Characteristics and Prognosis of 8p11.23-Amplified Squamous Lung Carcinomas
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atal, S.; Asokan, P.; Jhaj, R. Recent advances in targeted small-molecule inhibitor therapy for non-small-cell lung cancer-An update. J. Clin. Pharm. Ther. 2020, 45, 580–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012, 489, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Voutsadakis, I.A. Clinical Implications of Chromosomal Instability (CIN) and Kinetochore Abnormalities in Breast Cancers. Mol. Diagn. Ther. 2019, 23, 707–721. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 2016, 17, 183–193. [Google Scholar] [CrossRef]
- Morgensztern, D.; Devarakonda, S.; Govindan, R. Genomic Landscape of Squamous Cell Carcinoma of the Lung. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, 348–353. [Google Scholar] [CrossRef]
- Gelsi-Boyer, V.; Orsetti, B.; Cervera, N.; Finetti, P.; Sircoulomb, F.; Rougé, C.; Lasorsa, L.; Letessier, A.; Ginestier, C.; Monville, F.; et al. Comprehensive Profiling of 8p11-12 Amplification in Breast Cancer. Mol. Cancer Res. 2005, 3, 655–667. [Google Scholar] [CrossRef] [Green Version]
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 6, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.M.; Shih, J.; Ha, G.; Gao, G.F.; Zhang, X.; Berger, A.C.; Schumacher, S.E.; Wang, C.; Hu, H.; Liu, J.; et al. Genomic and Functional Approaches to Understanding Cancer Aneuploidy. Cancer Cell 2018, 33, 676–689.e673. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Lánczky, A.; Menyhárt, O.; Győrffy, B. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci. Rep. 2018, 8, 9227. [Google Scholar] [CrossRef] [Green Version]
- Slorach, E.M.; Chou, J.; Werb, Z. Zeppo1 is a novel metastasis promoter that represses E-cadherin expression and regulates p120-catenin isoform expression and localization. Genes Dev. 2011, 25, 471–484. [Google Scholar] [CrossRef] [Green Version]
- Baykara, O.; Dalay, N.; Kaynak, K.; Buyru, N. ZNF703 Overexpression may act as an oncogene in non-small cell lung cancer. Cancer Med. 2016, 5, 2873–2878. [Google Scholar] [CrossRef]
- White, R.J. RNA polymerases I and III, growth control and cancer. Nat. Rev. Mol. Cell Biol. 2005, 6, 69–78. [Google Scholar] [CrossRef]
- Liu, H.; Han, L.; Liu, Z.; Gao, N. Long noncoding RNA MNX1-AS1 contributes to lung cancer progression through the miR-527/BRF2 pathway. J. Cell. Physiol. 2019, 234, 13843–13850. [Google Scholar] [CrossRef]
- Wang, L.; Qu, J.; Zhou, L.; Liao, F.; Wang, J. MicroRNA-373 Inhibits Cell Proliferation and Invasion via Targeting BRF2 in Human Non-small Cell Lung Cancer A549 Cell Line. Cancer Res. Treat. 2018, 50, 936–949. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.Q.; Streicher, K.L.; Ray, M.E.; Abrams, J.; Ethier, S.P. Multiple Interacting Oncogenes on the 8p11-p12 Amplicon in Human Breast Cancer. Cancer Res 2006, 66, 11632–11643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rooney, C.; Geh, C.; Williams, V.; Heuckmann, J.M.; Menon, R.; Schneider, P.; Al-Kadhimi, K.; Dymond, M.; Smith, N.R.; Baker, D.; et al. Characterization of FGFR1 Locus in sqNSCLC Reveals a Broad and Heterogeneous Amplicon. PLoS ONE 2016, 11, e0149628. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Hollebecque, A.; Bahleda, R.; Loriot, Y.; Olaussen, K.A.; Massard, C.; Friboulet, L. Facts and New Hopes on Selective FGFR Inhibitors in Solid Tumors. Clin. Cancer Res. 2020, 26, 764–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moes-Sosnowska, J.; Chorostowska-Wynimko, J. Fibroblast Growth Factor Receptor 1-4 Genetic Aberrations as Clinically Relevant Biomarkers in Squamous Cell Lung Cancer. Front. Oncol. 2022, 12, 780650. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC). Thorac. Cancer 2020, 11, 511–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voutsadakis, I.A. HER2 in stemness and epithelial–mesenchymal plasticity of breast cancer. Clin. Transl. Oncol. 2018, 21, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Flores, N.M.; Hausmann, S.; Lofgren, S.M.; Kharchenko, V.; Angulo-Ibanez, M.; Sengupta, D.; Lu, X.; Czaban, I.; Azhibek, D.; et al. Elevated NSD3 histone methylation activity drives squamous cell lung cancer. Nature 2021, 590, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tian, W.; Yuan, G.; Deng, P.; Sengupta, D.; Cheng, Z.; Cao, Y.; Ren, J.; Qin, Y.; Zhou, Y.; et al. Molecular basis of nucleosomal H3K36 methylation by NSD methyltransferases. Nature 2021, 590, 498–503. [Google Scholar] [CrossRef]
- Pearson, A.; Smyth, E.; Babina, I.S.; Herrera-Abreu, M.T.; Tarazona, N.; Peckitt, C.; Turner, N.C. High-Level Clonal FGFR Amplification and Response to FGFR Inhibition in a Translational Clinical Trial. Cancer Discov. 2016, 6, 838–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
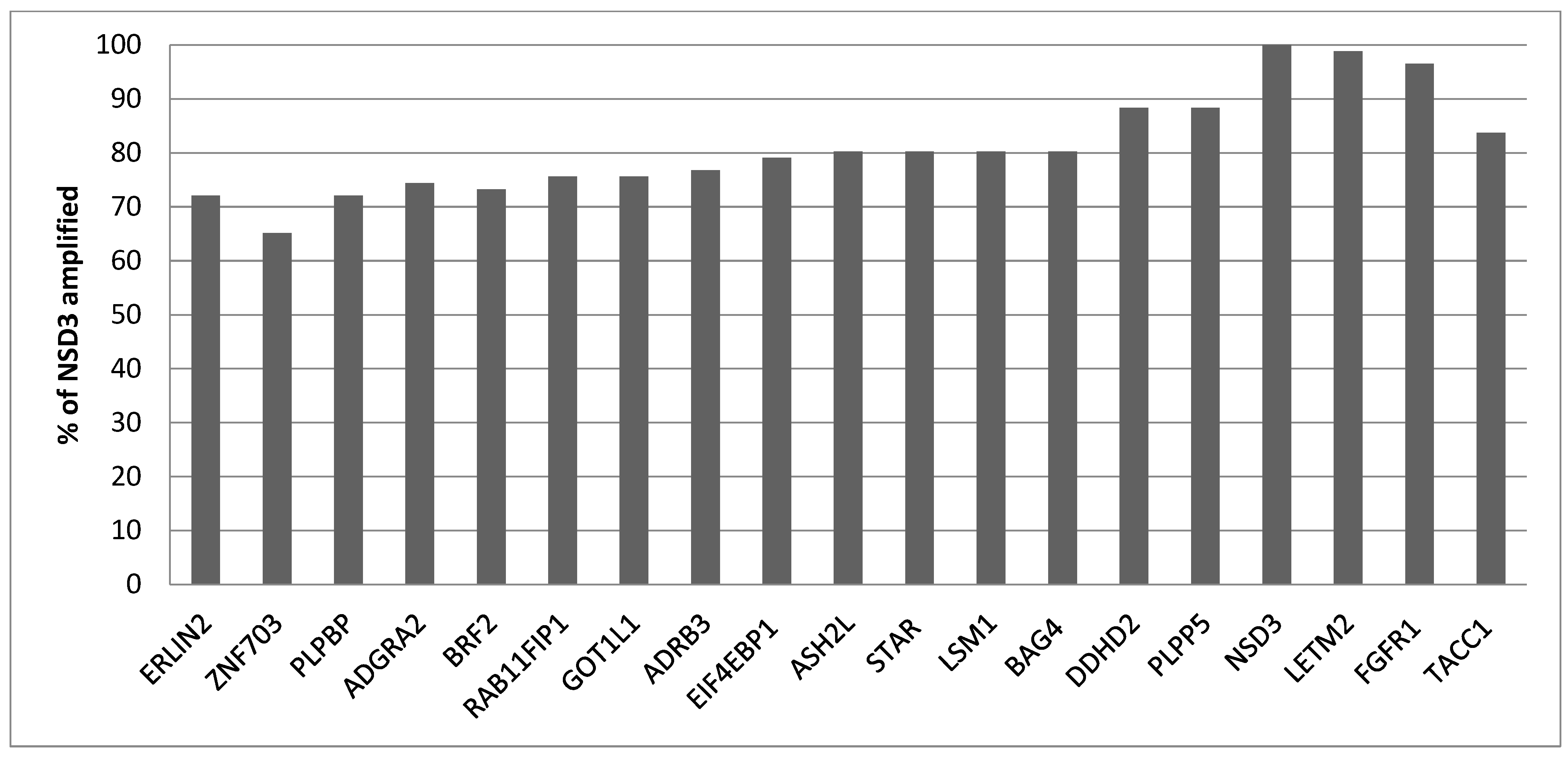

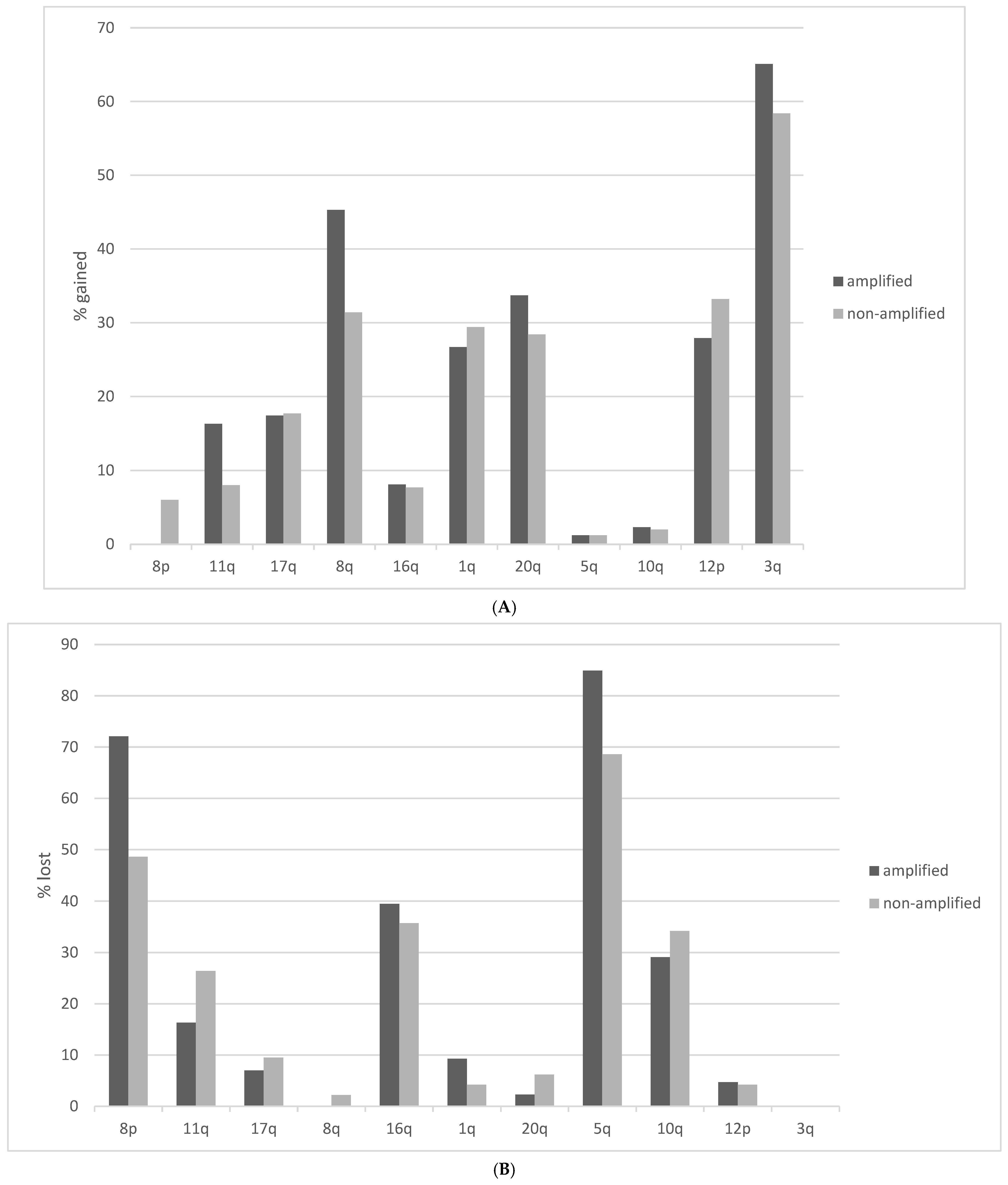
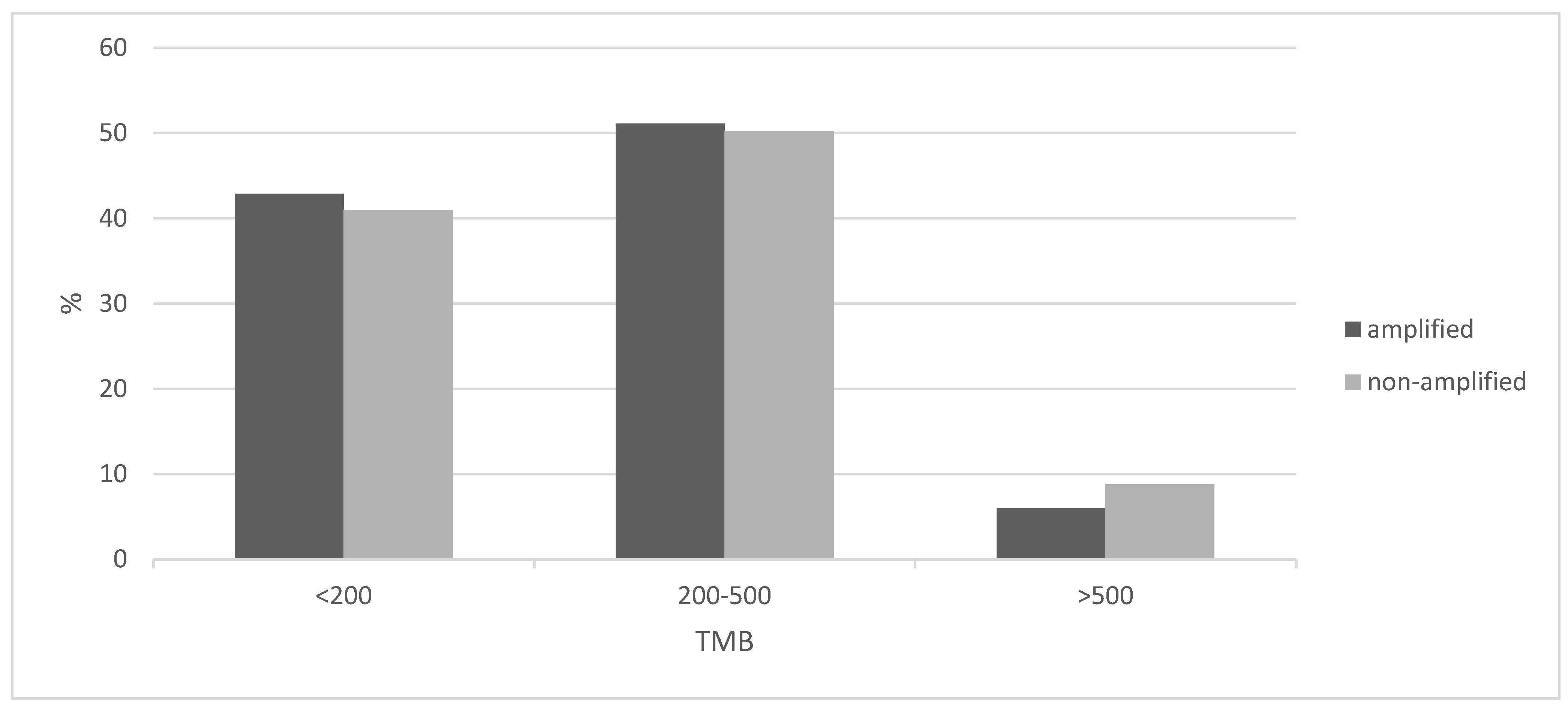
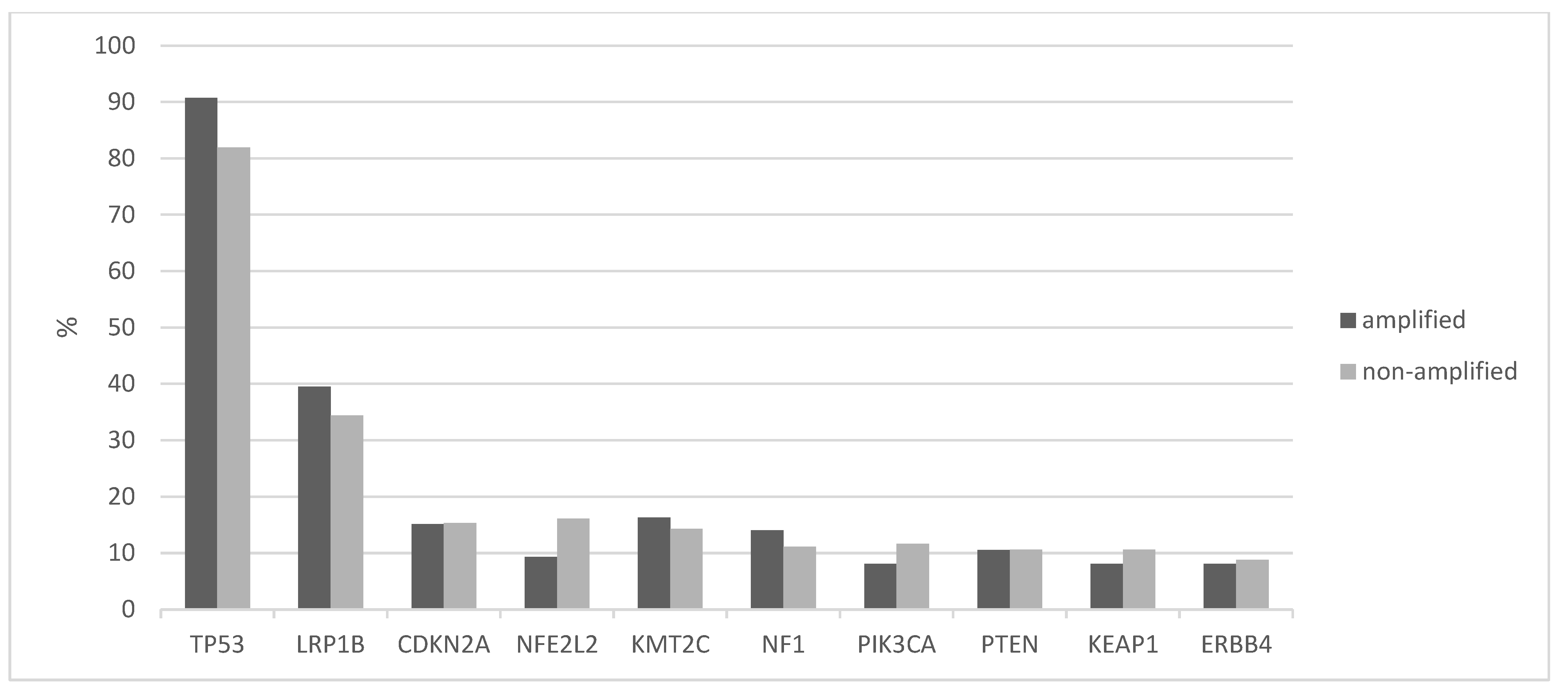


| Characteristic | Whole Group (n = 487) (%) | 8p11.23 Amplified (n = 86) (%) | 8p11.23 Nonamplified (n= 401) (%) | p |
|---|---|---|---|---|
| Age at diagnosis (mean ± SD) | 67.3 ± 8.5 | 67.3 ± 7.7 | 67.3 ± 8.7 | 1.0 |
| Age | ||||
| ≤65 years old | 182 (37.4) | 31 (36) | 151 (37.6) | 0.8 |
| >65 years old | 294 (60.4) | 53 (61.7) | 241 (60.1) | |
| NA | 11 (2.2) | 2 (2.3) | 9 (2.3) | |
| Sex | ||||
| Male | 358 (73.5) | 68 (79.1) | 290 (72.3) | 0.27 |
| Female | 127 (26.1) | 18 (20.9) | 109 (27.2) | |
| NA | 2 (0.4) | 2 (0.5) | ||
| Race | ||||
| White | 337 (69.2) | 56 (65.1) | 281 (70.1) | 0.84 |
| Black | 29 (6) | 4 (4.7) | 25 (6.2) | |
| Asian | 9 (1.8) | 1 (1.2) | 8 (2) | |
| NA | 112 (23) | 25 (29.1) | 87 (21.7) | |
| Stage at diagnosis | ||||
| I | 236 (48.5) | 37 (43) | 199 (49.7) | 0.53 |
| II | 158 (32.4) | 29 (33.7) | 129 (32.2) | |
| III | 83 (17) | 18 (21) | 65 (16.1) | |
| IV | 7 (1.4) | 2 (2.3) | 5 (1.2) | |
| NA | 3 (0.6) | 3 (0.7) |
| Official Name | Alternative Names | Position | Lung Squamous Carcinoma, n = 487 (%) |
|---|---|---|---|
| ERLIN2 (Endoplasmic Reticulum Lipid Raft Associated 2) | SPFH2 | 37,736,601–37,758,422 | 62 (12.7) |
| ZNF703 (Zinc Finger Protein 703) | FLJ14299, ZEPPO1 | 37,695,782–37,700,019 | 56 (11.5) |
| PLPBP (Pyridoxal phosphate binding protein) | PROSC | 37,762,595–37,779,768 | 62 (12.7) |
| ADGRA2 (Adhesion G protein coupled receptor A2) | GPR124 | 37,784,191–37,844,896 | 64 (13.1) |
| BRF2 (RNA polymerase III transcription initiation factor subunit) | TFIIIB50 | 37,843,268–37,849,861 | 63 (12.9) |
| RAB11FIP1 (RAB11 Family Interacting Protein 1) | 37,858,618–37,899,497 | 65 (13.3) | |
| GOT1L1 (Glutamic-oxaloacetic transaminase 1-like 1) | MGC33309 | 37,934,281–37,940,124 | 65 (13.3) |
| ADRB3 (Adrenoreceptor beta 3) | 37,962,990–37,966,599 | 66 (13.6) | |
| EIF4EBP1 (Eukaryotic transcription initiation factor 4E binding protein 1) | 4E-BP1 | 38,030,534–38,060,365 | 68 (14) |
| ASH2L (ASH2-like histone lysine methyltransferase complex subunit) | 38,105,493–38,144,076 | 69 (14.2) | |
| STAR (Steroidogenic acute regulatory protein) | STARD1 | 38,142,700–38,150,992 | 69 (14.2) |
| LSM1 (LSM1 homolog, mRNA-degradation-associated) | CASM | 38,163,335–38,176,730 | 69 (14.2) |
| BAG4 (BAG cochaperone 4) | SODD | 38,176,533–38,213,301 | 69 (14.2) |
| DDHD2 (DDHD domain containing 2) | 38,225,218–38,275,558 | 76 (15.6) | |
| PLPP5 (Phospholipid phosphatase 5) | PPAPDC1B, HTPAP | 38,263,130–38,269,243 | 76 (15.6) |
| NSD3 (Nuclear receptor binding SET domain protein 3) | WHSC1L1 | 38,269,704–38,382,272 | 86 (17.7) |
| LETM2 (Leucine zipper and EF-hand containing transmembrane protein 2) | SLC55A2 | 38,386,207–38,409,527 | 85 (17.5) |
| FGFR1 (Fibroblast Growth Factor Receptor 1) | CD331 | 38,400,215–38,468,834 | 83 (17) |
| TACC1 (Transforming Acidic Coiled Coil Containing protein 1) | 38,728,186–38,853,028 | 72 (14.8) |
| Official Name | Primary Antibody | IHC Staining (Number of Samples) | |
|---|---|---|---|
| None–Low | Medium–High | ||
| ERLIN2 | HPA002025 (r pAb) Sigma-Aldrich | 4 | 8 |
| CAB014894 (r mAb) Origene | 0 | 11 | |
| ZNF703 | HPA023930 (r pAb) Sigma-Aldrich | 10 | 1 |
| CAB068249 (m mAb) Sigma-Aldrich | 0 | 12 | |
| PLPBP | HPA023646 (r pAb) Sigma-Aldrich | 11 | 1 |
| HPA023733 (r pAb) Sigma-Aldrich | 11 | 1 | |
| CAB017033 (m mAb) Origene | 4 | 5 | |
| ADGRA2 | HPA012393 (r pAb) Sigma-Aldrich | 12 | 0 |
| BRF2 | HPA023378 (r pAb) Sigma-Aldrich | 0 | 11 |
| CAB019269 (r mAb) Origene | 2 | 9 | |
| RAB11FIP1 | HPA023904 (r pAb) Sigma-Aldrich | 3 | 8 |
| HPA024010 (r pAb) Sigma-Aldrich | 9 | 1 | |
| HPA025960 (r pAb) Sigma-Aldrich | 2 | 9 | |
| CAB017037 (r mAb) Origene | 5 | 5 | |
| GOT1L1 | HPA028778 (r pAb) Sigma-Aldrich | 7 | 4 |
| ADRB3 | HPA061969 (r pAb) Sigma-Aldrich | 12 | 0 |
| EIF4EBP1 | HPA023501 (r pAb) Sigma-Aldrich | 4 | 7 |
| CAB005032 (r mAb) Epitomics | 3 | 8 | |
| CAB005039 (r mAb) Epitomics | 8 | 2 | |
| ASH2L | HPA042289 (r pAb) Sigma-Aldrich | 3 | 7 |
| STAR | HPA023644 (r pAb) Sigma-Aldrich | 11 | 0 |
| HPA027318 (r pAb) Sigma-Aldrich | 11 | 0 | |
| CAB032598 (r pAb) Santa Cruz Biotechnology | 11 | 0 | |
| LSM1 | NA | ||
| BAG4 | HPA018951 (r pAb) Sigma-Aldrich | 3 | 7 |
| CAB013716 (r mAb) Origene | 1 | 11 | |
| DDHD2 | HPA023143 (r pAb) Sigma-Aldrich | 9 | 1 |
| HPA023147 (r pAb) Sigma-Aldrich | 8 | 3 | |
| CAB015202 (r mAb) Origene | 4 | 8 | |
| PLPP5 | NA | ||
| NSD3 | HPA005659 (r pAb) Sigma-Aldrich | 5 | 7 |
| HPA018893 (r pAb) Sigma-Aldrich | 0 | 10 | |
| CAB013721 (r mAb) Origene | 0 | 10 | |
| LETM2 | HPA025032 (r pAb) Sigma-Aldrich | 11 | 1 |
| FGFR1 | HPA056402 (r pAb) Sigma-Aldrich | 12 | 0 |
| CAB033614 (m mAb) Santa Cruz Biotechnology | 5 | 5 | |
| TACC1 | HPA024702 (r pAb) Sigma-Aldrich | 11 | 1 |
| CAB017041 (r mAb) Origene | 2 | 7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voutsadakis, I.A. Characteristics and Prognosis of 8p11.23-Amplified Squamous Lung Carcinomas. J. Clin. Med. 2023, 12, 1711. https://doi.org/10.3390/jcm12051711
Voutsadakis IA. Characteristics and Prognosis of 8p11.23-Amplified Squamous Lung Carcinomas. Journal of Clinical Medicine. 2023; 12(5):1711. https://doi.org/10.3390/jcm12051711
Chicago/Turabian StyleVoutsadakis, Ioannis A. 2023. "Characteristics and Prognosis of 8p11.23-Amplified Squamous Lung Carcinomas" Journal of Clinical Medicine 12, no. 5: 1711. https://doi.org/10.3390/jcm12051711





