Betting on Your Feelings: The Interplay between Emotion and Cognition in Gambling Affective Task
Abstract
1. Introduction
1.1. Impulsivity
1.2. Emotional and Affective States
1.3. The Effect of Affective States on Decision-Making Processes
1.4. The Present Study
2. Materials and Methods
2.1. Participants and Procedure
2.2. Questionnaires
2.2.1. South Oaks Gambling Screen (SOGS, Italian Version, [56])
2.2.2. Drug Abuse Screening Test (DAST, [57])
2.2.3. Alcohol Use Disorder Identification Test (AUDIT, [58])
2.2.4. Barratt Impulsiveness Scale (BIS-11, Italian Version, [59])
2.2.5. Toronto Alexithymia Scale (TAS-20, Italian Version, [60])
2.2.6. Difficulties in Emotion Regulation Scale (DERS-36, [61])
2.2.7. Emotion Regulation Questionnaire (ERQ, Italian Version, [62])
2.2.8. Positive and Negative Affective Schedule (PANAS, Italian Version, [63])
2.3. Behavioral Tasks
2.3.1. Iowa Gambling Task (IGT, [64])
2.3.2. Game of Dice Task (GDT, [65])
2.3.3. Gambling Affective Task (GAT; Figure 2)
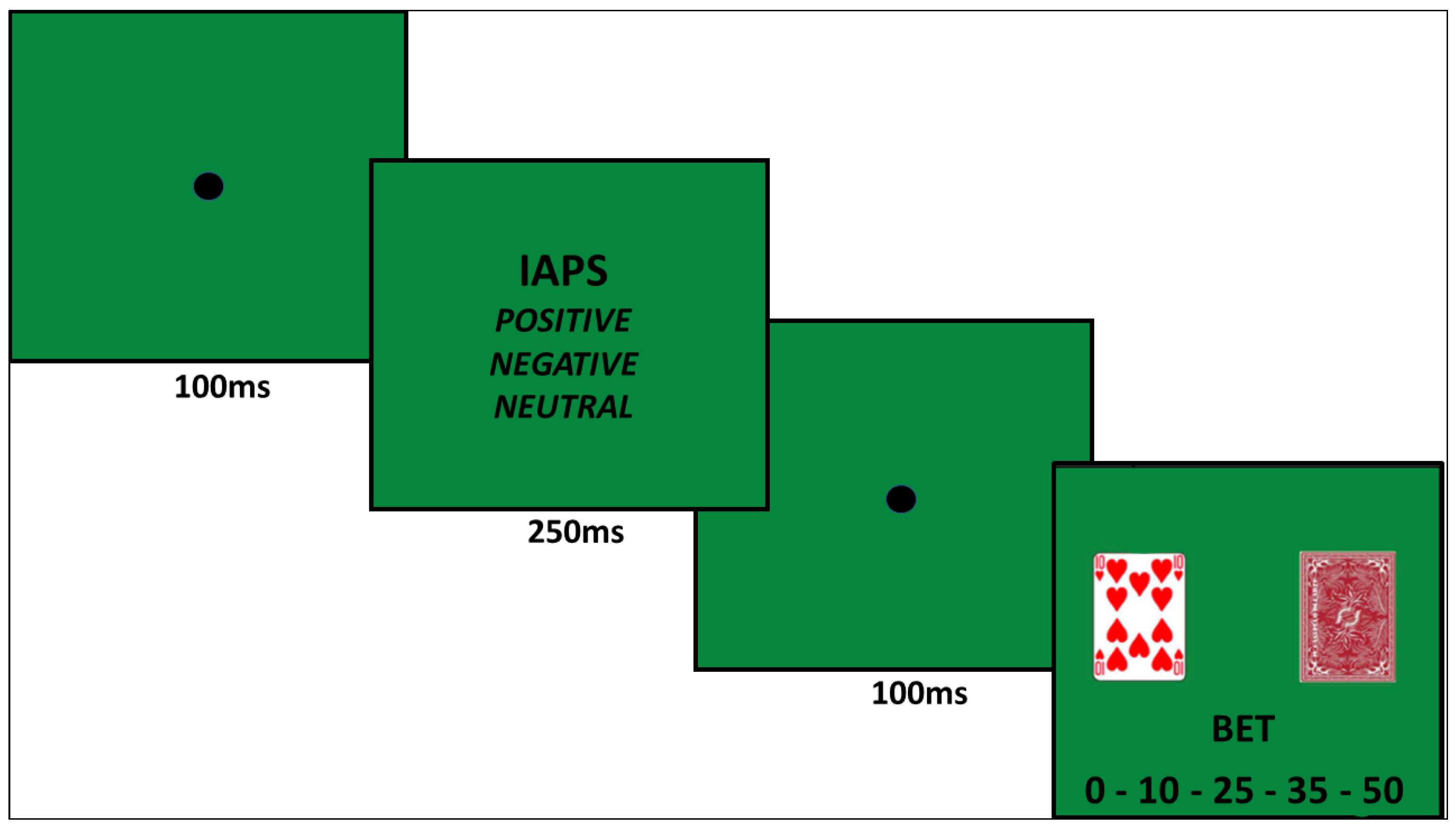
2.4. Statistical Analysis
3. Results
3.1. Impulsivity
3.2. Emotional and Affective States
3.3. Cognitive Aspects Underlying Decision-Making and Risk-Taking
3.4. Iowa Gambling Task
3.5. Game of Dice Task
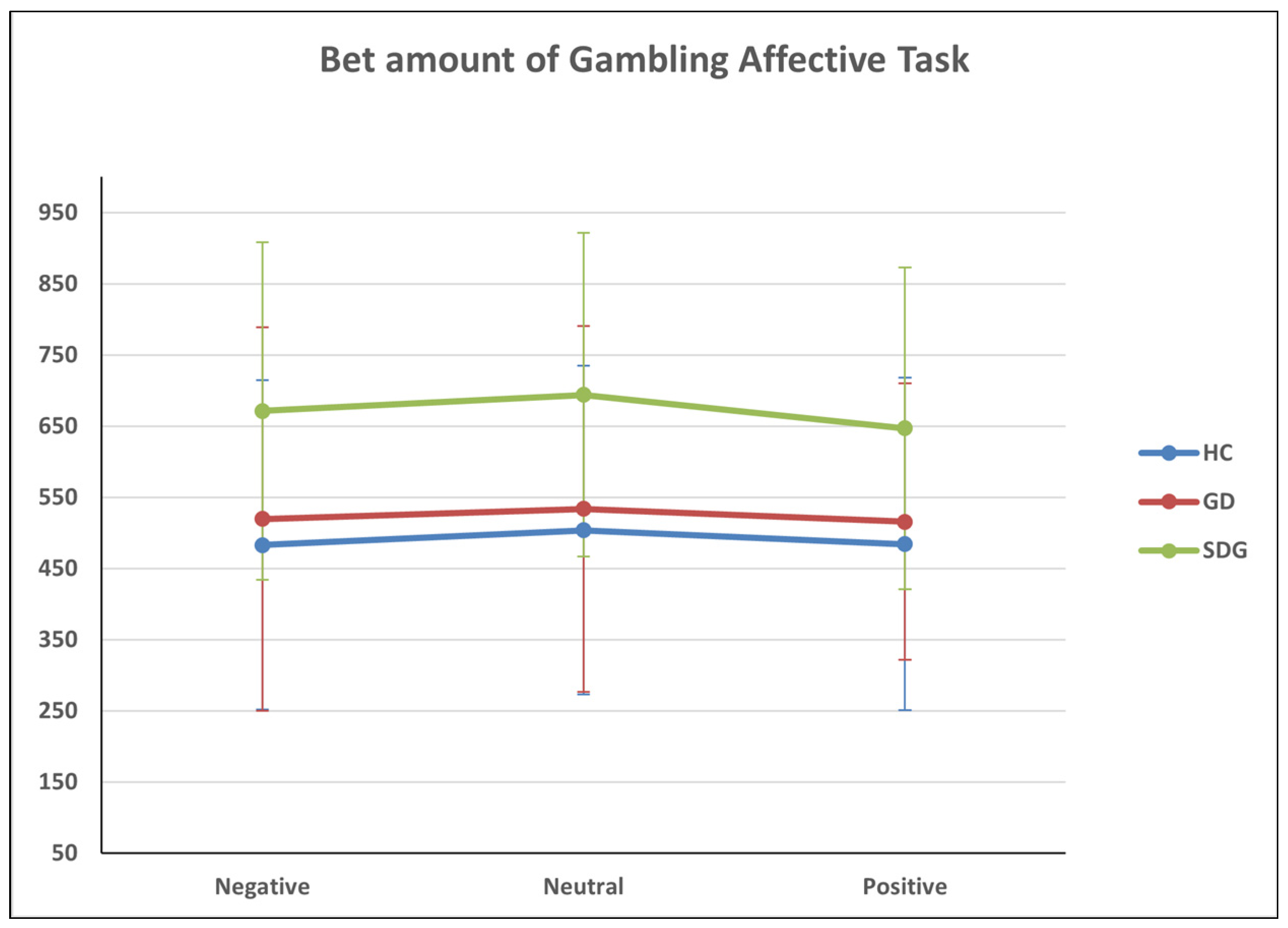
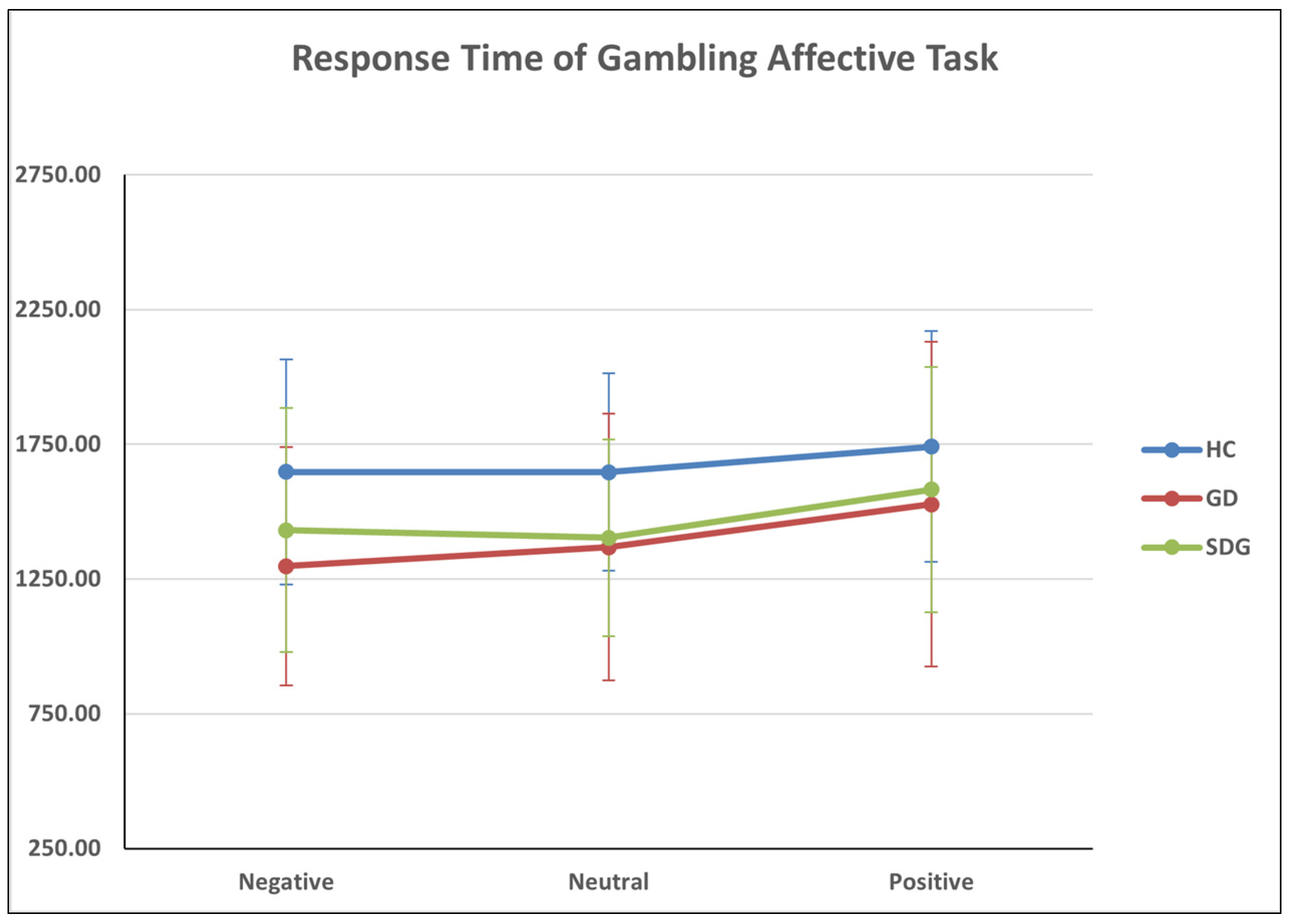
3.6. Gambling Affective Task
Amount of Risky and Safety Bets
3.7. Convergent Validity of Gambling Affective Task
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaffer, H.J.; Martin, R. Disordered Gambling: Etiology, Trajectory, and Clinical Considerations. Annu. Rev. Clin. Psychol. 2011, 7, 483–510. [Google Scholar] [CrossRef]
- Blaszczynski, A.; Nower, L. A Pathways Model of Problem and Pathological Gambling. Addiction 2002, 97, 487–499. [Google Scholar] [CrossRef]
- Clark, L.; Goudriaan, A.E. The Neuroscience and Neuropsychology of Gambling and Gambling Addiction: An Introduction to the Special Issue. Int. Gambl. Stud. 2018, 18, 173–177. [Google Scholar] [CrossRef]
- Buth, S.; Wurst, F.M.; Thon, N.; Lahusen, H.; Kalke, J. Comparative Analysis of Potential Risk Factors for At-Risk Gambling, Problem Gambling and Gambling Disorder among Current Gamblers—Results of the Austrian Representative Survey 2015. Front. Psychol. 2017, 8, 2188. [Google Scholar] [CrossRef]
- Allami, Y.; Hodgins, D.C.; Young, M.; Brunelle, N.; Currie, S.; Dufour, M.; Flores-Pajot, M.; Nadeau, L. A Meta-analysis of Problem Gambling Risk Factors in the General Adult Population. Addiction 2021, 116, 2968–2977. [Google Scholar] [CrossRef]
- Brunborg, G.S.; Hanss, D.; Mentzoni, R.A.; Molde, H.; Pallesen, S. Problem Gambling and the Five-factor Model of Personality: A Large Population-based Study. Addiction 2016, 111, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Sancho, M.; de Gracia, M.; Granero, R.; González-Simarro, S.; Sánchez, I.; Fernández-Aranda, F.; Trujols, J.; Mallorquí-Bagué, N.; Mestre-Bach, G.; del Pino-Gutiérrez, A.; et al. Differences in Emotion Regulation Considering Gender, Age, and Gambling Preferences in a Sample of Gambling Disorder Patients. Front. Psychiatry 2019, 10, 625. [Google Scholar] [CrossRef]
- Sleczka, P.; Braun, B.; Grüne, B.; Bühringer, G.; Kraus, L. Proactive Coping and Gambling Disorder among Young Men. J. Behav. Addict. 2016, 5, 639–648. [Google Scholar] [CrossRef]
- Johansson, A.; Grant, J.E.; Kim, S.W.; Odlaug, B.L.; Götestam, K.G. Risk Factors for Problematic Gambling: A Critical Literature Review. J. Gambl. Stud. 2009, 25, 67–92. [Google Scholar] [CrossRef] [PubMed]
- Dowling, N.A.; Youssef, G.J.; Greenwood, C.; Merkouris, S.S.; Suomi, A.; Room, R. The Identification of Low-Risk Gambling Limits for Specific Gambling Activities. J. Gambl. Stud. 2022, 38, 559–590. [Google Scholar] [CrossRef]
- Billieux, J.; Van der Linden, M.; Khazaal, Y.; Zullino, D.; Clark, L. Trait Gambling Cognitions Predict Near-miss Experiences and Persistence in Laboratory Slot Machine Gambling. Br. J. Psychol. 2012, 103, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Spurrier, M.; Blaszczynski, A. Risk Perception in Gambling: A Systematic Review. J. Gambl. Stud. 2014, 30, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Evenden, J. Impulsivity: A Discussion of Clinical and Experimental Findings. J. Psychopharmacol. 1999, 13, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Gerbing, D.W.; Ahadi, S.A.; Patton, J.H. Toward a Conceptualization of Impulsivity: Components across the Behavioral and Self-Report Domains. Multivar. Behav. Res. 1987, 22, 357–379. [Google Scholar] [CrossRef] [PubMed]
- Castellani, B.; Rugle, L. A Comparison of Pathological Gamblers to Alcoholics and Cocaine Misusers on Impulsivity, Sensation Seeking, and Craving. Int. J. Addict. 1995, 30, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Vitaro, F.; Brendgen, M.; Ladouceur, R.; Tremblay, R.E. Gambling, Delinquency, and Drug Use During Adolescence: Mutual Influences and Common Risk Factors. J. Gambl. Stud. 2001, 17, 171–190. [Google Scholar] [CrossRef]
- Chamberlain, S.R.; Derbyshire, K.; Daws, R.E.; Odlaug, B.L.; Leppink, E.W.; Grant, J.E. White Matter Tract Integrity in Treatment-Resistant Gambling Disorder. Br. J. Psychiatry 2016, 208, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Tiego, J.; Testa, R.; Bellgrove, M.A.; Pantelis, C.; Whittle, S. A Hierarchical Model of Inhibitory Control. Front. Psychol. 2018, 9, 1339. [Google Scholar] [CrossRef]
- Michalczuk, R.; Bowden-Jones, H.; Verdejo-Garcia, A.; Clark, L. Impulsivity and Cognitive Distortions in Pathological Gamblers Attending the UK National Problem Gambling Clinic: A Preliminary Report. Psychol. Med. 2011, 41, 2625–2635. [Google Scholar] [CrossRef]
- Goudriaan, A.E.; Yücel, M.; van Holst, R.J. Getting a Grip on Problem Gambling: What Can Neuroscience Tell Us? Front. Behav. Neurosci. 2014, 8, 141. [Google Scholar] [CrossRef]
- Moccia, L.; Pettorruso, M.; De Crescenzo, F.; De Risio, L.; Di Nuzzo, L.; Martinotti, G.; Bifone, A.; Janiri, L.; Di Nicola, M. Neural Correlates of Cognitive Control in Gambling Disorder: A Systematic Review of fMRI Studies. Neurosci. Biobehav. Rev. 2017, 78, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Mattick, R.P.; Jamadar, S.D.; Iredale, J.M. Deficits in Behavioural Inhibition in Substance Abuse and Addiction: A Meta-Analysis. Drug Alcohol Depend. 2014, 145, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Brevers, D.; Cleeremans, A.; Verbruggen, F.; Bechara, A.; Kornreich, C.; Verbanck, P.; Noël, X. Impulsive Action but Not Impulsive Choice Determines Problem Gambling Severity. PLoS ONE 2012, 7, e50647. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Robbins, T.W. Inhibition and Impulsivity: Behavioral and Neural Basis of Response Control. Prog. Neurobiol. 2013, 108, 44–79. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, K.; Hook, R.; Wickham, K.; Grant, J.E.; Chamberlain, S.R. Impulsivity in Gambling Disorder and Problem Gambling: A Meta-Analysis. Neuropsychopharmacology 2019, 44, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Bottesi, G.; Ghisi, M. Pathological Gambling: Compulsive-Impulsive Spectrum Disorder, Behavioural Addiction or Both? Phenotypic and Endophenotypic Evidence. Psychopathol. Rev. 2014, a1, 2–25. [Google Scholar] [CrossRef]
- Lorains, F.K.; Dowling, N.A.; Enticott, P.G.; Bradshaw, J.L.; Trueblood, J.S.; Stout, J.C. Strategic and Non-strategic Problem Gamblers Differ on Decision-making under Risk and Ambiguity. Addiction 2014, 109, 1128–1137. [Google Scholar] [CrossRef]
- Kräplin, A.; Goudriaan, A.E. Characteristics and Risk Factorsof Gambling Disorder as Basisfor Responsible Gambling Strategies. SUCHT 2018, 64, 247–256. [Google Scholar] [CrossRef]
- Wiehler, A.; Petzschner, F.H.; Stephan, K.E.; Peters, J. Episodic Tags Enhance Striatal Valuation Signals during Temporal Discounting in Pathological Gamblers. eNeuro 2017, 4, ENEURO.0159-17.2017. [Google Scholar] [CrossRef]
- Mallorquí-Bagué, N.; Vintró-Alcaraz, C.; Verdejo-García, A.; Granero, R.; Fernández-Aranda, F.; Magaña, P.; Mena-Moreno, T.; Aymamí, N.; Gómez-Peña, M.; Del Pino-Gutiérrez, A.; et al. Impulsivity and Cognitive Distortions in Different Clinical Phenotypes of Gambling Disorder: Profiles and Longitudinal Prediction of Treatment Outcomes. Eur. Psychiatry 2019, 61, 9–16. [Google Scholar] [CrossRef]
- Gohm, C.L.; Clore, G.L. Individual Differences in Emotional Experience: Mapping Available Scales to Processes. Pers. Soc. Psychol. Bull. 2000, 26, 679–697. [Google Scholar] [CrossRef]
- Adolphs, R. How Should Neuroscience Study Emotions? By Distinguishing Emotion States, Concepts, and Experiences. Soc. Cogn. Affect. Neurosci. 2017, 12, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Celeghin, A.; Diano, M.; Bagnis, A.; Viola, M.; Tamietto, M. Basic Emotions in Human Neuroscience: Neuroimaging and Beyond. Front. Psychol. 2017, 8, 1432. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A. A Circumplex Model of Affect. J. Pers. Soc. Psychol. 1980, 39, 1161–1178. [Google Scholar] [CrossRef]
- Stone, A.A. The Association between Perceptions of Daily Experiences and Self- and Spouse-Rated Mood. J. Res. Personal. 1981, 15, 510–522. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and Validation of Brief Measures of Positive and Negative Affect: The PANAS Scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Gendolla, G.H.E. Comment: Do Emotions Influence Action?—Of Course, They Are Hypo-Phenomena of Motivation. Emot. Rev. 2017, 9, 348–350. [Google Scholar] [CrossRef]
- Loewenstein, G.; Lerner, J.S. The Role of Affect in Decision Making. Handb. Affect. Sci. 2003, 619, 3. [Google Scholar]
- Hudson, A.; Jacques, S.; Stewart, S.H. Selective Attention to Emotional Pictures as a Function of Gambling Motives in Problem and Nonproblem Gamblers. Psychol. Addict. Behav. 2013, 27, 1079–1091. [Google Scholar] [CrossRef]
- Slovic, P.; Peters, E. Risk Perception and Affect. Curr. Dir. Psychol. Sci. 2006, 15, 322–325. [Google Scholar] [CrossRef]
- Isen, A.M.; Shalker, T.E.; Clark, M.; Karp, L. Affect, Accessibility of Material in Memory, and Behavior: A Cognitive Loop? J. Pers. Soc. Psychol. 1978, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Forgas, J.P. Mood and Judgment: The Affect Infusion Model (AIM). Psychol. Bull. 1995, 117, 39–66. [Google Scholar] [CrossRef] [PubMed]
- Isen, A.M.; Patrick, R. The Effect of Positive Feelings on Risk Taking: When the Chips Are Down. Organ. Behav. Hum. Perform. 1983, 31, 194–202. [Google Scholar] [CrossRef]
- Isen, A.M. An Influence of Positive Affect on Decision Making in Complex Situations: Theoretical Issues With Practical Implications. J. Consum. Psychol. 2001, 11, 75–85. [Google Scholar] [CrossRef]
- Lerner, J.S.; Keltner, D. Fear, Anger, and Risk. J. Pers. Soc. Psychol. 2001, 81, 146–159. [Google Scholar] [CrossRef]
- Gehring, W.J.; Willoughby, A.R. The Medial Frontal Cortex and the Rapid Processing of Monetary Gains and Losses. Science 2002, 295, 2279–2282. [Google Scholar] [CrossRef]
- Hudgens-Haney, M.E.; Hamm, J.P.; Goodie, A.S.; Krusemark, E.A.; McDowell, J.E.; Clementz, B.A. Neural Correlates of the Impact of Control on Decision Making in Pathological Gambling. Biol. Psychol. 2013, 92, 365–372. [Google Scholar] [CrossRef]
- Stanton, K.; Watson, D. Positive and Negative Affective Dysfunction in Psychopathology. Soc. Personal. Psychol. Compass 2014, 8, 555–567. [Google Scholar] [CrossRef]
- Kahneman, D. A Perspective on Judgment and Choice: Mapping Bounded Rationality. Am. Psychol. 2003, 58, 697–720. [Google Scholar] [CrossRef]
- McClure, S.M.; Bickel, W.K. A Dual-systems Perspective on Addiction: Contributions from Neuroimaging and Cognitive Training. Ann. N. Y. Acad. Sci. 2014, 1327, 62–78. [Google Scholar] [CrossRef]
- Ledgerwood, D.M.; Orr, E.S.; Kaploun, K.A.; Milosevic, A.; Frisch, G.R.; Rupcich, N.; Lundahl, L.H. Executive Function in Pathological Gamblers and Healthy Controls. J. Gambl. Stud. 2012, 28, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Berkman, E.T.; Hutcherson, C.A.; Livingston, J.L.; Kahn, L.E.; Inzlicht, M. Self-Control as Value-Based Choice. Curr. Dir. Psychol. Sci. 2017, 26, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Field, M.; Heather, N.; Murphy, J.G.; Stafford, T.; Tucker, J.A.; Witkiewitz, K. Recovery from Addiction: Behavioral Economics and Value-Based Decision Making. Psychol. Addict. Behav. 2020, 34, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Quaglieri, A.; Mari, E.; Cordellieri, P.; Paoli, E.; Dimarco, F.; Postiglione, M.; Nicolasi, G.; Fontanella, T.; Guidoni, U.; Vedovi, S.; et al. An Exploratory Study in Gambling Recovery Communities: A Comparison between “Pure” and Substance-Abusing Gamblers. J. Gambl. Issues 2021, 18–45. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013; Volume 5. [Google Scholar]
- Guerreschi, C.; Gander, S. Versione Italiana Del South Oaks Gambling Screen (SOGS). In Giocati Dal Gioco Quando Il Divertim. Diventa Una Mal. Il Gioco D’azzardo Patol; Lesieur, D.H.R., Blume, S.B., Eds.; San Paolo: Milano, Italy, 2000; pp. 137–142. [Google Scholar]
- Skinner, H.A. The Drug Abuse Screening Test. Addict. Behav. 1982, 7, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Piccinelli, M.; Tessari, E.; Bortolomasi, M.; Piasere, O.; Semenzin, M.; Garzotto, N.; Tansella, M. Efficacy of the Alcohol Use Disorders Identification Test as a Screening Tool for Hazardous Alcohol Intake and Related Disorders in Primary Care: A Validity Study. BMJ 1997, 314, 420. [Google Scholar] [CrossRef] [PubMed]
- Fossati, A.; Di Ceglie, A.; Acquarini, E.; Barratt, E.S. Psychometric Properties of an Italian Version of the Barratt Impulsiveness Scale-11 (BIS-11) in Nonclinical Subjects. J. Clin. Psychol. 2001, 57, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Bressi, C.; Taylor, G.; Parker, J.; Bressi, S.; Brambilla, V.; Aguglia, E.; Allegranti, I.; Bongiorno, A.; Giberti, F.; Bucca, M.; et al. Cross Validation of the Factor Structure of the 20-Item Toronto Alexithymia Scale: An Italian Multicenter Study. J. Psychosom. Res. 1996, 41, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Sighinolfi, C.; Norcini Pala, A.; Chiri, L.R.; Marchetti, I.; Sica, C. Difficulties in Emotion Regulation Scale (DERS): The Italian Translation and Adaptation. Psicoter. Cogn. Comport. 2010, 16, 141–170. [Google Scholar]
- Balzarotti, S.; John, O.P.; Gross, J.J. An Italian Adaptation of the Emotion Regulation Questionnaire. Eur. J. Psychol. Assess. 2010, 26, 61–67. [Google Scholar] [CrossRef]
- Terraciano, A.; McCrae, R.R.; Costa Jr, P.T. Factorial and Construct Validity of the Italian Positive and Negative Affect Schedule (PANAS). Eur. J. Psychol. Assess. 2003, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A.; Damasio, H.; Tranel, D.; Damasio, A.R. Deciding Advantageously Before Knowing the Advantageous Strategy. Science 1997, 275, 1293–1295. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.; Recknor, E.C.; Grabenhorst, F.; Bechara, A. Decisions under Ambiguity and Decisions under Risk: Correlations with Executive Functions and Comparisons of Two Different Gambling Tasks with Implicit and Explicit Rules. J. Clin. Exp. Neuropsychol. 2007, 29, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. NIMH Cent. Study Emot. Atten. 1997, 1, 3. [Google Scholar]
- Quaglieri, A.; Mari, E.; Boccia, M.; Piccardi, L.; Guariglia, C.; Giannini, A.M. Brain Network Underlying Executive Functions in Gambling and Alcohol Use Disorders: An Activation Likelihood Estimation Meta-Analysis of fMRI Studies. Brain Sci. 2020, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Zois, E.; Kortlang, N.; Vollstädt-Klein, S.; Lemenager, T.; Beutel, M.; Mann, K.; Fauth-Bühler, M. Decision-making Deficits in Patients Diagnosed with Disordered Gambling Using the Cambridge Gambling Task: The Effects of Substance Use Disorder Comorbidity. Brain Behav. 2014, 4, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Rogier, G.; Velotti, P. Conceptualizing Gambling Disorder with the Process Model of Emotion Regulation. J. Behav. Addict. 2018, 7, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Vitaro, F.; Arseneault, L.; Tremblay, R.E. Dispositional Predictors of Problem Gambling in Male Adolescents. Am. J. Psychiatry 1997, 154, 1769–1770. [Google Scholar] [CrossRef] [PubMed]
- Eysenck, S.B.G.; Eysenck, H.J.; Barrett, P. A Revised Version of the Psychoticism Scale. Personal. Individ. Differ. 1985, 6, 21–29. [Google Scholar] [CrossRef]
- Navas, J.F.; Verdejo-García, A.; LÓpez-GÓmez, M.; Maldonado, A.; Perales, J.C. Gambling with Rose-Tinted Glasses on: Use of Emotion-Regulation Strategies Correlates with Dysfunctional Cognitions in Gambling Disorder Patients. J. Behav. Addict. 2016, 5, 271–281. [Google Scholar] [CrossRef]
- Rogier, G.; Capone, A.; Velotti, P. Emotion Regulation Strategies and Dissociation in Gambling Disorder. Int. Gambl. Stud. 2022, 22, 18–36. [Google Scholar] [CrossRef]
- Jara-Rizzo, M.F.; Navas, J.F.; Catena, A.; Perales, J.C. Types of Emotion Regulation and Their Associations with Gambling: A Cross-Sectional Study with Disordered and Non-Problem Ecuadorian Gamblers. J. Gambl. Stud. 2019, 35, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Lara, C.M.; Navas, J.F.; Perales, J.C. The Paradoxical Relationship between Emotion Regulation and Gambling-Related Cognitive Biases. PLoS ONE 2019, 14, e0220668. [Google Scholar] [CrossRef] [PubMed]
- Velotti, P.; Rogier, G. An Exploratory Study of the Role Played by Hedonic Dysregulation in Gambling Disorder. Int. Gambl. Stud. 2021, 21, 1–16. [Google Scholar] [CrossRef]
- Bechara, A. The Role of Emotion in Decision-Making: Evidence from Neurological Patients with Orbitofrontal Damage. Brain Cogn. 2004, 55, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A.; Damasio, A.R.; Damasio, H.; Anderson, S.W. Insensitivity to Future Consequences Following Damage to Human Prefrontal Cortex. Cognition 1994, 50, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A. Decision Making, Impulse Control and Loss of Willpower to Resist Drugs: A Neurocognitive Perspective. Nat. Neurosci. 2005, 8, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Goudriaan, A.E.; Oosterlaan, J.; de Beurs, E.; van den Brink, W. Decision Making in Pathological Gambling: A Comparison between Pathological Gamblers, Alcohol Dependents, Persons with Tourette Syndrome, and Normal Controls. Cogn. Brain Res. 2005, 23, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.E.; Brewer, J.A.; Potenza, M.N. The Neurobiology of Substance and Behavioral Addictions. CNS Spectr. 2006, 11, 924–930. [Google Scholar] [CrossRef]
- Leeman, R.F.; Potenza, M.N. Similarities and Differences between Pathological Gambling and Substance Use Disorders: A Focus on Impulsivity and Compulsivity. Psychopharmacology 2012, 219, 469–490. [Google Scholar] [CrossRef]
- Rash, C.; Weinstock, J.; Van Patten, R. A Review of Gambling Disorder and Substance Use Disorders. Subst. Abus. Rehabil. 2016, 3, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Cavedini, P.; Riboldi, G.; Keller, R.; D’Annucci, A.; Bellodi, L. Frontal Lobe Dysfunction in Pathological Gambling Patients. Biol. Psychiatry 2002, 51, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.J.; Usdan, S.; Cremeens, J.; Vail-Smith, K. Disordered Gambling and Co-Morbidity of Psychiatric Disorders among College Students: An Examination of Problem Drinking, Anxiety and Depression. J. Gambl. Stud. 2014, 30, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Silveri, M.M.; Dager, A.D.; Cohen-Gilbert, J.E.; Sneider, J.T. Neurobiological Signatures Associated with Alcohol and Drug Use in the Human Adolescent Brain. Neurosci. Biobehav. Rev. 2016, 70, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Arndt, C.; Lischetzke, T.; Crayen, C.; Eid, M. The Assessment of Emotional Clarity via Response Times to Emotion Items: Shedding Light on the Response Process and Its Relation to Emotion Regulation Strategies. Cogn. Emot. 2018, 32, 530–548. [Google Scholar] [CrossRef]
- Isen, A.M.; Nygren, T.E.; Ashby, F.G. Influence of Positive Affect on the Subjective Utility of Gains and Losses: It Is Just Not Worth the Risk. J. Pers. Soc. Psychol. 1988, 55, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Hill, D. Affect Regulation Theory: A Clinical Model (Norton Series on Interpersonal Neurobiology); WW Norton & Company: New York, NY, USA, 2015; ISBN 0-393-71132-3. [Google Scholar]
- Rothermund, K. Motivation and Attention: Incongruent Effects of Feedback on the Processing of Valence. Emotion 2003, 3, 223–238. [Google Scholar] [CrossRef]
- Rothermund, K.; Voss, A.; Wentura, D. Counter-Regulation in Affective Attentional Biases: A Basic Mechanism That Warrants Flexibility in Emotion and Motivation. Emotion 2008, 8, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Frijda, N.H. The Emotions; Cambridge University Press: Cambridge, UK, 1986; ISBN 0-521-31600-6. [Google Scholar]
- Alessi, S.M.; Petry, N.M. Pathological Gambling Severity Is Associated with Impulsivity in a Delay Discounting Procedure. Behav. Process. 2003, 64, 345–354. [Google Scholar] [CrossRef]
- Dixon, M.R.; Marley, J.; Jacobs, E.A. Delay Discounting by Pathological Gamblers. J. Appl. Behav. Anal. 2003, 36, 449–458. [Google Scholar] [CrossRef]
- Reynolds, B. A Review of Delay-Discounting Research with Humans: Relations to Drug Use and Gambling. Behav. Pharmacol. 2006, 17, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Blaszczynski, A. The Longitudinal Relationships Between Psychiatric Disorders and Gambling Disorders. Int. J. Ment. Health Addict. 2018, 16, 16–44. [Google Scholar] [CrossRef]
- van Holst, R.J.; van den Brink, W.; Veltman, D.J.; Goudriaan, A.E. Why Gamblers Fail to Win: A Review of Cognitive and Neuroimaging Findings in Pathological Gambling. Neurosci. Biobehav. Rev. 2010, 34, 87–107. [Google Scholar] [CrossRef] [PubMed]
- van Holst, R.J.; van den Brink, W.; Veltman, D.J.; Goudriaan, A.E. Brain Imaging Studies in Pathological Gambling. Curr. Psychiatry Rep. 2010, 12, 418–425. [Google Scholar] [CrossRef]
- Conversano, C.; Marazziti, D.; Carmassi, C.; Baldini, S.; Barnabei, G.; Dell’Osso, L. Pathological Gambling: A Systematic Review of Biochemical, Neuroimaging, and Neuropsychological Findings. Harv. Rev. Psychiatry 2012, 20, 130–148. [Google Scholar] [CrossRef]


| GD Means (SD) | SDG Means (SD) | HC Means (SD) | F | p | ||
|---|---|---|---|---|---|---|
| Age | 37.33 (11.18) | 35.10 (11.88) | 34.95 (12.80) | 0.282 | 0.755 | |
| χ2 | p | |||||
| Educational level | Primary school | 0 (0%) | 1 (5%) | 0 (0%) | 27.234 | 0.001 |
| Secondary school 1° | 9 (37.5%) | 11 (55%) | 0 (0%) | |||
| Secondary school 2° | 15 (62.5%) | 7 (35%) | 13 (65%) | |||
| Bachelor’s degree | 0 (0%) | 1 (5%) | 3 (15%) | |||
| Master’s degree | 0 (0%) | 0 (0%) | 4 (20%) | |||
| GD Means (SD) | SDG Means (SD) | HC Means (SD) | F | p | |
|---|---|---|---|---|---|
| SOGS | 13.33 (3.54) | 10.95 (3.97) | 0.35 (0.59) | 15.866 | <0.001 |
| AUDIT | 2.17 (2.70) | 13.70 (12.91) | 2.70 (1.46) | 103.364 | <0.001 |
| DAST-10 | - | 6.85 (1.87) | - | - | - |
| Groups | M | SD | F | p | η2 | |
|---|---|---|---|---|---|---|
| BIS-11(II) Attentional Impulsivity | HC | 12.55 | 3.27 | 2.435 | 0.096 | 0.075 |
| GD | 14.96 | 3.37 | ||||
| SDG | 14.55 | 3.07 | ||||
| BIS-11(II) Motor Impulsivity | HC | 17.90 | 3.45 | 8.979 | <0.001 | 0.230 |
| GD | 24.42 | 6.06 | ||||
| SDG | 24.65 | 4.67 | ||||
| BIS-11(II) Non Planning Impulsivity | HC | 22.25 | 4.49 | 10.554 | <0.001 | 0.260 |
| GD | 29.54 | 5.36 | ||||
| SDG | 29.15 | 4.30 |
| Groups | M | SD | F | p | η2 | |
|---|---|---|---|---|---|---|
| DERS Non acceptance | HC | 12.00 | 4.90 | 1.679 | 0.195 | 0.053 |
| GD | 13.75 | 5.25 | ||||
| SDG | 12.40 | 4.44 | ||||
| DERS Goals | HC | 12.60 | 486 | 0.210 | 0.811 | 0.007 |
| GD | 11.50 | 4.10 | ||||
| SDG | 11.95 | 3.25 | ||||
| DERS Strategies | HC | 14.50 | 5.46 | 1.251 | 0.294 | 0.040 |
| GD | 17.25 | 7.52 | ||||
| SDG | 18.50 | 6.50 | ||||
| DERS Impulse | HC | 9.80 | 3.87 | 1.473 | 0.238 | 0.047 |
| GD | 12.00 | 5.32 | ||||
| SDG | 13.45 | 5.47 | ||||
| DERS Clarity | HC | 9.25 | 3.95 | 2.019 | 0.142 | 0.063 |
| GD | 11.58 | 5.68 | ||||
| SDG | 12.70 | 4.04 | ||||
| DERS Awareness | HC | 12.45 | 3.75 | 1.839 | 0.168 | 0.058 |
| GD | 15.88 | 5.23 | ||||
| SDG | 15.60 | 3.36 | ||||
| PANAS Positive | HC | 34.95 | 6.45 | 0.189 | 0.828 | 0.006 |
| GD | 35.79 | 7.37 | ||||
| SDG | 35.75 | 5.93 | ||||
| PANAS Negative | HC | 20.35 | 5.24 | 3.534 | 0.035 | 0.105 |
| GD | 27.21 | 9.61 | ||||
| SDG | 22.40 | 7.88 | ||||
| ERQ Reappraisal | HC | 30.50 | 7.21 | 0.150 | 0.861 | 0.005 |
| GD | 27.67 | 8.77 | ||||
| SDG | 27.45 | 9.53 | ||||
| ERQ Suppression | HC | 16.05 | 5.46 | 0.636 | 0.533 | 0.021 |
| GD | 13.04 | 6.69 | ||||
| SDG | 13.30 | 4.99 | ||||
| TAS Difficulty identifies feeling | HC | 12.90 | 5.17 | 1.077 | 0.347 | 0.035 |
| GD | 16.96 | 7.92 | ||||
| SDG | 17.95 | 6.21 | ||||
| TAS Difficulty Describing Feelings | HC | 13.55 | 5.49 | 1.158 | 0.321 | 0.037 |
| GD | 15.29 | 6.20 | ||||
| SDG | 13.60 | 4.63 | ||||
| TAS External-oriented thinking | HC | 16.00 | 4.41 | 1.879 | 0.162 | 0.059 |
| GD | 18.00 | 5.78 | ||||
| SDG | 19.80 | 4.77 |
| Group | M | SD | F | p | η2 | |
|---|---|---|---|---|---|---|
| N_wins | HC | 10.30 | 1.867 | 0.841 | 0.436 | 0.027 |
| GD | 9.46 | 3.092 | ||||
| SDG | 9.20 | 3.238 | ||||
| N_losses | HC | 7.70 | 1.867 | 0.841 | 0.436 | 0.027 |
| GD | 8.54 | 3.092 | ||||
| SDG | 8.80 | 3.238 | ||||
| RaW | HC | 0.60 | 1.046 | 1.433 | 0.246 | 0.045 |
| GD | 1.25 | 1.595 | ||||
| SDG | 1.35 | 1.843 | ||||
| RaL | HC | 1.45 | 1.356 | 3.462 | 0.038 | 0.102 |
| GD | 3.63 | 4.470 | ||||
| SDG | 4.40 | 4.210 | ||||
| SaW | HC | 9.10 | 2.269 | 1.187 | 0.312 | 0.039 |
| GD | 8.50 | 3.700 | ||||
| SDG | 7.40 | 4.321 | ||||
| SaL | HC | 5.85 | 1.348 | 7.534 | 0.001 | 0.203 |
| GD | 4.73 | 1.486 | ||||
| SDG | 3.85 | 2.007 | ||||
| N_R | HC | 2.40 | 2.210 | 3.179 | 0.049 | 0.094 |
| GD | 5.38 | 5.948 | ||||
| SDG | 6.20 | 5.827 | ||||
| N_S | HC | 15.60 | 2.210 | 3.540 | 0.035 | 0.107 |
| GD | 13.77 | 4.710 | ||||
| SDG | 11.80 | 5.827 |
| Group | Mean | SD | |
|---|---|---|---|
| BET_NEGATIVE | HC | 483.25 | 8.26 |
| GD | 519.56 | 8.63 | |
| SDG | 671.25 | 7.86 | |
| Total | 556.19 | 256.51 | |
| BET_NEUTRAL | HC | 504.00 | 227.26 |
| GD | 533.70 | 257.13 | |
| SDG | 694.00 | 230.99 | |
| Total | 575.16 | 249.93 | |
| BET_POSITIVE | HC | 484.50 | 226.31 |
| GD | 515.87 | 194.47 | |
| SDG | 647.00 | 233.77 | |
| Total | 547.54 | 225.18 |
| Group | Mean | SD | |
|---|---|---|---|
| RT_NEGATIVE | HC | 1647.81 | 418.28 |
| GD | 1297.78 | 441.76 | |
| SDG | 1431.36 | 452.71 | |
| Total | 1451.31 | 454.97 | |
| RT_NEUTRAL | HC | 1646.8 | 365.75 |
| GD | 1368.72 | 494.53 | |
| SDG | 1402.71 | 364.92 | |
| Total | 1467.80 | 428.89 | |
| RT_POSITIVE | HC | 1741.59 | 428.59 |
| GD | 1527.82 | 602.53 | |
| SDG | 1582.70 | 455.32 | |
| Total | 1613.10 | 506.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mari, E.; Cricenti, C.; Boccia, M.; Zucchelli, M.M.; Nori, R.; Piccardi, L.; Giannini, A.M.; Quaglieri, A. Betting on Your Feelings: The Interplay between Emotion and Cognition in Gambling Affective Task. J. Clin. Med. 2024, 13, 2990. https://doi.org/10.3390/jcm13102990
Mari E, Cricenti C, Boccia M, Zucchelli MM, Nori R, Piccardi L, Giannini AM, Quaglieri A. Betting on Your Feelings: The Interplay between Emotion and Cognition in Gambling Affective Task. Journal of Clinical Medicine. 2024; 13(10):2990. https://doi.org/10.3390/jcm13102990
Chicago/Turabian StyleMari, Emanuela, Clarissa Cricenti, Maddalena Boccia, Micaela Maria Zucchelli, Raffaella Nori, Laura Piccardi, Anna Maria Giannini, and Alessandro Quaglieri. 2024. "Betting on Your Feelings: The Interplay between Emotion and Cognition in Gambling Affective Task" Journal of Clinical Medicine 13, no. 10: 2990. https://doi.org/10.3390/jcm13102990
APA StyleMari, E., Cricenti, C., Boccia, M., Zucchelli, M. M., Nori, R., Piccardi, L., Giannini, A. M., & Quaglieri, A. (2024). Betting on Your Feelings: The Interplay between Emotion and Cognition in Gambling Affective Task. Journal of Clinical Medicine, 13(10), 2990. https://doi.org/10.3390/jcm13102990










