Bone Mineral Density and All-Cause Mortality in Patients with Nondialysis Chronic Kidney Disease: Results from KNOW-CKD Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection from Participants
2.3. Measurement of BMD
2.4. Exposure and Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Association of Low BMD with All-Cause Mortality in Patients with CKD
3.3. Sensitivity Analyses
3.4. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorente Ramos, R.M.; Azpeitia Armán, J.; Arévalo Galeano, N.; Muñoz Hernández, A.; García Gómez, J.M.; Gredilla Molinero, J. Dual energy X-ray absorptimetry: Fundamentals, methodology and clinical applications. Radiologia 2012, 54, 410–423. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. 2009, 76, S1–S130. [Google Scholar] [CrossRef]
- Moe, S.; Drüeke, T.; Cunningham, J.; Goodman, W.; Martin, K.; Olgaard, K.; Ott, S.; Sprague, S.; Lameire, N.; Eknoyan, G. Definition, Evaluation and Classification of Renal Osteodystrophy: A Position Statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006, 69, 1945–1953. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.E.; Choi, H.Y.; Kim, S.; Ryu, D.R.; Oh, H.J. Fracture risk in chronic kidney disease: A Korean population-based cohort study. Kidney Res. Clin. Pract. 2019, 38, 220–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fidan, N.; Inci, A.; Coban, M.; Ulman, C.; Kursat, S. Bone mineral density and biochemical markers of bone metabolism in predialysis patients with chronic kidney disease. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2016, 64, 861–866. [Google Scholar] [CrossRef]
- Kim, C.S.; Bae, E.H.; Ma, S.K.; Han, S.H.; Lee, K.-B.; Lee, J.; Oh, K.-H.; Chae, D.W.; Kim, S.W. Chronic Kidney Disease-Mineral Bone Disorder in Korean Patients: A Report from the KoreaN Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD). J. Korean Med. Sci. 2017, 32, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Yenchek, R.H.; Ix, J.H.; Shlipak, M.G.; Bauer, D.C.; Rianon, N.J.; Kritchevsky, S.B.; Harris, T.B.; Newman, A.B.; Cauley, J.A.; Fried, L.F. Bone mineral density and fracture risk in older individuals with CKD. Clin. J. Am. Soc. Nephrol. 2012, 7, 1130–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iimori, S.; Mori, Y.; Akita, W.; Kuyama, T.; Takada, S.; Asai, T.; Kuwahara, M.; Sasaki, S.; Tsukamoto, Y. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients—A single-center cohort study. Nephrol. Dial. Transplant. 2012, 27, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, D.C.; Winkelmayer, W.C. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef] [Green Version]
- Browner, W.S.; Seeley, D.G.; Vogt, T.M.; Cummings, S.R. Non-trauma mortality in elderly women with low bone mineral density. Lancet 1991, 338, 355–358. [Google Scholar] [CrossRef]
- Sennerby, U.; Melhus, H.; Gedeborg, R.; Byberg, L.; Garmo, H.; Ahlbom, A.; Pedersen, N.L.; Michaëlsson, K. Cardiovascular diseases and risk of hip fracture. JAMA 2009, 302, 1666–1673. [Google Scholar] [CrossRef]
- Shen, C.; Deng, J.; Zhou, R.; Chen, J.; Fan, S.; Li, Z.; Hu, Y.; Zhong, Q. Relation between bone mineral density, bone loss and the risk of cardiovascular disease in a Chinese cohort. Am. J. Cardiol. 2012, 110, 1138–1142. [Google Scholar] [CrossRef]
- Wiklund, P.; Nordström, A.; Jansson, J.H.; Weinehall, L.; Nordström, P. Low bone mineral density is associated with increased risk for myocardial infarction in men and women. Osteoporos. Int. 2012, 23, 963–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.H.; An, J.H.; Lim, S.; Koo, B.K.; Park, S.E.; Chang, H.J.; Choi, S.I.; Park, Y.J.; Park, K.S.; Jang, H.C.; et al. Lower bone mineral density is associated with higher coronary calcification and coronary plaque burdens by multidetector row coronary computed tomography in pre- and postmenopausal women. Clin. Endocrinol. 2009, 71, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.; Lee, K.B.; Kim, Y.H.; Hong, N.; Park, J.T.; Han, S.H.; Kang, S.W.; Choi, K.H.; Oh, K.H.; et al. Low bone mineral density is associated with coronary arterial calcification progression and incident cardiovascular events in patients with chronic kidney disease. Clin. Kidney J. 2022, 15, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Hyun, Y.Y.; Lee, K.B.; Han, S.H.; Choi, K.H.; Park, H.C.; Oh, Y.K.; Park, S.K.; Oh, K.H.; Ahn, C. Risk factors and renal outcomes of low bone mineral density in patients with non-dialysis chronic kidney disease. Osteoporos. Int. 2020, 31, 2373–2382. [Google Scholar] [CrossRef]
- Jiang, C.; Yan, C.; Duan, J. Bone Mineral Density Is Inversely Associated with Mortality in Chronic Kidney Disease Patients: A Meta-Analysis. J. Bone Miner. Res. 2022, 37, 2094–2102. [Google Scholar] [CrossRef]
- Mizuiri, S.; Nishizawa, Y.; Doi, T.; Yamashita, K.; Shigemoto, K.; Usui, K.; Arita, M.; Naito, T.; Doi, S.; Masaki, T. Association and predictive value of geriatric nutritional risk index, body composition, or bone mineral density in haemodialysis patients. Nephrology 2021, 26, 341–349. [Google Scholar] [CrossRef]
- Chen, Z.; Qureshi, A.R.; Ripsweden, J.; Wennberg, L.; Heimburger, O.; Lindholm, B.; Barany, P.; Haarhaus, M.; Brismar, T.B.; Stenvinkel, P. Vertebral bone density associates with coronary artery calcification and is an independent predictor of poor outcome in end-stage renal disease patients. Bone 2016, 92, 50–57. [Google Scholar] [CrossRef]
- Jaques, D.A.; Henderson, S.; Davenport, A. Association between bone mineral density at different anatomical sites and both mortality and fracture risk in patients receiving renal replacement therapy: A longitudinal study. Clin. Kidney J. 2022, 15, 1188–1195. [Google Scholar] [CrossRef]
- Oh, K.H.; Park, S.K.; Park, H.C.; Chin, H.J.; Chae, D.W.; Choi, K.H.; Han, S.H.; Yoo, T.H.; Lee, K.; Kim, Y.S.; et al. KNOW-CKD (KoreaN cohort study for Outcome in patients with Chronic Kidney Disease): Design and methods. BMC Nephrol. 2014, 15, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Eckardt, K.U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; De Zeeuw, D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [CrossRef] [PubMed]
- Alem, A.M.; Sherrard, D.J.; Gillen, D.L.; Weiss, N.S.; Beresford, S.A.; Heckbert, S.R.; Wong, C.; Stehman-Breen, C. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000, 58, 396–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tentori, F.; McCullough, K.; Kilpatrick, R.D.; Bradbury, B.D.; Robinson, B.M.; Kerr, P.G.; Pisoni, R.L. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int. 2014, 85, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Von der Recke, P.; Hansen, M.A.; Hassager, C. The association between low bone mass at the menopause and cardiovascular mortality. Am. J. Med. 1999, 106, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Hyder, J.A.; Allison, M.A.; Wong, N.; Papa, A.; Lang, T.F.; Sirlin, C.; Gapstur, S.M.; Ouyang, P.; Carr, J.J.; Criqui, M.H. Association of coronary artery and aortic calcium with lumbar bone density: The MESA Abdominal Aortic Calcium Study. Am. J. Epidemiol. 2009, 169, 186–194. [Google Scholar] [CrossRef] [Green Version]
- London, G.M.; Marty, C.; Marchais, S.J.; Guerin, A.P.; Metivier, F.; de Vernejoul, M.C. Arterial calcifications and bone histomorphometry in end-stage renal disease. J. Am. Soc. Nephrol. 2004, 15, 1943–1951. [Google Scholar] [CrossRef]
- Persy, V.; D’Haese, P. Vascular calcification and bone disease: The calcification paradox. Trends Mol. Med. 2009, 15, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000, 87, E10–E17. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.L.; Khosla, S. Physiology of bone loss. Radiol. Clin. N. Am. 2010, 48, 483–495. [Google Scholar] [CrossRef]
- Osterhoff, G.; Morgan, E.F.; Shefelbine, S.J.; Karim, L.; McNamara, L.M.; Augat, P. Bone mechanical properties and changes with osteoporosis. Injury 2016, 47, S11–S20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickolas, T.L.; Stein, E.M.; Dworakowski, E.; Nishiyama, K.K.; Komandah-Kosseh, M.; Zhang, C.A.; McMahon, D.J.; Liu, X.S.; Boutroy, S.; Cremers, S.; et al. Rapid cortical bone loss in patients with chronic kidney disease. J. Bone Miner. Res. 2013, 28, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.C.; Costa, A.G.; Cusano, N.E.; Kousteni, S.; Bilezikian, J.P. Catabolic and anabolic actions of parathyroid hormone on the skeleton. J. Endocrinol. Investig. 2011, 34, 801–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iseri, K.; Dai, L.; Chen, Z.; Qureshi, A.R.; Brismar, T.B.; Stenvinkel, P.; Lindholm, B. Bone mineral density and mortality in end-stage renal disease patients. Clin. Kidney J. 2020, 13, 307–321. [Google Scholar] [CrossRef] [PubMed]

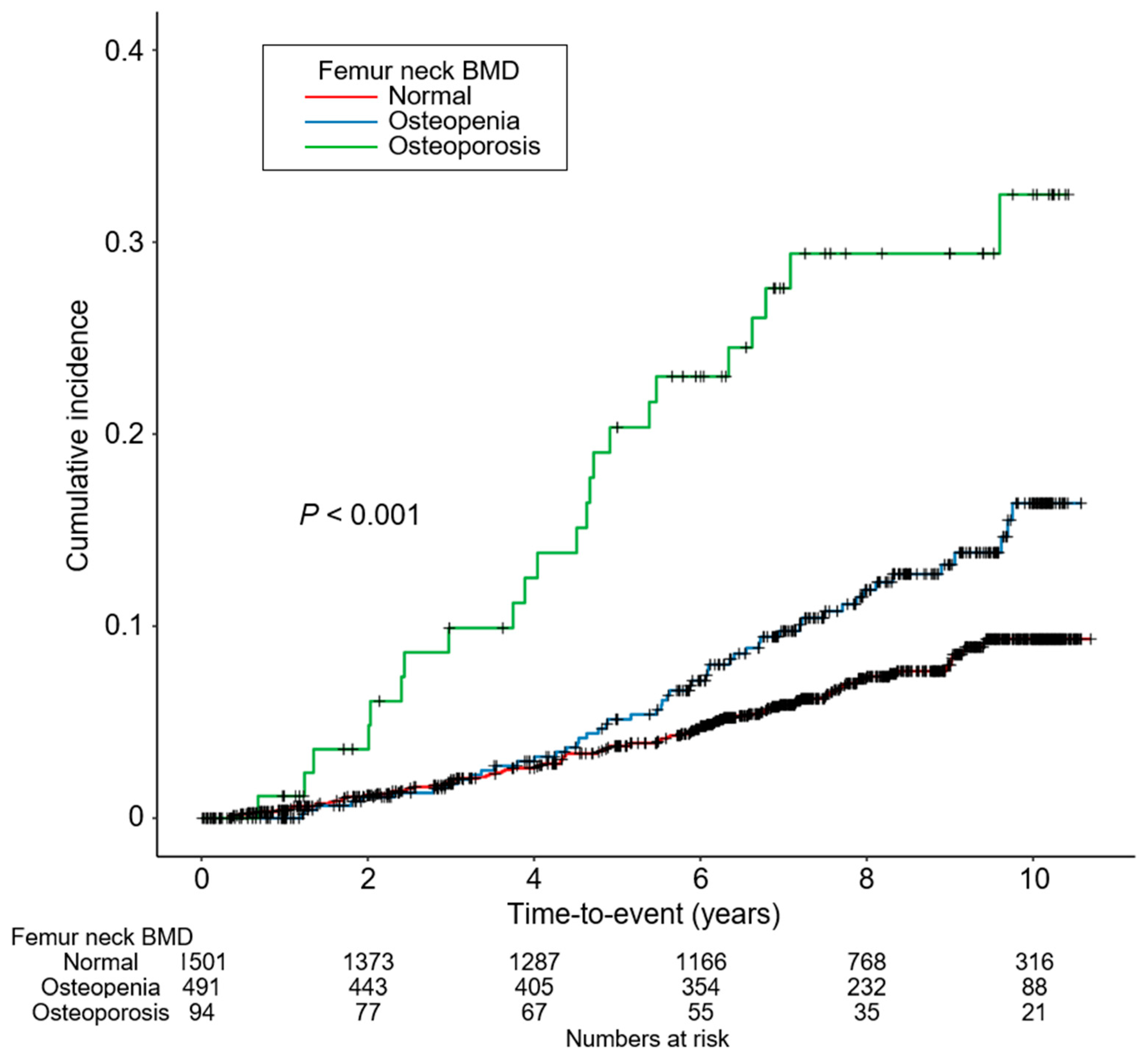

| Femur Neck BMD | ||||
|---|---|---|---|---|
| Normal | Osteopenia | Osteoporosis | p-Value | |
| Follow-up duration (year) | 7.281 ± 2.805 | 7.011 ± 2.853 | 6.173 ± 3.421 | 0.003 |
| Age (year) | 54.312 ± 12.399 | 50.890 ± 12.004 | 56.989 ± 9.978 | <0.001 |
| Male | 931 (62.0) | 297 (60.5) | 55 (58.5) | 0.690 |
| CCI | <0.001 | |||
| 0–3 | 1126 (75.0) | 315 (64.2) | 52 (55.3) | |
| 4–5 | 357 (23.8) | 166 (33.8) | 38 (40.4) | |
| 6–7 | 17 (1.1) | 10 (2.0) | 4 (4.3) | |
| ≥8 | 1 (0.1) | 0 (0.0) | 0 (0.0) | |
| Primary renal disease | <0.001 | |||
| DM | 341 (22.7) | 145 (29.5) | 36 (38.3) | |
| HTN | 279 (18.6) | 114 (23.2) | 17 (18.1) | |
| GN | 483 (32.2) | 157 (32.0) | 24 (25.5) | |
| TID | 7 (0.5) | 5 (1.0) | 1 (1.1) | |
| PKD | 297 (19.8) | 38 (7.7) | 4 (4.3) | |
| Others | 93 (6.2) | 32 (6.5) | 12 (12.8) | |
| Smoking status | <0.001 | |||
| Nonsmoker | 746 (49.7) | 287 (58.5) | 75 (79.8) | |
| Exsmoker | 274 (18.3) | 58 (11.8) | 5 (5.3) | |
| Current smoker | 480 (32.0) | 146 (29.7) | 14 (14.9) | |
| Medication | ||||
| ACEIs/ARBs | 1285 (85.6) | 422 (85.9) | 78 (83.0) | 0.752 |
| Diuretics | 430 (28.6) | 184 (37.5) | 41 (43.6) | <0.001 |
| Anti-HTN drugs ≥3 | 409 (27.2) | 168 (34.2) | 31 (33.0) | 0.009 |
| Statins | 727 (48.4) | 297 (60.5) | 61 (64.9) | <0.001 |
| BMI (kg/m2) | 24.9 ± 3.4 | 23.9 ± 3.3 | 23.4 ± 3.4 | <0.001 |
| Waist circumference (cm) | 88.1 ± 9.8 | 85.9 ± 9.5 | 84.1 ± 10.2 | <0.001 |
| SBP (mmHg) | 128.1 ± 16.1 | 127.2 ± 15.5 | 127.1 ± 19.6 | 0.526 |
| DBP (mmHg) | 77.7 ± 11.1 | 75.6 ± 10.8 | 73.5 ± 11.6 | <0.001 |
| Laboratory findings | ||||
| Hemoglobin (g/dL) | 13.19 ± 2.00 | 12.15 ± 1.88 | 11.482 ± 1.56 | <0.001 |
| Albumin (g/dL) | 4.20 ± 0.42 | 4.14 ± 0.42 | 4.07 ± 0.46 | 0.001 |
| Total cholesterol (mg/dL) | 175 ± 38 | 171 ± 40 | 169 ± 42 | 0.165 |
| HDL-C (mg/dL) | 49 ± 15 | 50 ± 16 | 48 ± 16 | 0.614 |
| LDL-C (mg/dL) | 98 ± 32 | 93 ± 31 | 91 ± 33 | 0.004 |
| TG (mg/dL) | 161 ± 104 | 151 ± 89 | 145 ± 75 | 0.040 |
| Fasting glucose (mg/dL) | 112 ± 41 | 107 ± 28 | 116 ± 52 | 0.026 |
| 25(OH)D (ng/mL) | 17.70 ± 7.18 | 18.36 ± 9.66 | 16.50 ± 9.06 | 0.156 |
| Total calcium (mg/dL) | 9.15 ± 0.52 | 9.07 ± 0.57 | 9.00 ± 0.55 | 0.001 |
| Phosphorus (mg/dL) | 3.62 ± 0.67 | 3.85 ± 0.69 | 3.91 ± 0.68 | <0.001 |
| iPTH (pg/mL) | 46 (29, 75) | 60 (40, 106) | 83 (51, 136) | <0.001 |
| hs-CRP (mg/dL) | 0.60 (0.20, 1.70) | 0.67 (0.23, 1.60) | 0.60 (0.30, 1.65) | 0.761 |
| Spot urine ACR (mg/g) | 301.78 (58.89, 2213.74) | 478.45 (127.23, 1183.61) | 501.49 (172.33, 4876.12) | 0.001 |
| Creatinine (mg/dL) | 1.69 ± 1.08 | 2.09 ± 1.27 | 2.34 ± 1.25 | <0.001 |
| eGFR (mL/min./1.73 m2) | 55.24 ± 30.91 | 40.21 ± 25.06 | 32.60 ± 24.13 | <0.001 |
| CKD stages | <0.001 | |||
| Stage 1 | 301 (20.1) | 38 (7.7) | 3 (3.2) | |
| Stage 2 | 325 (21.7) | 65 (13.2) | 6 (6.4) | |
| Stage 3a | 263 (17.5) | 67 (13.6) | 12 (12.8) | |
| Stage 3b | 296 (19.7) | 127 (25.9) | 16 (17.0) | |
| Stage 4 | 259 (17.3) | 143 (29.1) | 40 (42.6) | |
| Stage 5 | 57 (3.8) | 51 (10.4) | 17 (18.1) |
| BMD | Events, n (%) | Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | |||
| Femur neck BMD | Normal | 100 (6.7) | Reference | Reference | Reference | Reference | ||||
| Osteopenia | 52 (10.6) | 1.798 | 0.001 | 1.104 | 0.589 | 1.090 | 0.657 | 1.115 | 0.534 | |
| (1.256, 2.575) | (0.771, 1.582) | (0.745, 1.596) | (0.748, 1.661) | |||||||
| Osteoporosis | 23 (24.5) | 4.191 | <0.001 | 3.273 | <0.001 | 2.922 | <0.001 | 2.963 | <0.001 | |
| (2.520, 6.971) | (1.998, 5.361) | (1.707, 5.001) | (1.655, 5.307) | |||||||
| Femur Neck BMD | Events, n (%) | Unadjusted HR (95%CI) | p for Interaction | Adjusted HR (95%CI) | p for Interaction | |
|---|---|---|---|---|---|---|
| Age <60 years | Normal | 62 (6.6) | Reference | 0.888 | Reference | 0.321 |
| Osteopenia | 40 (11.0) | 1.747 (1.174, 2.600) | 1.397 (0.872, 2.236) | |||
| Osteoporosis | 12 (25.0) | 4.577 (2.466, 8.495) | 1.933 (0.808, 4.625) | |||
| Age ≥60 years | Normal | 38 (6.7) | Reference | Reference | ||
| Osteopenia | 12 (9.5) | 1.466 (0.766, 2.807) | 1.706 (0.727, 4.006) | |||
| Osteoporosis | 11 (23.9) | 4.309 (2.202, 8.433) | 9.676 (3.723, 25.151) | |||
| Male | Normal | 56 (6.0) | Reference | 0.845 | Reference | 0.798 |
| Osteopenia | 26 (8.8) | 1.517 (0.953, 2.415) | 1.399 (0.789, 2.479) | |||
| Osteoporosis | 11 (20.0) | 4.295 (2.250, 8.200) | 3.744 (1.635, 8.573) | |||
| Female | Normal | 44 (7.7) | Reference | Reference | ||
| Osteopenia | 26 (13.4) | 1.845 (1.136, 2.996) | 1.408 (0.785, 2.526) | |||
| Osteoporosis | 12 (30.8) | 4.407 (2.327, 8.346) | 3.433 (1.435, 8.215) | |||
| BMI < 23 kg/m2 | Normal | 27 (6.1) | Reference | 0.770 | Reference | 0.226 |
| Osteopenia | 19 (9.6) | 1.737 (0.965, 3.124) | 1.556 (0.736, 3.287) | |||
| Osteoporosis | 9 (20.5) | 3.928 (1.846, 8.355) | 2.253 (0.691, 7.350) | |||
| BMI ≥ 23 kg/m2 | Normal | 73 (7.3) | Reference | Reference | ||
| Osteopenia | 32 (11.1) | 1.604 (1.059, 2.431) | 1.364 (0.824, 2.257) | |||
| Osteoporosis | 14 (28.0) | 5.127 (2.893, 9.087) | 4.326 (2.145, 8.721) | |||
| eGFR ≥ 45 mL/min./1.73 m2 | Normal | 31 (3.7) | Reference | 0.434 | Reference | 0.156 |
| Osteopenia | 6 (3.8) | 1.079 (0.450, 2.586) | 0.656 (0.209, 2.061) | |||
| Osteoporosis | 3 (18.8) | 6.306 (1.927, 20.636) | 11.773 (2.768, 50.073) | |||
| eGFR < 45 mL/min./1.73 m2 | Normal | 69 (10.5) | Reference | Reference | ||
| Osteopenia | 46 (13.7) | 1.348 (0.928, 1.958) | 1.780 (1.142, 2.774) | |||
| Osteoporosis | 20 (25.6) | 2.838 (1.725, 4.670) | 3.775 (1.957, 7.283) | |||
| Spot urine ACR < 300 mg/g | Normal | 33 (4.6) | Reference | 0.072 | Reference | 0.248 |
| Osteopenia | 17 (8.9) | 2.129 (1.186, 3.822) | 1.276 (0.628, 2.593) | |||
| Osteoporosis | 10 (29.4) | 8.526 (4.198, 17.315) | 4.538 (1.853, 11.113) | |||
| Spot urine ACR ≥ 300 mg/g | Normal | 63 (8.5) | Reference | Reference | ||
| Osteopenia | 34 (11.9) | 1.423 (0.938, 2.160) | 1.603 (0.966, 2.658) | |||
| Osteoporosis | 12 (21.4) | 2.831 (1.527, 5.249) | 2.135 (0.851, 5.356) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, S.H.; Oh, T.R.; Choi, H.S.; Yang, E.M.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Oh, K.-H.; Hyun, Y.Y.; Sung, S.; et al. Bone Mineral Density and All-Cause Mortality in Patients with Nondialysis Chronic Kidney Disease: Results from KNOW-CKD Study. J. Clin. Med. 2023, 12, 1850. https://doi.org/10.3390/jcm12051850
Suh SH, Oh TR, Choi HS, Yang EM, Kim CS, Bae EH, Ma SK, Oh K-H, Hyun YY, Sung S, et al. Bone Mineral Density and All-Cause Mortality in Patients with Nondialysis Chronic Kidney Disease: Results from KNOW-CKD Study. Journal of Clinical Medicine. 2023; 12(5):1850. https://doi.org/10.3390/jcm12051850
Chicago/Turabian StyleSuh, Sang Heon, Tae Ryom Oh, Hong Sang Choi, Eun Mi Yang, Chang Seong Kim, Eun Hui Bae, Seong Kwon Ma, Kook-Hwan Oh, Young Youl Hyun, Suah Sung, and et al. 2023. "Bone Mineral Density and All-Cause Mortality in Patients with Nondialysis Chronic Kidney Disease: Results from KNOW-CKD Study" Journal of Clinical Medicine 12, no. 5: 1850. https://doi.org/10.3390/jcm12051850





