Abstract
Introduction: Comorbid insomnia and obstructive sleep apnea (COMISA) is not a well-identified sleep disorder, despite having a significant impact on health. This study investigates the relationship between sleep bruxism (SB) and sleep architecture in patients with COMISA, obstructive sleep apnea (OSA), and in those without any sleep disorders. Methods: 119 patients were included in the study and divided into three groups: OSA, COMISA, and a control group. Polysomnographic (PSG) examination provided parameters related to sleep architecture, OSA, and characteristics of SB. Results: The bruxism episode index (BEI) and other SB parameters were not found to be statistically different between the three groups (p > 0.05). There was no statistical difference in measured sleep architecture between the COMISA and OSA groups (p > 0.05). In comparison to the control group, participants in the COMISA group were found to have an increased apnea–hypopnea index (AHI), oxygen desaturation index (ODI), respiratory disturbance index (RDI), all arousals (AA), and respiratory arousals (RA) (p < 0.05). Among COMISA patients, AA and RA were shown to have a positive linear correlation with the number of bradycardia events per hour (r = 0.49, r = 0.48, p < 0.05). Conclusions: SB does not occur in patients with COMISA more frequently than in patients with OSA or those without any sleep disorders. PSG parameters are not specific for COMISA; therefore, in order to differentiate this disorder from OSA alone, a comprehensive patient assessment has to be performed.
1. Introduction
The term “COMISA”, which refers to concurrent insomnia and obstructive sleep apnea (OSA), is a relatively new term, having first been mentioned in the literature only in 2017 [1]. It is worth noting that the first study describing the co-existence of insomnia and OSA had been published almost 45 years earlier, in 1973 [2]. Unfortunately, despite the high prevalence of OSA coexisting with insomnia, there have been very few studies over the years regarding this subject [3]. OSA is a sleep disorder characterized by a repetitive collapse of the upper airway, leading to partial cessation or a complete block of airflow during sleep [4]. This leads to arterial oxygen desaturation and changes in intrathoracic pressure, ultimately generating nocturnal arousals; OSA affects about 9% to 38% of the population [5,6]. Clinical symptoms include snoring, fatigue, excessive daytime sleepiness, and a reduced quality of life [7,8]. Insomnia is the second most commonly occurring sleep disorder worldwide and consists of problems in initiating or maintaining sleep, despite having optimal conditions and opportunities for sleep [9]. Thirty percent of the population will experience symptoms of insomnia at least once in their lifetime. Waking up too early and experiencing poor quality of sleep are symptoms that are also indicative of insomnia [10].
After researchers’ attention had been drawn to the occurrence of OSA with insomnia and after further research had been conducted, a number of discoveries were published. First of all, the prevalence of COMISA continues to be high, varying from 29.2% to 38%, and up to as many as 50% of participants in certain studies [11,12]. Patients have reported more intense symptoms (such as increased daytime sleepiness or daytime impairments, for instance, fatigue, memory difficulties, and decreased mood) and an overall reduced quality of life and sleep compared to patients suffering from either insomnia or OSA alone [13,14]. Males have been found to be more commonly affected by the decreased quality of life and fatigue than females. Furthermore, these characteristics have been found to occur more frequently in posttraumatic stress disorder (PTSD) patients, military personnel, or war veterans [15,16,17]. Additionally, COMISA has been found to significantly increase the risk of cardiovascular disease (CVD), hypertension, and mortality. It has also been associated with depression, stress, anxiety, or diabetes type 2 more so than OSA or insomnia alone [18,19,20,21].
However, in the literature, the relationship between COMISA and sleep bruxism (SB) has yet to be explored. SB is a condition characterized by rhythmic (phasic) or nonrhythmic (tonic) masticatory muscle activity during sleep that ultimately results in the grinding or clenching of teeth and/or bracing or thrusting of the mandible [22]. Bruxism has been estimated to affect around 13% of the population; however, it is not considered to be a movement or sleep disorder in otherwise healthy individuals [23]. SB has been found to occur more frequently in patients with particular sleep disorders, such as OSA or restless leg syndrome (RLS) [24,25]. Additionally, several studies have shown a positive connection between OSA and SB [26,27]; however, OSA has not always been linked with SB [28]. There exists a theoretical “temporal” mechanism against OSA in concomitant SB and OSA where, during bruxism episodes, mandible protrusion maintains the patency of the upper airway tract [29,30]. COMISA syndrome consists of OSA and insomnia alone but it has not been estimated as to whether SB could have any connection in COMISA using a gold standard method to diagnose sleep disorders such as polysomnography with video-recording [31].
Therefore, to examine the relationship between SB and COMISA syndrome using valid methods, the objective of our study was to investigate the potential characteristics of SB in patients with COMISA syndrome vs. an OSA group vs. patients without any sleep disorders. Additionally, the full sleep architecture from the PSG examination was compared between these groups.
2. Materials and Methods
Qualified researchers from the Sleep Laboratory of the Department and Clinic of Internal Medicine, Occupational Diseases, Hypertension and Clinical Oncology at Wroclaw Medical University, Poland, recruited 119 patients for the study. Among these patients, 73 were male and 60 female. The patients were divided into three groups: an obstructive sleep apnea group consisting of 42 patients, a COMISA group including 24 patients, and a control group (patients with no sleep disorders) with 53 subjects. (Figure 1).
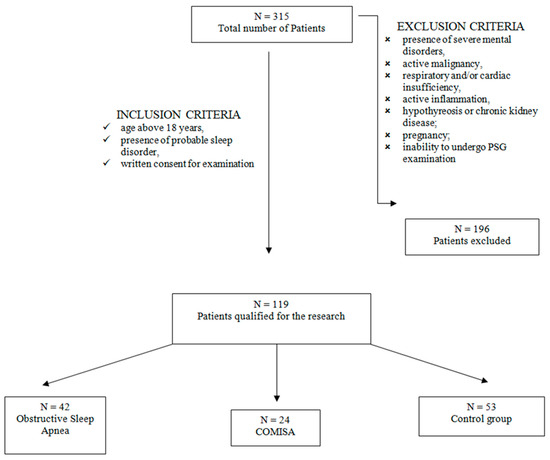
Figure 1.
Flowchart for accepting patients into the study.
This study was in accordance with the Declaration of Helsinki. Before participating in the examination, all patients had signed an informed written consent form. The study obtained approval from the Ethical Committee at Wroclaw Medical University (no. KB-523/2021). The research is registered at clinicaltrials.gov, and the examination reference number is NCT04937036.
2.1. Study Participants
In this present study, patients were admitted to the Sleep Laboratory at the Department and Clinic of Internal Medicine, Occupational Diseases, Hypertension, and Clinical Oncology at Wroclaw Medical University in Poland for video PSG examination. Patients that were included in the study were 18 years of age or older, had a probable sleep disorder, and had signed an informed consent form for the examination.
Among the exclusion criteria was the presence of severe mental illness, active malignant process, cardiac and/or respiratory disease, and active inflammation, as well as other severe metabolic diseases. Also, pregnant women were excluded from the study as were those unable to undergo polysomnographic examination. Qualified medical doctors divided the participants into the above-mentioned study groups on the basis of detailed criteria. The presence of insomnia in COMISA was diagnosed according to the fifth edition criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) and the International Classification of Sleep Disorders (3rd edition, ICSD-3). The criteria specify that there is the presence of a nocturnal sleep disturbance (criterion A) and a daytime-related impairment (criterion B). Furthermore, there is an occurrence of the sleep disorder for at least three nights a week for a minimum three-month period of time, thus enabling a diagnosis of a clinically relevant disorder [32].
Regarding the treatment of insomnia, there is a fast-screening tool, the Insomnia Severity Index (ISI), which is used by researchers as an outcome measure. The ISI contains seven factors that assess the difficulties of sleep maintenance (both nocturnal and early morning awakenings) and sleep onset. These include satisfaction with the current sleep pattern, interference with daily functioning, noticeable impairment (by a second or third party) that can be attributed to the sleep problem, and the stage of distress or any concern that can be caused by the sleep problem. Each factor is scored on a scale from 0 to 4 with the total maximum score being 28 points. A higher score can indicate more severe insomnia [33]. Categories of insomnia are defined based on ISI scores as follows: no insomnia (0–7) and mild to severe insomnia (8–28) [34].
2.2. Polysomnographic Examination
Polysomnograms were performed in accordance with the standard sleep scoring criteria of the American Academy of Sleep Medicine (AASM, 2013) using Nox-A1 (Nox Medical, Reykjavík, Iceland). We recorded the bioelectrical activity of the brain with the use of electroencephalography (EEG), whereby shifts changes in the EEG to a higher frequency (frequencies > 16 Hz) lasting ≥3 s occurring after ≥10 s of stable sleep were defined as cortical arousals [35]. Electro-oculography (EOG) was used to record eye movement. In addition, the airflow was recorded with a nasal pressure sensor; inductive plethysmography was used for chest and abdominal movements and an electromyogram showed the muscular tension from the tibial electrodes. Moreover, the body position of the patient was recorded and lateral masseter electromyography (EMC) was also performed.
After examination, a qualified physician (author HM) from the Sleep Laboratory, Wroclaw Medical University, Poland, manually analyzed the collected data on the Noxturnal system (version 2.6). Scoring of the sleep disorders was performed in accordance with the AASM guidelines. Participants assigned to the OSA group were also selected according to AASM guidelines.
According to the International Classification of Sleep Disorders (3rd edition, ICSD-3), the definition of OSA is having a PSG-determined obstructive respiratory disturbance Index (RDI) ≥ five events/h that is associated with the typical symptoms of OSA. There can be a presence of daytime sleepiness, fatigue, insomnia, ineffective sleep, awakenings with a choking sensation, witnessed apneas, or loud snoring. When the obstructive RDI score is higher than 15 events/h, the OSA is recognized even with the absence of symptoms [36]. In addition to this, rhythmic masseter muscle activity (RMMA) was assessed according to the recorded audio sounds with the electromyography (EMG) of the bilateral masseters and chin region using surface electrodes. After inspection of the skin and making sure that it is in a dry and clean location that has no wounds or abrasions, the skin was cleaned with water and abrasive skin-prepping gel. If the skin was very oily, wipes with alcohol were used. The electrodes were applied to the skin using paste ensuring biocompatibility and electrical contact. When diagnosing the SB, an amplitude at least double that of the background EMG activity was required. Pauses between the EMG bursts that indicate the same SB episode should not be longer than three seconds. Additionally, at least two audible tooth-grinding episodes that coexisted with the EMG bursts were required to establish the bruxism diagnosis. In accordance with the ASM guidelines, bruxism episodes were classified into phasic, tonic, and mixed forms. The bruxism episode index (BEI) was obtained based on the number of bruxism events per hour of sleep. The intensity of the sleep bruxism was divided into insignificant (BEI < 2), mild or moderate (BEI = 2–4), or severe (BEI > 4).
2.3. Statistical Analysis of the Results
The statistical analysis was performed with Statistica 13.3 (Statsoft, Cracow, Poland). The obtained data are presented as means and standard deviations. To determine any correlations, the Spearman rank test was used. Variance analysis (ANOVA) followed by a post hoc test was used for all parametric data and Kruskal–Wallis ANOVA for the remaining data, i.e., non-parametric data. A p value of <0.05 was considered statistically significant. The differences between all groups, i.e., COMISA vs. OSA, COMISA vs. control, and OSA vs. Control, were analyzed in the study but, according to the study objectives, only results from comparisons between COMISA and OSA and the control group are shown.
3. Results
A total of 119 patients were enrolled in the study, 59 males and 60 females, with an average age of 47.0 ± 6.8 years. The OSA group comprised 42 patients, with a mean age of 52.8 ± 14.4 years. Twenty-four participants were included in the COMISA group, with an average age of 59.8 ± 13.8 years. Finally, 53 patients made up the control group, with a mean age of 36.0 ± 12.9 years. BMI was found to be highest in the COMISA group (31.7 ± 5.0 kg/m2) and lowest in the control group (23.9 ± 4.1 kg/m2). OSA patients had an average BMI of 29.4 ± 4.1 kg/m2. The fact that participants in the COMISA group had a higher BMI than patients in the control group was found to be statistically significant (p < 0.05). These data are presented in Table 1.

Table 1.
Main characteristics of the included patients.
In terms of sleep architecture, the COMISA group did not differ significantly from the OSA group (p > 0.05). However, in comparison to participants in the control group, patients with COMISA were found to have higher respiratory parameters such as AHI, ODI, RDI, apneas, hypopneas per hour, all arousals (AA), and respiratory arousals (RA) with snoring. This was found to be statistically significant (p < 0.05). PSG parameters that were assessed in the examined groups are presented in Table 2.

Table 2.
PSG parameters among COMISA, OSA, and control group. The p value was presented as a comparison between patients diagnosed with COMISA and other groups.
A Bruxism episode index (BEI) > 2 was observed in 80 out of the 119 patients (67%), of which 36 were male (61%) and 41 were female (72%) participants. In total, 27 OSA patients (64%), 13 COMISA (54%), and 40 control patients (71%) received an SB diagnosis based on the aforementioned BEI value. BEI and other SB parameters, however, were not found to be statistically different between COMISA and the comparator groups (p > 0.05). The results are presented in Table 3.

Table 3.
Bruxism parameters measured during polysomnography studies in patients. p value was calculated as a comparison between COMISA patients and other individual groups.
4. Discussion
The main result of this study is that SB does not occur more frequently in patients with COMISA. There is only one existing study that reports the lack of an association between SB and COMISA in comparison to OSA alone [37]. The results of that study are consistent with our observations. However, in those studies, SB diagnosis was based on self-reported symptoms of bruxism and did not include a control group that would allow for a comparison of results [37]. Due to the limited number of publications addressing the relationship between bruxism and COMISA, it is challenging to discuss our results in relation to those mentioned in other studies. There is a need to conduct further research on this topic. Comparing our observations to individual components of COMISA, such as OSA, it appears that patients with OSA often have fewer SB episodes [28,38,39]. This relationship appears to be so significant that some authors have even referred to SB as a “protective factor” against the development of OSA [32]. Articles found in our analysis of the existing literature have not shown a positive correlation between AHI and BEI in OSA [40,41,42]. Our COMISA group with an increased AHI value also lacks a correlation between respiratory disturbances and BEI, which could indicate that COMISA may be a protective factor against the development of SB. However, we did not attempt a categorization of COMISA according to OSA severity; therefore, further studies are required to explain the relationship between the AHI value and SB.
Previous studies examining patients with COMISA have presented inconclusive results on sleep structure. In our article, there were no differences in sleep structure between COMISA and OSA patients. However, we did observe that, in comparison to the control group, respiratory PSG parameters characteristic for OSA (such as AHI, ODI, RDI, respiratory issues, or apnea arousals) were significantly higher than in the COMISA group. The opposite respiratory results were obtained in a study conducted by Mysliwiec et al. [43], whose OSA patients had the highest levels for the above breathing parameters, followed by the COMISA participants. In other studies, only AHI differed between groups and, once again, this was higher in OSA than the COMISA groups [16]. Wulterkens et al. [44] defined the difference in COMISA, OSA, and insomnia with additional “insomnia” features, such as increased WASO or Sleep Onset Latency without changes in respiratory parameters [45]. Some articles have also noticed changes in both insomnia and respiratory parameters [43]. We only observed significant differences in respiratory parameters when comparing the COMISA and OSA patients to our control group. Unfortunately, the previously mentioned studies did not contain control groups that would allow for a direct comparison of our results. Our analysis of the existing literature found that PSG variables such as total sleep time (TST) and sleep efficiency were found to be different in certain groups of patients [16,45,46]. On the other hand, we did not observe these correlations in our study because these differences found in other research may influence group profiles such that they consist of only insomnia patients who were not present in our study. We would like to emphasize that some of the cited studies were conducted among soldiers and PTSD patients, as opposed to our study, which did not include patients with these characteristics. These features could influence the final results. It is also worth mentioning that concomitant SB could change sleep architecture and may play a role in changing particular parameters [47].
The presented study has a few limitations. First, the COMISA group consisted of only 24 patients; therefore, future studies should include a larger group of participants to avoid the potential risk of bias. Our study also did not include a group of patients suffering from insomnia alone, thus restricting our ability to provide a comparison between COMISA and insomnia patients. For these reasons, our results may be different from studies related to this topic. It is also worth mentioning that the PSG examination was conducted without an adaptive night in the hospital; therefore, the obtained PSG results may contain a risk of bias.
5. Conclusions
SB does not occur more frequently in COMISA than in OSA or the control group. COMISA did not show specific deviations in PSG parameters in comparison to OSA subjects; however, it was found to cause serious respiratory disturbances when comparing the results to the control group. To distinguish COMISA from OSA, a comprehensive patient assessment needs to be conducted, as PSG alone does not allow for the diagnosis of COMISA.
Author Contributions
Conceptualization and visualization: H.M., M.M.e.C., M.W. and B.B.; methodology: D.N., H.M., J.S. and A.W; investigation: H.M., M.M.-Z. and A.W.; writing the manuscript: B.B., J.P., K.A. and J.K.; revision of the manuscript: G.L., M.W., M.W.-P. and H.M.; supervision: H.M., M.W., M.W.-P. and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Ethical Committee at Wroclaw Medical University, no. KB-523/2021. The research is registered online at clinicaltrials.gov and the number of the examination is NCT04937036.
Informed Consent Statement
Written informed consent was obtained from the patients to publish this paper.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sweetman, A.M.; Lack, L.C.; Catcheside, P.G.; Antic, N.A.; Chai-Coetzer, C.L.; Smith, S.S.; Douglas, J.A.; McEvoy, R.D. Developing a Successful Treatment for Co-Morbid Insomnia and Sleep Apnoea. Sleep. Med. Rev. 2017, 33, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Eldridge, F.L.; Dement, W.C. Insomnia with Sleep Apnea: A New Syndrome. Science 1973, 181, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, A.; Lack, L.; Bastien, C. Co-Morbid Insomnia and Sleep Apnea (COMISA): Prevalence, Consequences, Methodological Considerations, and Recent Randomized Controlled Trials. Brain Sci. 2019, 9, 371. [Google Scholar] [CrossRef] [PubMed]
- Wieckiewicz, M.; Bogunia-Kubik, K.; Mazur, G.; Danel, D.; Smardz, J.; Wojakowska, A.; Poreba, R.; Dratwa, M.; Chaszczewska-Markowska, M.; Winocur, E.; et al. Genetic Basis of Sleep Bruxism and Sleep Apnea-Response to a Medical Puzzle. Sci. Rep. 2020, 10, 7497. [Google Scholar] [CrossRef] [PubMed]
- Martynowicz, H.; Porębska, I.; Poręba, R.; Mazur, G.; Brzecka, A. Nocturnal Blood Pressure Variability in Patients with Obstructive Sleep Apnea Syndrome. In Advancements in Clinical Research; Pokorski, M., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; Volume 952, pp. 9–15. ISBN 978-3-319-48032-9. [Google Scholar]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of Obstructive Sleep Apnea in the General Population: A Systematic Review. Sleep. Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, B.; Martynowicz, H.; Więckiewicz, M.; Straburzyński, M.; Antolak, M.; Budrewicz, S.; Staszkiewicz, M.; Kopszak, A.; Waliszewska-Prosół, M. Prevalence of Headaches and Their Relationship with Obstructive Sleep Apnea (OSA)—Systematic Review and Meta-Analysis. Sleep. Med. Rev. 2024, 73, 101889. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R. Obstructive Sleep Apnea. Ann. Intern. Med. 2019, 171, ITC81–ITC96. [Google Scholar] [CrossRef] [PubMed]
- Roach, M.; Juday, T.; Tuly, R.; Chou, J.W.; Jena, A.B.; Doghramji, P.P. Challenges and Opportunities in Insomnia Disorder. Int. J. Neurosci. 2021, 131, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Roth, T. Insomnia: Definition, Prevalence, Etiology, and Consequences. J. Clin. Sleep. Med. 2007, 3, S7–S10. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, R.; Lei, F.; Zhou, J.; Zhang, J.; Wing, Y.-K.; Sanford, L.D.; Tang, X. Worldwide and Regional Prevalence Rates of Co-Occurrence of Insomnia and Insomnia Symptoms with Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Sleep. Med. Rev. 2019, 45, 1–17. [Google Scholar] [CrossRef]
- Cho, Y.W.; Kim, K.T.; Moon, H.-J.; Korostyshevskiy, V.R.; Motamedi, G.K.; Yang, K.I. Comorbid Insomnia With Obstructive Sleep Apnea: Clinical Characteristics and Risk Factors. J. Clin. Sleep. Med. 2018, 14, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, A.; Lack, L.; McEvoy, R.D.; Smith, S.; Eckert, D.J.; Osman, A.; Carberry, J.C.; Wallace, D.; Nguyen, P.D.; Catcheside, P. Bi-Directional Relationships between Co-Morbid Insomnia and Sleep Apnea (COMISA). Sleep. Med. Rev. 2021, 60, 101519. [Google Scholar] [CrossRef] [PubMed]
- Luyster, F.S.; Buysse, D.J.; Strollo, P.J. Comorbid Insomnia and Obstructive Sleep Apnea: Challenges for Clinical Practice and Research. J. Clin. Sleep. Med. 2010, 6, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, S.-A.; Lee, G.; Ryu, H.-S.; Chung, S.; Chung, Y.-S.; Kim, W.S. Gender Differences in the Effect of Comorbid Insomnia Symptom on Depression, Anxiety, Fatigue, and Daytime Sleepiness in Patients with Obstructive Sleep Apnea. Sleep. Breath. 2014, 18, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mysliwiec, V.; Gill, J.; Lee, H.; Baxter, T.; Pierce, R.; Barr, T.L.; Krakow, B.; Roth, B.J. Sleep Disorders in US Military Personnel: A High Rate of Comorbid Insomnia and Obstructive Sleep Apnea. Chest 2013, 144, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Krakow, B.; Melendrez, D.; Pedersen, B.; Johnston, L.; Hollifield, M.; Germain, A.; Koss, M.; Warner, T.D.; Schrader, R. Complex Insomnia: Insomnia and Sleep-Disordered Breathing in a Consecutive Series of Crime Victims with Nightmares and PTSD. Biol. Psychiatry 2001, 49, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.A.; Knapp, K. Cardiovascular and Psychiatric Morbidity in Obstructive Sleep Apnea (OSA) with Insomnia (Sleep Apnea plus) versus Obstructive Sleep Apnea without Insomnia: A Case-Control Study from a Nationally Representative US Sample. PLoS ONE 2014, 9, e90021. [Google Scholar] [CrossRef] [PubMed]
- Lechat, B.; Loffler, K.A.; Wallace, D.M.; Reynolds, A.; Appleton, S.L.; Scott, H.; Vakulin, A.; Lovato, N.; Adams, R.; Eckert, D.J.; et al. All-Cause Mortality in People with Co-Occurring Insomnia Symptoms and Sleep Apnea: Analysis of the Wisconsin Sleep Cohort. Nat. Sci. Sleep 2022, 14, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.; Lanquart, J.-P.; Mungo, A.; Loas, G. Cardiovascular Risk Associated with Co-Morbid Insomnia and Sleep Apnoea (COMISA) in Type 2 Diabetics. Sleep Sci. 2022, 15, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, A.; Lack, L.; McEvoy, R.D.; Catcheside, P.G.; Antic, N.A.; Chai-Coetzer, C.L.; Douglas, J.; O’Grady, A.; Dunn, N.; Robinson, J.; et al. Effect of Depression, Anxiety, and Stress Symptoms on Response to Cognitive Behavioral Therapy for Insomnia in Patients with Comorbid Insomnia and Sleep Apnea: A Randomized Controlled Trial. J. Clin. Sleep Med. 2021, 17, 545–554. [Google Scholar] [CrossRef]
- Martynowicz, H.; Wieczorek, T.; Macek, P.; Wojakowska, A.; Poręba, R.; Gać, P.; Mazur, G.; Skomro, R.; Smardz, J.; Więckiewicz, M. The Effect of Continuous Positive Airway Pressure and Mandibular Advancement Device on Sleep Bruxism Intensity in Obstructive Sleep Apnea Patients. Chron. Respir. Dis. 2022, 19, 14799731211052301. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.G.; Wetselaar, P.; Glaros, A.G.; Kato, T.; Santiago, V.; Winocur, E.; De Laat, A.; De Leeuw, R.; et al. International Consensus on the Assessment of Bruxism: Report of a Work in Progress. J. Oral. Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Kuang, B.; Aarab, G.; Wei, Y.; Blanken, T.F.; Lobbezoo, F.; Someren, E.J.W.V.; Ramautar, J.R.; Wassing, R. Associations between Signs of Sleep Bruxism and Insomnia: A Polysomnographic Study. J. Sleep Res. 2023, 32, e13827. [Google Scholar] [CrossRef] [PubMed]
- Martynowicz, H.; Gac, P.; Brzecka, A.; Poreba, R.; Wojakowska, A.; Mazur, G.; Smardz, J.; Wieckiewicz, M. The Relationship between Sleep Bruxism and Obstructive Sleep Apnea Based on Polysomnographic Findings. J. Clin. Med. 2019, 8, 1653. [Google Scholar] [CrossRef] [PubMed]
- Massahud, M.L.d.B.; Bruzinga, F.F.B.; Diniz, S.A.d.M.; Seraidarian, K.K.d.A.; Lopes, R.d.M.; Barros, V.d.M.; Seraidarian, P.I. Association between Sleep Bruxism, Use of Antidepressants, and Obstructive Sleep Apnea Syndrome: A Cross-Sectional Study. J. Oral. Rehabil. 2022, 49, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, H.; Kitaura, H.; Hashimoto, T.; Ito, M.; Kinbara, M.; Deguchi, T.; Irokawa, T.; Ohisa, N.; Ogawa, H.; Takano-Yamamoto, T. Relationship between Sleep Bruxism and Sleep Respiratory Events in Patients with Obstructive Sleep Apnea Syndrome. Sleep Breath. 2014, 18, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Yamaguchi, T.; Mikami, S.; Watanabe, K.; Gotouda, A.; Okada, K.; Hishikawa, R.; Shibuya, E.; Shibuya, Y.; Lavigne, G. Weak Association between Sleep Bruxism and Obstructive Sleep Apnea. A Sleep Laboratory Study. Sleep Breath. 2016, 20, 703–709. [Google Scholar] [CrossRef]
- Beddis, H.; Pemberton, M.; Davies, S. Sleep Bruxism: An Overview for Clinicians. Br. Dent. J. 2018, 225, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Guarda-Nardini, L.; Marchese-Ragona, R.; Lobbezoo, F. Theories on Possible Temporal Relationships between Sleep Bruxism and Obstructive Sleep Apnea Events. An Expert Opinion. Sleep Breath. 2015, 19, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Smardz, J.; Wieckiewicz, M.; Michalek-Zrabkowska, M.; Gac, P.; Poreba, R.; Wojakowska, A.; Blaszczyk, B.; Mazur, G.; Martynowicz, H. Is Camera Recording Crucial for the Correct Diagnosis of Sleep Bruxism in Polysomnography? J. Sleep Res. 2023, 32, e13858. [Google Scholar] [CrossRef]
- Riemann, D.; Baglioni, C.; Bassetti, C.; Bjorvatn, B.; Dolenc Groselj, L.; Ellis, J.G.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Gonçalves, M.; et al. European Guideline for the Diagnosis and Treatment of Insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef] [PubMed]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an Outcome Measure for Insomnia Research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Ragnoli, B.; Pochetti, P.; Raie, A.; Malerba, M. Comorbid Insomnia and Obstructive Sleep Apnea (COMISA): Current Concepts of Patient Management. Int. J. Environ. Res. Public Health 2021, 18, 9248. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Sateia, M.J. International Classification of Sleep Disorders-Third Edition: Highlights and Modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Hesselbacher, S.E.; Nye, P.; Aiyer, A.A.; Surani, S.R. Comorbid Insomnia and Sleep Apnea: Characterization of the Syndrome and Understanding Its Associations with Comorbid Sleep Conditions. Sleep Breath. 2021, 25, 1995–2000. [Google Scholar] [CrossRef] [PubMed]
- Cid-Verdejo, R.; Domínguez Gordillo, A.A.; Hallal-Peche, F.; Ardizone García, I.; Martínez Orozco, F.J. Is There an Association between Sleep Bruxism and Obstructive Sleep Apnea? A Case-Control Polysomnographic Investigation. Sleep Med. 2023, 114, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, G.M.; Guerra, P.; Di Giovanni, G.; Boselli, M.; Parrino, L.; Terzano, M.G. Sleep Bruxism Is a Disorder Related to Periodic Arousals During Sleep. J. Dent. Res. 1998, 77, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kuang, B.; Lobbezoo, F.; de Vries, N.; Hilgevoord, A.; Aarab, G. Sleep Bruxism Is Highly Prevalent in Adults with Obstructive Sleep Apnea: A Large-Scale Polysomnographic Study. J. Clin. Sleep Med. 2023, 19, 443–451. [Google Scholar] [CrossRef]
- Yap, A.U.; Tan, M.W.Y.; Tan, S.H.X.; Chua, A.P. Sleep Bruxism Events: An Epiphenomenon of Severe Obstructive Sleep Apnea? Clin. Oral. Investig. 2023, 27, 4633–4642. [Google Scholar] [CrossRef]
- Tan, M.W.Y.; Yap, A.U.-J.; Chua, A.P.; Wong, J.C.M.; Parot, M.V.J.; Tan, K.B.C. Prevalence of Sleep Bruxism and Its Association with Obstructive Sleep Apnea in Adult Patients: A Retrospective Polysomnographic Investigation. J. Oral. Facial Pain. Headache 2019, 33, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Mysliwiec, V.; Brock, M.S.; Pruiksma, K.E.; Straud, C.L.; Taylor, D.J.; Hansen, S.; Foster, S.N.; Mithani, S.; Zwetzig, S.; Gerwell, K.; et al. A Comprehensive Evaluation of Insomnia, Obstructive Sleep Apnea and Comorbid Insomnia and Obstructive Sleep Apnea in US Military Personnel. Sleep 2022, 45, zsac203. [Google Scholar] [CrossRef] [PubMed]
- Wulterkens, B.M.; Hermans, L.W.A.; Fonseca, P.; Asin, J.; Duis, N.; Janssen, H.C.J.P.; Overeem, S.; van Gilst, M.M. Sleep Structure in Patients with COMISA Compared to OSA and Insomnia. J. Clin. Sleep Med. 2023, 19, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Verkler, J.; Koshorek, G.; Roth, T.; Roehrs, T. 0335 Polysomnography in Insomnia Disorder. Sleep 2019, 42, A137. [Google Scholar] [CrossRef]
- Sigurdardottir, F.D.; Bertisch, S.M.; Reid, M.L.; deFilippi, C.R.; Lima, J.A.C.; Redline, S.; Omland, T. Association between Insomnia Phenotypes and Subclinical Myocardial Injury: The Multi-Ethnic Study of Atherosclerosis. Sleep 2023, 46, zsac318. [Google Scholar] [CrossRef]
- de Holanda, T.A.; Castagno, C.D.; Barbon, F.J.; Costa, Y.M.; Goettems, M.L.; Boscato, N. Sleep Architecture and Factors Associated with Sleep Bruxism Diagnosis Scored by Polysomnography Recordings: A Case-Control Study. Arch. Oral. Biol. 2020, 112, 104685. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).