1288. https://doi.org/10.3390/jcm12041288
Chicago/Turabian StyleGao, Yuan, Mengqi Wang, Yang Wu, Hao Deng, Yangyang Xu, Yan Ren, Chun Wang, and Wei Wang. 2023. "Gamma Knife Radiosurgery for Cushing’s Disease: Evaluation of Biological Effective Dose from a Single-Center Experience" Journal of Clinical Medicine 12, no. 4: 1288. https://doi.org/10.3390/jcm12041288




