Deep Brain Stimulation in the Treatment of Tardive Dyskinesia
Abstract
:1. Introduction
2. Etiology and Risk Factors
3. Assessment Tools
4. Pharmacological Treatment
5. Deep Brain Stimulation
5.1. Internal Globus Pallidus (GPi)
5.1.1. Motor Effects of GPi DBS
5.1.2. Side Effects of GPi DBS
5.2. Subthalamic Nucleus (STN)
5.2.1. Motor Symptoms of STN DBS
5.2.2. Side Effects of STN DBS
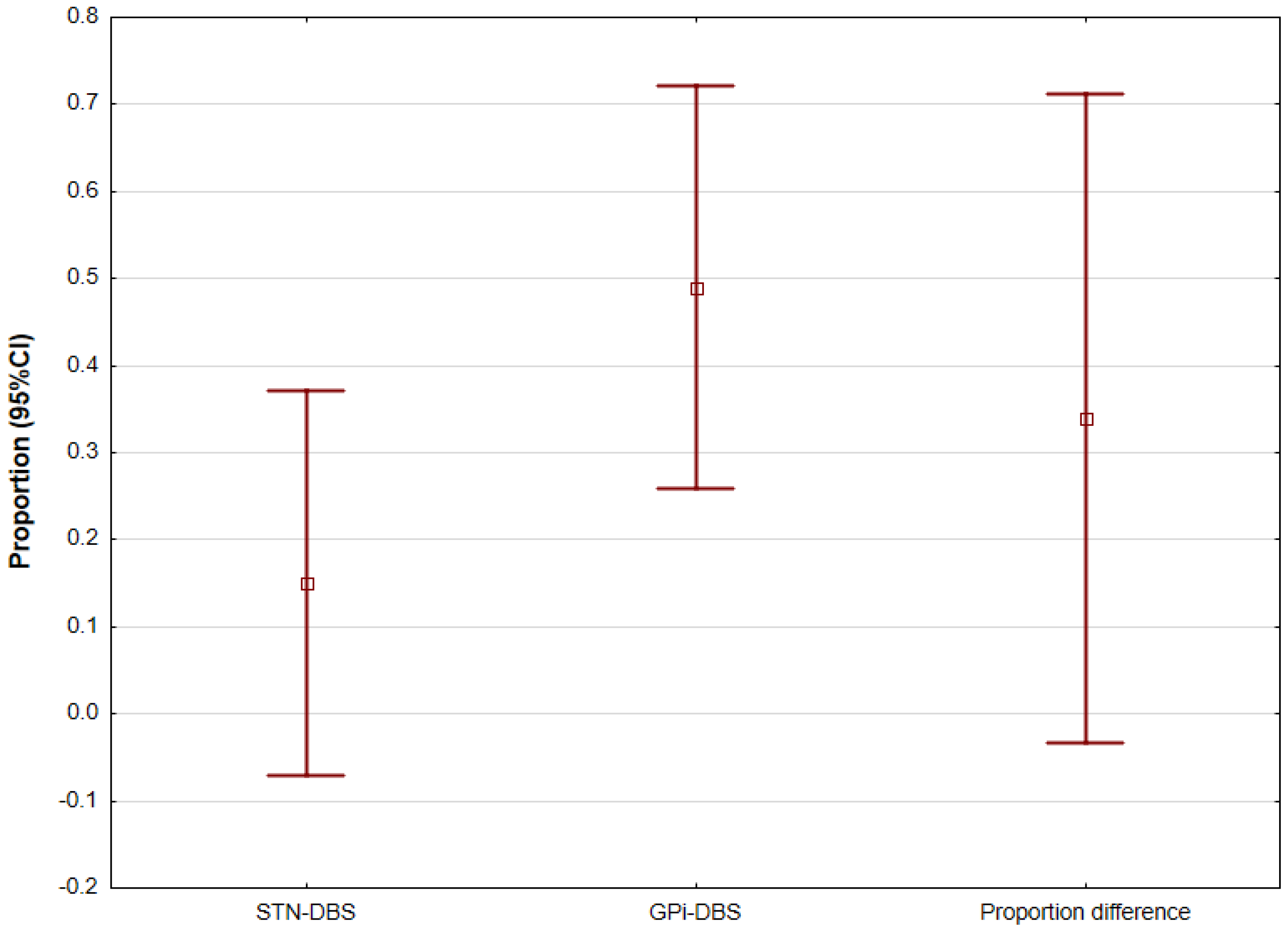
5.3. Internal Globus Pallidus (GPi) and Subthalamic Nucleus (STN) DBS Comparison
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Creed, M.C.; Hamani, C.; Bridgman, A.; Fletcher, P.J.; Nobrega, J.N. Contribution of decreased serotonin release to the antidyskinetic effects of deep brain stimulation in a rodent model of tardive dyskinesia: Comparison of the subthalamic and entopeduncular nuclei. J. Neurosci. 2012, 32, 9574–9581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciardi, L.; Pringsheim, T.; Barnes, T.R.E.; Martino, D.; Gardner, D.; Remington, G.; Addington, D.; Morgante, F.; Poole, N.; Carson, A.; et al. Treatment Recommendations for Tardive Dyskinesia. Can. J. Psychiatry 2019, 64, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Waln, O.; Jankovic, J. An update on tardive dyskinesia: From phenomenology to treatment. Tremor Other Hyperkinetic Mov. 2013, 3, tre-03-161-4138-1. [Google Scholar] [CrossRef]
- Meng, D.W.; Liu, H.G.; Yang, A.C.; Zhang, K.; Zhang, J.G. Long-term effects of subthalamic nucleus deep brain stimulation in tardive dystonia. Chin. Med. J. 2016, 129, 1257–1258. [Google Scholar] [CrossRef] [PubMed]
- Frei, K. Tardive dyskinesia: Who gets it and why. Park. Relat. Disord. 2019, 59, 151–154. [Google Scholar] [CrossRef]
- Deng, Z.D.; Li, D.Y.; Zhang, C.C.; Pan, Y.X.; Zhang, J.; Jin, H.; Zeljec, K.; Zhan, S.K.; Sun, B.M. Long-term follow-up of bilateral subthalamic deep brain stimulation for refractory tardive dystonia. Park. Relat. Disord. 2017, 41, 58–65. [Google Scholar] [CrossRef]
- Carroll, B.; Irwin, D.E. Health care resource utilization and costs for patients with tardive dyskinesia. J. Manag. Care Spec. Pharm. 2019, 25, 810–816. [Google Scholar] [CrossRef]
- Citrome, L.; Isaacson, S.H.; Larson, D.; Kremens, D. Tardive dyskinesia in older persons taking antipsychotics. Neuropsychiatry Dis. Treat. 2021, 17, 3127–3134. [Google Scholar] [CrossRef]
- Lerner, V.; Miodownik, C. Motor symptoms of schizophrenia: Is tardive dyskinesia a symptom or side effect? A modern treatment. Curr. Psychiatry Rep. 2011, 13, 295–304. [Google Scholar] [CrossRef]
- Sarró, S.; Pomarol-Clotet, E.; Canales-Rodríguez, E.J.; Salvador, R.; Gomar, J.J.; Ortiz-Gil, J.; Landín-Romero, R.; Vila-Rodríguez, F.; Blanch, J.; McKenna, P.J. Structural brain changes associated with tardive dyskinesia in schizophrenia. Br. J. Psychiatry 2013, 203, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Whitty, P.F.; Owoeye, O.; Waddington, J.L. Neurological signs and involuntary movements in schizophrenia: Intrinsic to and informative on systems pathobiology. Schizophr. Bull. 2009, 35, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.P.; Malhotra, A.K. Pharmacogenetics and antipsychotics: Therapeutic efficacy and side effects prediction. Expert Opin. Drug Metab. Toxicol. 2011, 7, 9–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Kang, S.G. Genetics of tardive dyskinesia. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 98. [Google Scholar]
- Thelma, B.K.; Srivastava, V.; Tiwari, A.K. Genetic underpinnings of tardive dyskinesia: Passing the baton to pharmacogenetics. Pharmacogenomics 2008, 9, 1285–1306. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.R.; Van Harten, P.N.; Van Os, J. Antipsychotic-induced tardive dyskinesia and polymorphic variations in COMT, DRD2, CYP1A2 and MnSOD genes: A meta-analysis of pharmacogenetic interactions. Mol. Psychiatry 2008, 13, 544–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Åberg, K.; Adkins, D.E.; Bukszár, J.; Webb, B.T.; Caroff, S.N.; Miller, D.D.; Sebat, J.; Stroup, S.; Fanous, A.H.; Vladimirov, V.I.; et al. Genomewide Association Study of Movement-Related Adverse Antipsychotic Effects. Biol. Psychiatry 2010, 67, 279–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inada, T.; Koga, M.; Ishiguro, H.; Horiuchi, Y.; Syu, A.; Yoshio, T.; Takahashi, N.; Ozaki, N.; Arinami, T. Pathway-based association analysis of genome-wide screening data suggest that genes associated with the γ-aminobutyric acid receptor signaling pathway are involved in neuroleptic-induced, treatment-resistant tardive dyskinesia. Pharm. Genom. 2008, 18, 317–323. [Google Scholar] [CrossRef]
- Greenbaum, L.; Alkelai, A.; Rigbi, A.; Kohn, Y.; Lerer, B. Evidence for association of the GLI2 gene with tardive dyskinesia in patients with chronic schizophrenia. Mov. Disord. 2010, 25, 2809–2817. [Google Scholar] [CrossRef]
- Syu, A.; Ishiguro, H.; Inada, T.; Horiuchi, Y.; Tanaka, S.; Ishikawa, M.; Arai, M.; Itokawa, M.; Niizato, K.; Iritani, S.; et al. Association of the HSPG2 gene with neuroleptic-induced tardive dyskinesia. Neuropsychopharmacology 2010, 35, 1155–1164. [Google Scholar] [CrossRef]
- Aquino, C.C.H.; Lang, A.E. Tardive dyskinesia syndromes: Current concepts. Park. Relat. Disord. 2014, 20, S113–S117. [Google Scholar] [CrossRef]
- Ferentinos, P.; Dikeos, D. Genetic correlates of medical comorbidity associated with schizophrenia and treatment with antipsychotics. Curr. Opin. Psychiatry 2012, 25, 381–390. [Google Scholar] [CrossRef]
- Souza, R.P.; Remington, G.; Chowdhury, N.I.; Lau, M.K.; Voineskos, A.N.; Lieberman, J.A.; Meltzer, H.Y.; Kennedy, J.L. Association study of the GSK-3B gene with tardive dyskinesia in European Caucasians. Eur. Neuropsychopharmacol. 2010, 20, 688–694. [Google Scholar] [CrossRef]
- Ethier, I.; Kagechika, H.; Shudo, K.; Rouillard, C.; Lévesque, D. Docosahexaenoic acid reduces haloperidol-induced dyskinesias in mice: Involvement of Nur77 and retinoid receptors. Biol. Psychiatry 2004, 56, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Teo, J.T.; Edwards, M.J.; Bhatia, K. Tardive dyskinesia is caused by maladaptive synaptic plasticity: A hypothesis. Mov. Disord. 2012, 27, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Thobois, S.; Poisson, A.; Damier, P. Surgery for tardive dyskinesia. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 98. [Google Scholar]
- Trugman, J.M.; Leadbetter, R.; Zalis, M.E.; Burgdorf, R.O.; Wooten, G.F. Treatment of severe axial tardive dystonia with clozapine: Case report and hypothesis. Mov. Disord. 1994, 9, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Tsai, G.; Goff, D.C.; Chang, R.W.; Flood, J.; Baer, L.; Coyle, J.T. Markers of glutamatergic neurotransmission and oxidative stress associated with tardive dyskinesia. Am. J. Psychiatry 1998, 155, 1207–1213. [Google Scholar] [CrossRef]
- Lu, R.B.; Ko, H.C.; Lin, W.L.; Lin, Y.T.; Ho, S.L. CSF neurochemical study of tardive dyskinesia. Biol. Psychiatry 1989, 25, 717–724. [Google Scholar]
- Hori, H.; Ohmori, O.; Shinkai, T.; Kojima, H.; Okano, C.; Suzuki, T.; Nakamura, J. Manganese superoxide dismutase gene polymorphism and schizophrenia: Relation to tardive dyskinesia. Neuropsychopharmacology 2000, 23, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Cloud, L.J.; Zutshi, D.; Factor, S.A. Tardive Dyskinesia: Therapeutic Options for an Increasingly Common Disorder. Neurotherapeutics 2014, 11, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.H.; Lee, H.J. Oxidative stress and tardive dyskinesia: Pharmacogenetic evidence. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 207–213. [Google Scholar] [CrossRef]
- Elkashef, A.M.; Wyatt, R.J. Tardive dyskinesia: Possible involvement of free radicals and treatment with vitamin E. Schizophr. Bull. 1999, 25, 731–740. [Google Scholar] [CrossRef]
- Sachdev, P.; Saharov, T.; Cathcart, S. The preventative role of antioxidants (selegiline and vitamin E) in a rat model of tardive dyskinesia. Biol. Psychiatry 1999, 46, 1672–1681. [Google Scholar] [CrossRef]
- Bartels, M.; Themelis, J. Computerized tomography in tardive dyskinesia—Evidence of structural abnormalities in the basal ganglia system. Archiv Für Psychiatrie UND Nervenkrankheiten Vereinigt MIT Zeitschrift Für Die Gesamte Neurologie UND Psychiatrie 1983, 233, 371–379. [Google Scholar] [CrossRef]
- Mion, C.C.; Andreasen, N.C.; Arndt, S.; Swayze, V.W.; Cohen, G.A. MRI abnormalities in tardive dyskinesia. Psychiatry Res. Neuroimaging 1991, 40, 157–166. [Google Scholar] [CrossRef]
- Guy, W.; Ban, T.A.; Wilson, W.H. The prevalence of abnormal involuntary movements among chronic schizophrenic. Int. Clin. Psychopharmacol. 1986, 1, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E.; Fahn, S.; Marsden, C.D.; Bressman, S.B.; Moskowitz, C.; Friedman, J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 1985, 35, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouclet-Courtemanche, H.; Rouaud, T.; Thobois, S.; Nguyen, J.M.; Brefel-Courbon, C.; Chereau, I.; Cuny, E.; Derost, P.; Eusebio, A.; Guehl, D.; et al. Long-term efficacy and tolerability of bilateral pallidal stimulation to treat tardive dyskinesia. Neurology 2016, 86, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Bhidayasiri, R.; Fahn, S.; Weiner, W.J.; Gronseth, G.S.; Sullivan, K.L.; Zesiewicz, T.A. Evidence-based guideline: Treatment of tardive syndromes: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013, 81, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Bhidayasiri, R.; Jitkritsadakul, O.; Friedman, J.H.; Fahn, S. Updating the recommendations for treatment of tardive syndromes: A systematic review of new evidence and practical treatment algorithm. J. Neurol. Sci. 2018, 389, 67–75. [Google Scholar] [CrossRef]
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia; American Psychiatric Association: Washington, DC, USA, 2020. [Google Scholar]
- Sako, W.; Goto, S.; Shimazu, H.; Murase, N.; Matsuzaki, K.; Tamura, T.; Mure, H.; Tomogane, Y.; Arita, N.; Yoshikawa, H.; et al. Bilateral deep brain stimulation of the globus pallidus internus in tardive dystonia. Mov. Disord. 2008, 23, 1929–1931. [Google Scholar] [CrossRef]
- Nandi, D.; Parkin, S.; Scott, R.; Winter, J.L.; Joint, C.; Gregory, R.; Stein, J.; Aziz, T.Z. Camptocormia treated with bilateral pallidal stimulation: Case report. Neurosurg. Focus 2002, 97, 461–466. [Google Scholar]
- Yianni, J.; Bain, P.; Giladi, N.; Auca, M.; Gregory, R.; Joint, C.; Nandi, D.; Stein, J.; Scott, R.; Aziz, T. Globus pallidus internus deep brain stimulation for dystonic conditions: A prospective audit. Mov. Disord. 2003, 18, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Gruber, D.; Trottenberg, T.; Kivi, A.; Schoenecker, T.; Kopp, U.A.; Hoffmann, K.T.; Schneider, G.H.; Kühn, A.A.; Kupsch, A. Long-term effects of pallidal deep brain stimulation in tardive dystonia. Neurology 2009, 73, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Capelle, H.H.; Blahak, C.; Schrader, C.; Baezner, H.; Kinfe, T.M.; Herzog, J.; Dengler, R.; Krauss, J.K. Chronic deep brain stimulation in patients with tardive dystonia without a history of major psychosis. Mov. Disord. 2010, 25, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.P.; Chang, W.S.; Chang, J.W. Treatment of secondary dystonia with a combined stereotactic procedure: Long-term surgical outcomes. Acta Neurochir. 2011, 153, 2319–2328. [Google Scholar] [CrossRef] [PubMed]
- Sobstyl, M.; Ząbek, M.; Mossakowski, Z.; Zaczyński, A. Deep brain stimulation of the internal globus pallidus for disabling haloperidol-induced tardive dystonia. Report of two cases. Neurol. Neurochir. Pol. 2016, 50, 258–261. [Google Scholar] [CrossRef]
- Mobin, F.; De Salles, A.A.F.; Behnke, E.J.; Frysinger, R. Correlation between MRI-based stereotactic thalamic deep brain stimulation electrode placement, macroelectrode stimulation and clinical response to tremor control. Stereotact. Funct. Neurosurg. 1999, 72, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.K.; Plaha, P.; O’Sullivan, K.; McCarter, R.; Heywood, P.; Gill, S.S. MRI directed bilateral stimulation of the subthalamic nucleus in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1631–1637. [Google Scholar] [CrossRef] [Green Version]
- Burchiel, K.J.; McCartney, S.; Lee, A.; Raslan, A.M. Accuracy of deep brain stimulation electrode placement using intraoperative computed tomography without microelectrode recording. J. Neurosurg. 2013, 119, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Von Langsdorff, D.; Paquis, P.; Fontaine, D. In vivo measurement of the frame-based application accuracy of the Neuromate neurosurgical robot. J. Neurosurg. 2015, 122, 191–194. [Google Scholar] [CrossRef] [Green Version]
- Lefranc, M.; Capel, C.; Pruvot, A.S.; Fichten, A.; Desenclos, C.; Toussaint, P.; Le Gars, D.; Peltier, J. The impact of the reference imaging modality, registration method and intraoperative flat-panel computed tomography on the accuracy of the ROSA® stereotactic robot. Stereotact. Funct. Neurosurg. 2014, 92, 242–250. [Google Scholar] [CrossRef]
- D’haese, P.F.; Pallavaram, S.; Konrad, P.E.; Neimat, J.; Fitzpatrick, J.M.; Dawant, B.M. Clinical accuracy of a customized stereotactic platform for deep brain stimulation after accounting for brain shift. Stereotact. Funct. Neurosurg. 2010, 88, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Bjartmarz, H.; Rehncrona, S. Comparison of accuracy and precision between frame-based and frameless stereotactic navigation for deep brain stimulation electrode implantation. Stereotact. Funct. Neurosurg. 2007, 85, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.; Tittgemeyer, M.; Schwarz, J.; Preul, C.; Strecker, K.; Meixensberger, J. The first evaluation of brain shift during functional neurosurgery by deformation field analysis. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1161–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.F.; Mewes, K.; Gross, R.E.; Škrinjar, O. Assessment of brain shift related to deep brain stimulation surgery. Stereotact. Funct. Neurosurg. 2007, 86, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Hunsche, S.; Sauner, D.; Maarouf, M.; Poggenborg, J.; Lackner, K.; Sturm, V.; Treuer, H. Intraoperative X-ray detection and MRI-based quantification of brain shift effects subsequent to implantation of the first electrode in bilateral implantation of deep brain stimulation electrodes. Stereotact. Funct. Neurosurg. 2009, 87, 322–329. [Google Scholar] [CrossRef]
- Okun, M.S.; Tagliati, M.; Pourfar, M.; Fernandez, H.H.; Rodriguez, R.L.; Alterman, R.L.; Foote, K.D. Management of Referred Deep Brain Stimulation Failures. Arch. Neurol. 2005, 62, 1250–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moro, E.; Poon, Y.Y.W.; Lozano, A.M.; Saint-Cyr, J.A.; Lang, A.E. Subthalamic nucleus stimulation: Improvements in outcome with reprogramming. Arch. Neurol. 2006, 63, 1266–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkmann, J.; Herzog, J.; Kopper, F.; Geuschl, G. Introduction to the programming of deep brain stimulators. Mov. Disord. 2002, 17, S181–S187. [Google Scholar] [CrossRef]
- Volkmann, J.; Moro, E.; Pahwa, R. Basic algorithms for the programming of deep brain stimulation in Parkinson’s disease. Mov. Disord. 2006, 21, S284–S289. [Google Scholar] [CrossRef]
- Bronstein, J.M.; Tagliati, M.; Alterman, R.L.; Lozano, A.M.; Volkmann, J.; Stefani, A.; Horak, F.B.; Okun, M.S.; Foote, K.D.; Krack, P.; et al. Deep brain stimulation for Parkinson disease an expert consensus and review of key issues. Arch. Neurol. 2011, 68, 165. [Google Scholar] [CrossRef]
- Temperli, P.; Ghika, J.; Villemure, J.G.; Burkhard, P.R.; Bogousslavsky, J.; Vingerhoets, F.J.G. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology 2003, 60, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.; Krafczyk, S.; Valkovič, P.; Eggert, T.; Claassen, J.; Bötzel, K. Objective measurement of muscle rigidity in Parkinsonian patients treated with subthalamic stimulation. Mov. Disord. 2009, 24, 57–63. [Google Scholar] [CrossRef]
- Thobois, S.; Ballanger, B.; Xie-Brustolin, J.; Damier, P.; Durif, F.; Azulay, J.P.; Derost, P.; Witjas, T.; Raoul, S.; Le Bars, D.; et al. Globus pallidus stimulation reduces frontal hyperactivity in tardive dystonia. J. Cereb. Blood Flow Metab. 2008, 28, 1127–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzini, A.; Marras, C.; Ferroli, P.; Zorzi, G.; Bugiani, O.; Romito, L.; Broggi, G. Long-term high-frequency bilateral pallidal stimulation for neuroleptic-induced tardive dystonia: Report of two cases. J. Neurosurg. 2005, 102, 721–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, N.; Balas, I.; Janszky, J.; Simon, M.; Fekete, S.; Komoly, S. Status dystonicus in tardive dystonia successfully treated by bilateral deep brain stimulation. Clin. Neurol. Neurosurg. 2011, 113, 808–809. [Google Scholar] [CrossRef] [PubMed]
- Starr, P.A.; Turner, R.S.; Rau, G.; Lindsey, N.; Heath, S.; Volz, M.; Ostrem, J.L.; Marks, W.J. Microelectrode-guided implantation of deep brain stimulators into the globus pallidus internus for dystonia: Techniques, electrode locations, and outcomes. J. Neurosurg. 2006, 104, 488–501. [Google Scholar] [CrossRef]
- Trottenberg, T.; Paul, G.; Meissner, W.; Maier-Hauff, K.; Taschner, C.; Kupsch, A. Pallidal and thalamic neurostimulation in severe tardive dystonia. J. Neurol. Neurosurg. Psychiatry 2001, 70, 557–559. [Google Scholar] [CrossRef] [Green Version]
- Hälbig, T.D.; Gruber, D.; Kopp, U.A.; Schneider, G.H.; Trottenberg, T.; Kupsch, A. Pallidal stimulation in dystonia: Effects on cognition, mood, and quality of life. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1713–1716. [Google Scholar] [CrossRef] [Green Version]
- Nambu, A. Somatotopic organization of the primate basal ganglia. Front. Neuroanat. 2011, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Spindler, M.A.; Galifianakis, N.B.; Wilkinson, J.R.; Duda, J.E. Globus pallidus interna deep brain stimulation for tardive dyskinesia: Case report and review of the literature. Park. Relat. Disord. 2013, 19, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Magariños-Ascone, C.M.; Regidor, I.; Gómez-Galán, M.; Cabañes-Martínez, L.; Figueiras-Méndez, R. Deep brain stimulation in the globus pallidus to treat dystonia: Electrophysiological characteristics and 2 years’ follow-up in 10 patients. Neuroscience 2008, 152, 558–571. [Google Scholar] [CrossRef]
- Eltahawy, H.A.; Feinstein, A.; Khan, F.; Saint-Cyr, J.; Lang, A.E.; Lozano, A.M. Bilateral globus pallidus internus deep brain stimulation in tardive dyskinesia: A case report. Mov. Disord. 2004, 19, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Trottenberg, T.; Volkmann, J.; Deuschl, G.; Kühn, A.A.; Schneider, G.H.; Müller, J.; Alesch, F.; Kupsch, A. Treatment of severe tardive dystonia with pallidal deep brain stimulation. Neurology 2005, 64, 344–346. [Google Scholar] [CrossRef]
- Katsakiori, P.F.; Kefalopoulou, Z.; Markaki, E.; Paschali, A.; Ellul, J.; Kagadis, G.C.; Chroni, E.; Constantoyannis, C. Deep brain stimulation for secondary dystonia: Results in 8 patients. Acta Neurochir. 2009, 151, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Kefalopoulou, Z.; Paschali, A.; Markaki, E.; Vassilakos, P.; Ellul, J.; Constantoyannis, C. A double-blind study on a patient with tardive dyskinesia treated with pallidal deep brain stimulation. Acta Neurol. Scand. 2009, 119, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Fogel, W.; Kloss, M.; Rasche, D.; Volkmann, J.; Tronnier, V. Pallidal stimulation for dystonia. Neurosurgery 2004, 55, 1361–1370. [Google Scholar] [CrossRef]
- Kosel, M.; Sturm, V.; Frick, C.; Lenartz, D.; Zeidler, G.; Brodesser, D.; Schlaepfer, T.E. Mood improvement after deep brain stimulation of the internal globus pallidus for tardive dyskinesia in a patient suffering from major depression. J. Psychiatry Res. 2007, 41, 801–803. [Google Scholar] [CrossRef]
- Shaikh, A.G.; Mewes, K.; DeLong, M.R.; Gross, R.E.; Triche, S.D.; Jinnah, H.A.; Boulis, N.; Willie, J.T.; Freeman, A.; Alexander, G.E.; et al. Temporal profile of improvement of tardive dystonia after globus pallidus deep brain stimulation. Park. Relat. Disord. 2015, 21, 116–119. [Google Scholar] [CrossRef] [Green Version]
- Schrader, C.; Peschel, T.; Petermeyer, M.; Dengler, R.; Hellwig, D. Unilateral deep brain stimulation of the internal globus pallidus alleviates tardive dyskinesia. Mov. Disord. 2004, 19, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Egidi, M.; Franzini, A.; Marras, C.; Cavallo, M.; Mondani, M.; Lavano, A.; Romanelli, P.; Castana, L.; Lanotte, M.; Farneti, M. A survey of Italian cases of dystonia treated by deep brain stimulation. J. Neurosurg. Sci. 2007, 51, 153. [Google Scholar]
- Morigaki, R.; Mure, H.; Kaji, R.; Nagahiro, S.; Goto, S. Therapeutic perspective on tardive syndrome with special reference to deep brain stimulation. Front. Psychiatry 2016, 7, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pretto, T.E.; Dalvi, A.; Kang, U.J.; Penn, R.D. A prospective blinded evaluation of deep brain stimulation for the treatment of secondary dystonia and primary torticollis syndromes. J. Neurosurg. 2008, 109, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Boulogne, S.; Danaila, T.; Polo, G.; Broussolle, E.; Thobois, S. Relapse of tardive dystonia after globus pallidus deep-brain stimulation discontinuation. J. Neurol. 2014, 261, 1636–1637. [Google Scholar] [CrossRef] [PubMed]
- Trinh, B.; Ha, A.D.; Mahant, N.; Kim, S.D.; Owler, B.; Fung, V.S.C. Dramatic improvement of truncal tardive dystonia following globus pallidus pars interna deep brain stimulation. J. Clin. Neurosci. 2014, 21, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Albassam, A.; Silver, B. Deep brain stimulation in the treatment of tardive dyskinesia. Psychiatr. Ann. 2014, 44, 123–125. [Google Scholar] [CrossRef]
- Ogata, E.; Ogura, M.; Nishibayashi, H.; Sasaki, T.; Kakishita, K.; Nakao, N. GPi-DBS for tardive dystonia: A case report. Jpn. J. Neurosurg. 2014, 23, 348–353. [Google Scholar] [CrossRef]
- Woo, P.Y.M.; Chan, D.T.M.; Zhu, X.L.; Yeung, J.H.M.; Chan, A.Y.Y.; Au, A.C.W.; Cheng, K.M.; Lau, K.Y.; Wing, Y.K.; Mok, V.C.T.; et al. Pallidal deep brain stimulation: An effective treatment in chinese patients with tardive dystonia. Hong Kong Med. J. 2014, 20, 455–459. [Google Scholar] [CrossRef] [Green Version]
- Cohen, O.S.; Hassin-Baer, S.; Spiegelmann, R. Deep brain stimulation of the internal globus pallidus for refractory tardive dystonia. Park. Relat. Disord. 2007, 13, 541–544. [Google Scholar] [CrossRef]
- Damier, P.; Thobois, S.; Witjas, T.; Cuny, E.; Derost, P.; Raoul, S.; Mertens, P.; Peragut, J.C.; Lemaire, J.J.; Burbaud, P.; et al. Bilateral deep brain stimulation of the globus pallidus to treat tardive dyskinesia. Arch. Gen. Psychiatry 2007, 64, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Chang, E.F.; Schrock, L.E.; Starr, P.A.; Ostrem, J.L. Long-term benefit sustained after bilateral pallidal deep brain stimulation in patients with refractory tardive dystonia. Stereotact. Funct. Neurosurg. 2010, 88, 304–310. [Google Scholar] [CrossRef]
- Krause, P.; Kroneberg, D.; Gruber, D.; Koch, K.; Schneider, G.H.; Kühn, A.A. Long-term effects of pallidal deep brain stimulation in tardive dystonia: A follow-up of 5–14 years. J. Neurol. 2022, 269, 3563–3568. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Mure, H.; Morigaki, R.; Miyamoto, R.; Miyake, K.; Matsuda, T.; Fujita, K.; Izumi, Y.; Kaji, R.; Goto, S.; et al. Long-term follow-up of 12 patients treated with bilateral pallidal stimulation for tardive dystonia. Life 2021, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Mentzel, C.L.; Tenback, D.E.; Tijssen, M.A.J.; Visser-Vandewalle, V.E.R.M.; Van Harten, P.N. Efficacy and safety of deep brain stimulation in patients with medication-induced tardive dyskinesia and/or dystonia: A systematic review. J. Clin. Psychiatry 2012, 73, 1434–1438. [Google Scholar] [CrossRef] [PubMed]
- Krauss, J.K.; Lipsman, N.; Aziz, T.; Boutet, A.; Brown, P.; Chang, J.W.; Davidson, B.; Grill, W.M.; Hariz, M.I.; Horn, A.; et al. Technology of deep brain stimulation: Current status and future directions. Nat. Rev. Neurol. 2021, 17, 75–87. [Google Scholar] [CrossRef]
- Foncke, E.M.J.; Schuurman, P.R.; Speelman, J.D. Suicide after deep brain stimulation of the internal globus pallidus for dystonia. Neurology 2006, 66, 142–143. [Google Scholar] [CrossRef]
- de Gusmao, C.M.; Pollak, L.E.; Sharma, N. Neuropsychological and psychiatric outcome of GPi-deep brain stimulation in dystonia. Brain Stimul. 2017, 10, 994–996. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Tsuboi, T.; Hegland, K.W.; Herndon, N.E.; Shukla, A.W.; Patterson, A.; Almeida, L.; Foote, K.D.; Okun, M.S.; Ramirez-Zamora, A. Dysarthria and Speech Intelligibility following Parkinson’s Disease Globus Pallidus Internus Deep Brain Stimulation. J. Park. Dis. 2020, 10, 1493–1502. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, F.; Li, W.; Wang, N.; Han, C.; Fan, S.; Li, P.; Xu, L.; Zhang, J.; Meng, F. Relationship between electrode position of deep brain stimulation and motor symptoms of Parkinson’s disease. BMC Neurol. 2021, 21, 847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, K.; Wang, Z.; Ge, M.; Ma, Y. Deep brain stimulation in the treatment of secondary dystonia. Chin. Med. J. 2006, 119, 2069–2074. [Google Scholar] [CrossRef]
- Sun, B.; Chen, S.; Zhan, S.; Le, W.; Krahl, S.E. Subthalamic nucleus stimulation for primary dystonia and tardive dystonia. Acta Neurochir. Suppl. 2007, 97, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.; Ceponiene, R.; Savla, P.; Bernstein, J.; Ghanchi, H.; Ananda, A. Resolution of tardive tremor after bilateral subthalamic nucleus deep brain stimulation placement. Surg. Neurol. Int. 2020, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Celiker, O.; Demir, G.; Kocaoglu, M.; Altug, F.; Acar, F. Comparison of subthalamic nucleus vs. globus pallidus interna deep brain stimulation in terms of gait and balance; A two year follow-up study. Turk. Neurosurg. 2019, 29, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Au, K.L.K.; Wong, J.K.; Tsuboi, T.; Eisinger, R.S.; Moore, K.; Lopes, J.L.M.L.J.; Holland, M.T.; Holanda, V.M.; Peng-Chen, Z.; Patterson, A.; et al. Globus Pallidus Internus (GPi) Deep Brain Stimulation for Parkinson’s Disease: Expert Review and Commentary. Neurol. Ther. 2021, 10, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.A.; Ouyang, B.; Goetz, S.; Metman, L.V. A Novel DBS Paradigm for Axial Features in Parkinson’s Disease: A Randomized Crossover Study. Mov. Disord. 2020, 35, 1369–1378. [Google Scholar] [CrossRef]
- Barbe, M.T.; Reker, P.; Hamacher, S.; Franklin, J.; Kraus, D.; Dembek, T.A.; Becker, J.; Steffen, J.K.; Allert, N.; Wirths, J.; et al. DBS of the PSA and the VIM in essential tremor. Neurology 2018, 91, e543–e550. [Google Scholar] [CrossRef]
- Blomstedt, P.; Persson, R.S.; Hariz, G.M.; Linder, J.; Fredricks, A.; Häggström, B.; Philipsson, J.; Forsgren, L.; Hariz, M. Deep brain stimulation in the caudal zona incerta versus best medical treatment in patients with Parkinson’s disease: A randomised blinded evaluation. J. Neurol. Neurosurg. Psychiatry 2018, 89, 710–716. [Google Scholar] [CrossRef] [Green Version]
| Nonmodifiable Factors | Modifiable Factors |
|---|---|
| Advanced age | Type of dopamine receptor blocking agents |
| Female sex | Duration of illness |
| Caucasian or African ethnicity | Dosage and length of exposure to a dopamine receptor blocker |
| Intellectual disability | Intermittent antipsychotic treatment |
| Brain damage | Anticholinergic treatment |
| Negative symptoms in schizophrenia | Smoking |
| Alcohol and cocaine abuse/dependence | |
| Akathisia |
| DRD2 and DRD3 |
| HTR2A (5-HT2A receptors) |
| COMT |
| MnSOD |
| Cytochrome P450 (CYP2D6) |
| GSK-3ß |
| 3′-Regulatory region of Nurr77 mRNA |
| SLC6A11, GABRB2, and GABRG3 related to GABAergic transmission |
| GRIN2A related to NMDA receptor and glutamatergic transmission |
| GSTM1, GSTP1, NOS3, and NQO1 involved in oxidative stress reactions |
| BDNF |
| GLI2 |
| HSPG2 |
| Author [Reference] | Localization | Mono-/Bipolar (N, When >1) | Scale (% of Improvement)/Follow Up (Months) |
|---|---|---|---|
| Pouclet-Courtemanche [38] | PV-GPi | M | AIMS (63)/12–132 |
| Sako [42] | PV-GPi | M/B (5) | BFMDRS-M (58–100), BFMDRS-D (67–100)/3–49 |
| Nandi [43] | PV-GPi | B | BFMDRS-M (28), BFMDRS-D (39), AIMS (42)/ 12 |
| Gruber [45] | PVL-GPi | M/B (8) | BFMDRS-M (64-100), BFMDRS-D (25–100), AIMS (33–100)/26–80 |
| Capelle [46] | PVL-GPi | B (4) | BFMDRS-M (70–91), BFMDRS-D (50–100)/16–36 |
| Kim [47] | PVL-GPi | M | BFMDRS-M (97), BFMDRS-D (100)/20 |
| Sobstyl [48] | PVL-GPi | B (2) | BFMDRS-M (69–78), BFMDRS-D (56–73)/12–24 |
| Franzini [67] | PVL-GPi | M (2) | BFMDRS-M (86–88)/12 |
| Kovacs [68] | PVL-GPi | ? | BFMDRS-M (97), BFMDRS-D (96)/12 |
| Starr [69] | PVL-GPi | ? (4) | BFMDRS-M (6–100)/9–27 |
| Trottenberg [70] | PV-GPi | M | BFMDRS-M (73), AIMS (54)/6 |
| Hälbig [71] | PVM-GPi | M (2) | BFMDRS-M (77–93)/? |
| Spindler [73] | GPi | M | AIMS (67)/<60 |
| Magariños-Ascone [74] | GPi | ? | BFMDRS-M (48), BFMDRS-D (44)/12 |
| Eltahawy [75] | PV-GPi | M | BFMDRS-M (60)/18 |
| Trottenberg [76] | PVM-GPi | M (5) | BFMDRS-M (75–98), BFMDRS-D (80–100)/6 |
| Katsakiori [77] | GPi | M | BFMDRS-M (94), BFMDRS-D (84)/12 |
| Kefalopoulou [78] | GPi | M | BFMDRS-M (91), AIMS (77)/6 |
| Krause [79] | GPi | M (3) | BFMDRS-M (−1–0), no benefit/≤36 |
| Kosel [80] | GPi | M | BFMDRS-M (35)/18 |
| Shaikh [81] | GPi | M (8) | BFMDRS-M (67–100)/6–60 |
| Schrader [82] | GPi | M | AIMS (63)/ 5 |
| Egidi [83] | GPi | M | BFMDRS-M (47), BFMDRS-D (55)/? |
| Pretto [85] | GPi | B | BFMDRS (~90)/6 |
| Boulogne [86] | PVL-GPi | M | AIMS (79)/120 |
| Trinh [87] | GPi | ? | BFMDRS-M (90), BFMDRS-D (87)/18 |
| Puri [88] | GPi | ? | AIMS (55)/6 |
| Ogata [89] | PL-GPi | B | BFMDRS-M (69), BFMDRS-D (64), AIMS (94)/7 |
| Woo [90] | PV-GPi | M (3) | BFMDRS-M (54–100)/3–120 |
| Cohen [91] | GPi | M (2) | BFMDRS-M (63–88), BFMDRS-D (53–100)/7–13 |
| Damier [92] | PVL-GPi | M (10) | AIMS (33–78)/6 |
| Chang [93] | PV-GPi | M | BFMDRS-M (71), BFMDRS-D (48), AIMS (77)/27–76 |
| Krause [94] | GPi | B (7) | BFMDRS-M (90), BFMDRS-D (79), AIMS (73)/63–171 |
| Koyama [95] | GPi | B (12) | BFMDRS (78)/6–186 |
| Side Effect | Brain Area |
|---|---|
| Mood and cognitive symptoms | Ventral part of GPi |
| Motor side effects (corticospinal and corticobulbar side, i.e., tonic muscle contractions) | Posterior part of GPi/capsular fibers |
| Phosphenes (seeing light without light entering the eye) | Ventral/optic tract |
| Low threshold for capsular side effects (i.e., muscle contractions) | Medial GPi |
| Speech impairment | Internal capsule, medial and posterior to GPi |
| Author [Reference] | Localization | Mono-/Bipolar (N, When >1) | Scale (% of Improvement) /Follow Up (Months) |
|---|---|---|---|
| Deng [6] | STN | B (10) | BFMDRS (88), AIMS (94)/12–105 |
| Zhang [102] | STN | B (2) | BFMDRS (>90)/3–36 |
| Sun [103] | STN | B (2) | AIMS (63) BFMDRS (>77)/6–42 |
| Kashyap [104] | STN | B | ?, “near-complete resolution of tremors”/24 |
| Side Effect | Brain Area |
|---|---|
| Spastic muscle contraction | Internal capsule |
| Uni- or bilateral gaze deviation | Fibers stemming from the frontal eye field running in the internal capsule, fibers of the third nerve (inferomedial to the STN and within the red nucleus), sympathetic fibers within the zona incerta or STN |
| Autonomic symptoms | Hypothalamus and red nucleus |
| Paresthesia | Medial lemniscus |
| Speech impairment | Internal capsule, the pallidal and cerebello-thalamic fiber tracts medial and dorsal of the STN, medial left-sided STN stimulation in right-handed patients, higher left STN voltage |
| Depression | Substantia nigra |
| Mania | Medial and ventral areas of STN |
| Impulse control disorder | Ventromedial and limbic areas of STN, SNr, medial forebrain bundle |
| Cognitive problems | Ventral and medial parts of STN, perforation of the caudate nucleus during surgery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczakowska, A.; Gabryelska, A.; Gawlik-Kotelnicka, O.; Strzelecki, D. Deep Brain Stimulation in the Treatment of Tardive Dyskinesia. J. Clin. Med. 2023, 12, 1868. https://doi.org/10.3390/jcm12051868
Szczakowska A, Gabryelska A, Gawlik-Kotelnicka O, Strzelecki D. Deep Brain Stimulation in the Treatment of Tardive Dyskinesia. Journal of Clinical Medicine. 2023; 12(5):1868. https://doi.org/10.3390/jcm12051868
Chicago/Turabian StyleSzczakowska, Adrianna, Agata Gabryelska, Oliwia Gawlik-Kotelnicka, and Dominik Strzelecki. 2023. "Deep Brain Stimulation in the Treatment of Tardive Dyskinesia" Journal of Clinical Medicine 12, no. 5: 1868. https://doi.org/10.3390/jcm12051868
APA StyleSzczakowska, A., Gabryelska, A., Gawlik-Kotelnicka, O., & Strzelecki, D. (2023). Deep Brain Stimulation in the Treatment of Tardive Dyskinesia. Journal of Clinical Medicine, 12(5), 1868. https://doi.org/10.3390/jcm12051868







