Significant Interactions between Adipokines and Vitamin D Combined with the Estimated Glomerular Filtration Rate: A Geriatric Case Study
Abstract
:1. Introduction
2. Materials and Methods
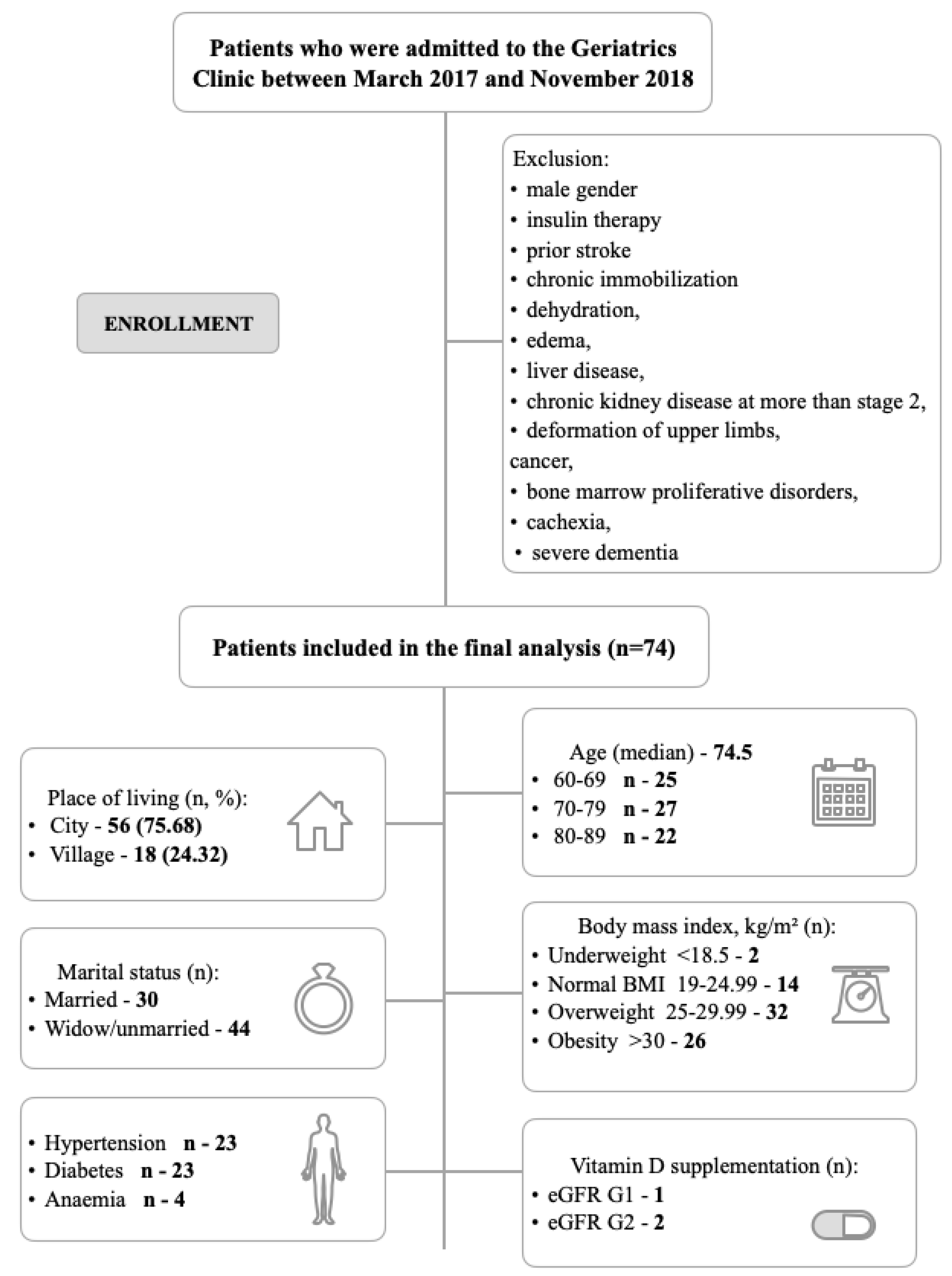
2.1. Recruitment and Participants
2.2. Ethical Approval
2.3. Demographic Profiles and Clinical Characteristics Collection
2.4. Biochemical and Hematological Assays
2.4.1. Serum Leptin Assays
2.4.2. Serum Adiponectin Measurement
2.4.3. Serum Omentin Analysis
2.4.4. Serum Ghrelin Assays
2.4.5. Serum Visfatin Measurement
2.5. Anthropometric Calculation
2.6. eGFR Calculation
2.7. Statistical/Data Analysis
3. Results
3.1. Baseline Characteristics
3.2. eGFR Assessment
3.3. Association between Vitamin D Concentration and Clinical Variables
3.4. Age Groups Analysis
3.5. Relationship between Clinical Variables
3.6. Tests of Sensitivity and Specificity
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Report from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the Implications of Demographic Change. Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/PDF/?uri=CELEX:52020DC0241&from=EN (accessed on 12 March 2022).
- The World Bank Data. Population Ages 65 and Above (% of Total Population). Available online: https://data.worldbank.org/indicator/SP.POP.65UP.TO.ZS (accessed on 12 March 2022).
- World Health Organization. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 12 March 2022).
- Alshahrani, F.; Aljohani, N. Vitamin D: Deficiency, sufficiency and toxicity. Nutrients 2013, 5, 3605–3616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szu-Wen, C.; Hung-Chang, L. Vitamin D and health-The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Ajabshir, S.; Asif, A.; Nayer, A. The effects of vitamin D on the renin-angiotensin system. J. Nephropathol. 2014, 3, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Zdrojewicz, Z.; Chruszczewska, E.; Miner, M. The influence of vitamin D on the man organism. Med. Rodz. 2015, 2, 61–66. [Google Scholar]
- Anyanwu, A.C.; Olopade, O.B.; Onung, S.I.; Odeniyi, I.A.; Coker, H.A.; Fasanmade, O.A.; Ohwovoriole, A.E. Serum Vitamin D Levels in Persons with Type 2 Diabetes Mellitus in Lagos, Nigeria. Int. J. Diabetes Clin. Res. 2020, 7, 133. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Aranow, C. Vitamin D and the Immune System. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef] [Green Version]
- Kalueff, A.V.; Eremin, K.O.; Tuohimaa, P. Mechanisms of Neuroprotective Action of Vitamin D3. Biochemistry (Moscow) 2004, 69, 738–741. [Google Scholar] [CrossRef]
- Chakraborti, C.K. Vitamin D as a promising anticancer agent. Indian J. Pharmacol. 2011, 43, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Wąsowski, M.; Czerwińska, E.; Marcinkowska-Suchowierska, E. Obesity–The condition predisposing to vitamin D deficiency. Post. Nauk Med. 2012, 3, 258–264. [Google Scholar]
- Meehan, M.; Penckofer, S. The Role of Vitamin D in the Aging Adult. J. Aging Gerontol. 2014, 2, 60–71. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, F.G.; Justice, J.N.; de Freitas, E.C.; Kershaw, E.E.; Sparks, L.M. Adipose Tissue Quality in Aging: How Structural and Functional Aspects of Adipose Tissue Impact Skeletal Muscle Quality. Nutrients 2019, 11, 2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waas, T.; Schulz, A.; Lotz, J.; Rossmann, H.; Pfeiffer, N.; Beutel, M.E.; Schmidtmann, I.; Münzel, T.; Wild, P.; Lackner, K. Distribution of estimated glomerular filtration rate and determinants of its age dependent loss in a German population-based study. Sci. Rep. 2021, 11, 10165. [Google Scholar] [CrossRef]
- Syed Ali Fathima, S.; Sasivathanam, N.; Nirmala Devi, K.; Arshiya Begum, A.; Vanitha, K.; Santhi, N. Serum Visfatin-A Novel Marker of Chronic Kidney Disease. JMSH 2017, 3, 19–25. [Google Scholar] [CrossRef]
- Kwon, J.; Suzuki, T.; Yoshida, H.; Kim, H.; Yoshida, Y.; Iwasa, H. Concomitant lower serum albumin and vitamin D levels are associated with decreased objective physical performance among Japanese community-dwelling elderly. Gerontology 2007, 53, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.J.; Ibrahim, K.; Sayer, A.; Robinson, S.M.; Roberts, H.C. Assessment and Treatment of the Anorexia of Aging: A Systematic Review. Nutrients 2019, 11, 144. [Google Scholar] [CrossRef] [Green Version]
- Lewiński, A.; Karbownik-Lewińska, M.; Wieczorek-Szukała, K.; Stasiak, M.; Stawerska, R. Contribution of Ghrelin to the Pathogenesis of Growth Hormone Deficiency. Int. J. Mol. Sci. 2021, 22, 9066. [Google Scholar] [CrossRef]
- Kelesidis, T.; Kelesidis, I.; Chou, S.; Mantzoros, C.S. Narrative review: The role of leptin in human physiology: Emerging clinical applications. Ann. Intern. Med. 2010, 152, 93–100. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. (Lausanne) 2021, 12, 585887. [Google Scholar] [CrossRef]
- Gangloff, A.; Bergeron, J.; Lemieux, I.; Tremblay, A.; Poirier, P.; Alméras, N.; Després, J.P. Relationships between circulating 25(OH) vitamin D, leptin levels and visceral adipose tissue volume: Results from a 1-year lifestyle intervention program in men with visceral obesity. Int. J. Obes. (Lond.) 2020, 44, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; McNulty, H.; Ward, M.; Hoey, L.; McSorley, E.; Wallace, J.M.; Carson, E.; Molloy, A.M.; Healy, M.; Casey, M.C.; et al. Vitamin D deficiency is associated with inflammation in older Irish adults. J. Clin. Endocrinol. Metab. 2014, 99, 1807–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, K.; Agrawal, D.K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar] [CrossRef] [Green Version]
- Meeker, S.; Seamons, A.; Maggio-Price, M.; Paik, J. Protective links between vitamin D, inflammatory bowel disease and colon cancer. World J. Gastroenterol. 2016, 22, 933–948. [Google Scholar] [CrossRef]
- Gallagher, J.C. Vitamin D and Aging. Endocrinol. Metab. Clin. North. Am. 2013, 42, 319–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonemura, K.; Fujimoto, T.; Fujigaki, Y.; Hishida, A. Vitamin D deficiency is implicated in reduced serum albumin concentrations in patients with end-stage renal disease. Am. J. Kidney Dis. 2000, 36, 337–344. [Google Scholar] [CrossRef]
- Refaie, M.R.; Sayed-Ahmed, N.A.; Bakr, A.M.; Aziz, M.Y.; El Kannishi, M.H.; Abdel-Gawad, S.S. Aging is an Inevitable Risk Factor for Insulin Resistance. J. Taibah Univ. Med. Sci. 2006, 1, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Ko, G.T.; Wai, H.P.; Tang, J.S. Effects of Age on Plasma Glucose Levels in Non-diabetic Hong Kong Chinese. Croat. Med. J. 2006, 47, 709–713. [Google Scholar]
- Akamizu, T.; Murayama, T.; Teramukai, S.; Miura, K.; Bando, I.; Irako, T.; Iwakura, H.; Ariyasu, H.; Hosoda, H.; Tada, H.; et al. Plasma ghrelin levels in healthy elderly volunteers: The levels of acylated ghrelin in elderly females correlate positively with serum IGF-I levels and bowel movement frequency and negatively with systolic blood pressure. J. Endocrinol. 2006, 188, 333–344. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, K.; Ouchi, N.; Matsuzawa, Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie. 2012, 94, 2137–2142. [Google Scholar] [CrossRef]






| Variable (Units) | Participants (N = 74) | Reference Values * |
|---|---|---|
| Vitamin D (ng/mL) | 18.51 ± 7.77 | 30.0–100.0 |
| Creatinine (mg/dL) | 0.76 ± 0.10 | 0.5–0.8 |
| eGFR (mL/min/1.73 m2) | 80.08 ± 11.37 | >60.0 |
| Calcium (mmol/L) | 2.28 ± 0.11 | 2.1–2.7 |
| Parathyroid hormone (pg/mL) | 64.33 ± 33.70 | 18.5–88.0 |
| Albumin (g/dL) | 3.86 ± 0.35 | 4.02–4.76 |
| Glucose (mg/dL) | 96.01 ± 26.24 | 70.0–99.0 |
| Insulin (µU/mL) | 10.04 ± 9.94 | 3.0–25.0 |
| HOMA-IR | 2.56 ± 3.19 | <2 (normal) |
| hsCRP (mg/L) | 3.13 ± 4.47 | 0–5.0 |
| Leptin (ng/mL) | 8.64 ± 5.01 | 0.156–10.0 |
| Adiponectin (ng/mL) | 24,112.81 ± 4839.17 | 0.156–10.0 |
| Omentin (ng/mL) | 108.83 ± 54.21 | 1.56–100.0 |
| Ghrelin (pg/mL) | 9602.92 ± 643.43 | 123.5–10,000.0 |
| Visfatin (ng/mL) | 24,393.77 ± 19,059.31 | 1.56–100.0 |
| Body mass index (kg/m2) | 28.59 ± 5.30 | 19–24.99 (normal) |
| WHR | 0.89 ± 0.07 | <0.8 (low) |
| Glomerular Filtration Rate Category | Estimated Glomerular Filtration Rate Range (mL/min/1.73 m2) | Severity Description |
|---|---|---|
| G1 | ≥90 | Normal or high |
| G2 | 60–89 | Mildly Decreased |
| G3a | 45–59 | Mildly to Moderately Decreased |
| G3b | 30–44 | Moderately to Severely Decreased |
| G4 | 15–29 | Severely Decreased |
| G5 | <15 | Kidney Failure |
| Variable/ Unit | eGFR | ||
|---|---|---|---|
| G1 n = 19 | G2 n = 55 | p-Value | |
| Age | 74.79 (7.74) | 74.42 (7.20) | 0.8497 |
| BMI kg/m2 | 28.20 25.30/33.70 | 28.10 25.50/31.20 | 0.6119 |
| Vitamin D ng/mL | 13.90 11.00/20.90 | 18.90 14.50/24.30 | 0.0757 |
| Creatinine mg/dL | 0.65 0.63/0.67 | 0.80 0.75/0.85 | <0.0001 |
| Calcium mmol/L | 2.23 2.18/2.31 | 2.28 2.22/2.33 | 0.1229 |
| PTH pg/mL | 60.60 38.30/77.70 | 62.20 39.40/80.90 | 0.6294 |
| Albumin g/dL | 3.82 (0.36) | 3.87 (0.35) | 0.5866 |
| Glucose mg/L | 88.00 84.00/100.00 | 89.00 83.00/100.00 | 0.9852 |
| HOMA-IR | 2.04 1.37/3.34 | 1.62 1.03/2.10 | 0.1311 |
| hsCRP mg/L | 1.98 0.87/4.53 | 1.39 0.78/3.28 | 0.4211 |
| Leptin ng/mL | 9.68 5.36/13.39 | 10.70 2.75/13.12 | 0.8917 |
| Adiponectin ng/mL | 24,790.00 21,165.00/26,050.00 | 24,340.00 21,920.00/26,575.00 | 0.6650 |
| Omentin ng/mL | 101.70 66.79/122.30 | 104.60 77.28/136.00 | 0.4691 |
| Ghrelin pg/mL | 1000.00 9433.00/1000.00 | 1000.00 9379.00/1000.00 | 0.7771 |
| Visfatin ng/mL | 13,510.00 10,575.00/22,310.00 | 22,270.00 12,675.00/30,605.00 | 0.0521 |
| WHR | 0.91 (0.06) | 0.88 (0.08) | 0.0905 |
| Variable/ Unit | Vitamin D (Concentration ng/mL) | ||||||
|---|---|---|---|---|---|---|---|
| >21 n = 24 | 15–21 n = 25 | <15.00 n = 25 | p-Value | I vs. II p-Value | I vs. III p-Value | II vs. III p-Value | |
| Age | 71.29 (6.26) | 72.92 (6.42) | 79.20 (6.85) | 0.0001 | 0.6584 | 0.0003 | 0.0032 |
| BMI kg/m2 | 28.61 (6.27) | 27.82 (4.31) | 29.33 (5.31) | 0.6078 | 0.8622 | 0.885 | 0.5787 |
| hsCRP mg/L | 1.20 0.51/3.28 | 1.35 0.78/2.95 | 1.98 1.07/5.06 | 0.0954 | 1.0000 | 0.1227 | 0.3046 |
| Creatinine mg/dL | 0.79 (0.08) | 0.77 (0.09) | 0.73 (0.11) | 0.0911 | 0.7033 | 0.079 | 0.3425 |
| eGFR (mL/min/1.73 m2) | 77.25 (9.34) | 79.60 (10.61) | 83.28 (13.33) | 0.1735 | 0.7458 | 0.1530 | 0.4826 |
| Albumin g/dL | 3.99 (0.33) | 3.94 (0.34) | 3.66 (0.30) | 0.0015 | 0.8465 | 0.0024 | 0.0109 |
| Glucose mg/dL | 90.00 84.00/104.50 | 90.00 84.00/93.00 | 87.00 84.00/94.00 | 0.6410 | 1.0000 | 1.0000 | 1.0000 |
| Insulin mU/mL | 8.05 5.05/12.90 | 7.20 5.60/10.50 | 7.30 5.80/9.50 | 0.8951 | 1.0000 | 1.0000 | 1.0000 |
| HOMA-IR | 1.89 1.16/3.11 | 1.67 1.27/2.34 | 1.62 1.22/2.07 | 0.7637 | 1.0000 | 1.0000 | 1.0000 |
| Ghrelin pg/mL | 10,000.00 9758.00/10,000.00 | 10,000.00 9626.00/10,000.00 | 9748.00 9012.00/10,000.00 | 0.0397 | 1.0000 | 0.0698 | 0.3390 |
| Leptin ng/mL | 9.17 2.24/12.17 | 8.22 2.76/12.56 | 12.32 7.71/14.15 | 0.0575 | 1.0000 | 0.1148 | 0.1187 |
| Adiponectin ng/mL | 23,982.50 19,090.00/25,622.50 | 24,790.00 23,110.00/26,185.00 | 24,825.00 22,175.00/28,355.00 | 0.1979 | 0.6362 | 0.2392 | 1.0000 |
| Omentin ng/mL | 94.79 75.60/125.80 | 107.50 79.97/122.30 | 104.60 74.90/151.90 | 0.8935 | 1.0000 | 1.0000 | 1.0000 |
| Visfatin ng/mL | 17,855.00 11,730.00/25,270.00 | 20,815.00 15,535.00/31,080.00 | 19,540.00 9660.00/26,950.00 | 0.4132 | 0.6718 | 1.0000 | 0.8558 |
| WHR | 0.87 (0.07) | 0.88 (0.08) | 0.91 (0.07) | 0.1279 | 0.8795 | 0.1281 | 0.2977 |
| Variable/ Unit | Age | ||||||
|---|---|---|---|---|---|---|---|
| 60–69 n = 25 | 70–79 n = 27 | 80–89 n = 22 | p-Value | I vs. II p-Value | I vs. III p-Value | II vs. III p-Value | |
| BMI kg/m2 | 29.00 25.70/32.80 | 27.80 25.30/32.00 | 27.35 22.00/31.20 | 0.3653 | 1.0000 | 0.487 | 1.0000 |
| hsCRP mg/L | 1.35 0.87/3.33 | 1.47 0.78/3.41 | 1.49 1.03/4.37 | 0.9085 | 1.0000 | 1.0000 | 1.0000 |
| Vitamin D ng/mL | 20.90 15.70.24.50 | 19.20 14.70/22.40 | 13.20 8.80/18.10 | 0.0082 | 1.0000 | 0.0081 | 0.0686 |
| Creatinine mg/dL | 0.78 (0.10) | 0.76 (0.08) | 0.75 (0.11) | 0.5374 | 0.9169 | 0.5335 | 0.7636 |
| eGFR (mL/min/1.73 m2) | 80.24 (10.82) | 79.33 (10.37) | 80.82 (13.44) | 0.9009 | 0.9566 | 0.9839 | 0.8950 |
| Albumin g/dL | 4.05 (0.36) | 3.85 (0.32) | 3.65 (0.28) | 0.0004 | 0.0855 | 0.0005 | 0.1108 |
| Glucose mg/dL | 91.00 85.00/105.00 | 92.00 85.00/100.00 | 85.00 82.00/89.00 | 0.0677 | 1.0000 | 0.1378 | 0.1148 |
| Insulin mU/mL | 7.80 6.20/10.70 | 8.20 5.80/14.50 | 6.35 4.40/7.80 | 0.0208 | 1.0000 | 0.0638 | 0.0315 |
| HOMA-IR | 2.04 1.39/2.40 | 1.84 1.37/3.72 | 1.35 0.97/1.66 | 0.0079 | 1.0000 | 0.0202 | 0.0178 |
| Ghrelin pg/mL | 10,000.00 10,000.00/10,000.00 | 9959.00 9214.00/10,000.00 | 9727.00 8394.00/10,000.00 | 0.0086 | 0.2077 | 0.0161 | 0.8437 |
| Leptin ng/mL | 12.56 5.23/13.36 | 10.48 2.76/12.56 | 8.21 2.75/12.32 | 0.4296 | 1.0000 | 0.5979 | 1.0000 |
| Adiponectin ng/mL | 23,675.00 19,515.00/25,145.00 | 25,170.00 23,750.00/26,755.00 | 24,187.00 21,165.00/27,380.00 | 0.0627 | 0.0582 | 0.3989 | 1.0000 |
| Omentin ng/mL | 103.30 79.47/124.00 | 101.70 69.41/115.40 | 111.05 59.22/154.50 | 0.4153 | 0.7743 | 1.0000 | 0.7653 |
| Visfatin ng/mL | 19,520.00 8955.00/24,655.00 | 20,625.00 12,519.00/29,225.00 | 21050.00 11,100.00/41,675.00 | 0.3391 | 0.7445 | 0.52 | 1.0000 |
| WHR | 0.89 (0.06) | 0.89 (0.09) | 0.89 (0.07) | 0.9933 | 0.9929 | 0.9984 | 0.9986 |
| ROC Data | Stimulant | Destimulant | Destimulant | Destimulant | Stimulant | Destimulant | Stimulant | Stimulant | Destimulant | Destimulant |
|---|---|---|---|---|---|---|---|---|---|---|
| hsCRP mg/L | Creatinine mg/dL | Albumin g/dL | Glucose mg/dL | Insulin mU/mL | Ghrelin pg/mL | Leptin ng/mL | Adiponectin ng/mL | Omentin ng/mL | Visfatin ng/mL | |
| AUC | 0.605 | 0.606 | 0.662 | 0.572 | 0.503 | 0.598 | 0.685 | 0.579 | 0.519 | 0.529 |
| Youden index | 0.26 | 0.26 | 0.28 | 0.19 | 0.15 | 0.18 | 0.31 | 0.21 | 0.18 | 0.17 |
| Cut-off point | 1.06 | 0.72 | 3.70 | 97.0 | 4.70 | 8394.00 | 7.38 | 19,670.00 | 88.13 | 9660.00 |
| Sensitivity (%) | 74.4 | 46.2 | 56.4 | 82.1 | 89.7 | 17.9 | 79.5 | 94.9 | 46.2 | 25.6 |
| Specificity (%) | 51.4 | 80.0 | 71.4 | 37.1 | 25.7 | 100 | 51.4 | 25.7 | 71.4 | 91.4 |
| Positive predictive value (%) | 63.0 | 72.0 | 68.8 | 59.3 | 57.4 | 100 | 64.6 | 58.7 | 64.3 | 76.9 |
| Negative predictive value (%) | 64.3 | 57.1 | 59.5 | 65.0 | 69.2 | 52.2 | 69.2 | 81.8 | 54.3 | 52.5 |
| Accuracy (%) | 63.5 | 62.2 | 63.5 | 60.8 | 59.5 | 56.8 | 66.2 | 62.2 | 58.1 | 56.8 |
| p-value | 0.1140 | 0.1079 | 0.0103 | 0.2877 | 0.9620 | 0.1374 | 0.0291 | 0.2407 | 0.7754 | 0.6731 |
| ROC Data | Destimulant | Destimulant | Destimulant | Destimulant | Destimulant | Destimulant | Stimulant | Stimulant | Destimulant | Stimulant | Destimulant | Destimulant |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D ng/mL | Creatinine mg/dL | Calcium mmol/L | PTH pg/mL | Albumin g/dL | Glucose mg/dL | hsCRP mg/L | Ghrelin pg/mL | Leptin ng/mL | Adiponectin ng/mL | Omentin ng/mL | Visfatin ng/mL | |
| AUC | 0.638 | 0.995 | 0.62 | 0.538 | 0.557 | 0.498 | 0.563 | 0.521 | 0.511 | 0.534 | 0.556 | 0.651 |
| Youden index | 0.31 | 0.96 | 0.22 | 0.13 | 0.16 | 0.11 | 0.23 | 0.10 | 0.14 | 0.14 | 0.19 | 0.33 |
| Cut-off point | 13.90 | 0.68 | 2.20 | 78.20 | 3.60 | 88.00 | 1.91 | 9820.00 | 10.55 | 24,385.00 | 74.01 | 22,450.00 |
| Sensitivity (%) | 52.6 | 100.00 | 42.1 | 84.2 | 47.4 | 57.9 | 57.9 | 68.4 | 63.2 | 63.2 | 36.8 | 84.2 |
| Specificity (%) | 78.2 | 96.4 | 80.0 | 29.1 | 69.1 | 52.7 | 65.5 | 41.8 | 50.9 | 50.9 | 81.8 | 49.1 |
| Positive predictive value (%) | 45.5 | 90.5 | 42.1 | 29.1 | 34.6 | 29.7 | 36.7 | 28.9 | 30.8 | 30.8 | 41.2 | 36.4 |
| Negative predictive value (%) | 82.7 | 100 | 80.0 | 84.2 | 79.2 | 78.4 | 81.8 | 79.3 | 80.0 | 80.0 | 78.9 | 90.0 |
| Accuracy (%) | 71.6 | 97.3 | 70.3 | 43.5 | 63.5 | 54.1 | 63.5 | 48.6 | 54.1 | 54.1 | 70.3 | 58.1 |
| p-value | 0.0668 | <0.0001 | 0.1134 | 0.6285 | 0.4608 | 0.9807 | 0.4298 | 0.7932 | 0.8857 | 0.6556 | 0.4637 | 0.0370 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biercewicz, M.; Kwiatkowska, K.; Kędziora-Kornatowska, K.; Krintus, M.; Ślusarz, R.; Ruszkowska-Ciastek, B. Significant Interactions between Adipokines and Vitamin D Combined with the Estimated Glomerular Filtration Rate: A Geriatric Case Study. J. Clin. Med. 2023, 12, 2370. https://doi.org/10.3390/jcm12062370
Biercewicz M, Kwiatkowska K, Kędziora-Kornatowska K, Krintus M, Ślusarz R, Ruszkowska-Ciastek B. Significant Interactions between Adipokines and Vitamin D Combined with the Estimated Glomerular Filtration Rate: A Geriatric Case Study. Journal of Clinical Medicine. 2023; 12(6):2370. https://doi.org/10.3390/jcm12062370
Chicago/Turabian StyleBiercewicz, Monika, Katarzyna Kwiatkowska, Kornelia Kędziora-Kornatowska, Magdalena Krintus, Robert Ślusarz, and Barbara Ruszkowska-Ciastek. 2023. "Significant Interactions between Adipokines and Vitamin D Combined with the Estimated Glomerular Filtration Rate: A Geriatric Case Study" Journal of Clinical Medicine 12, no. 6: 2370. https://doi.org/10.3390/jcm12062370






