Heart Rate Recovery: Up to Date in Heart Failure—A Literature Review
Abstract
:1. Introduction
2. Autonomic Influence in Exercise and the Recovery Period
3. Main HRR Parameters
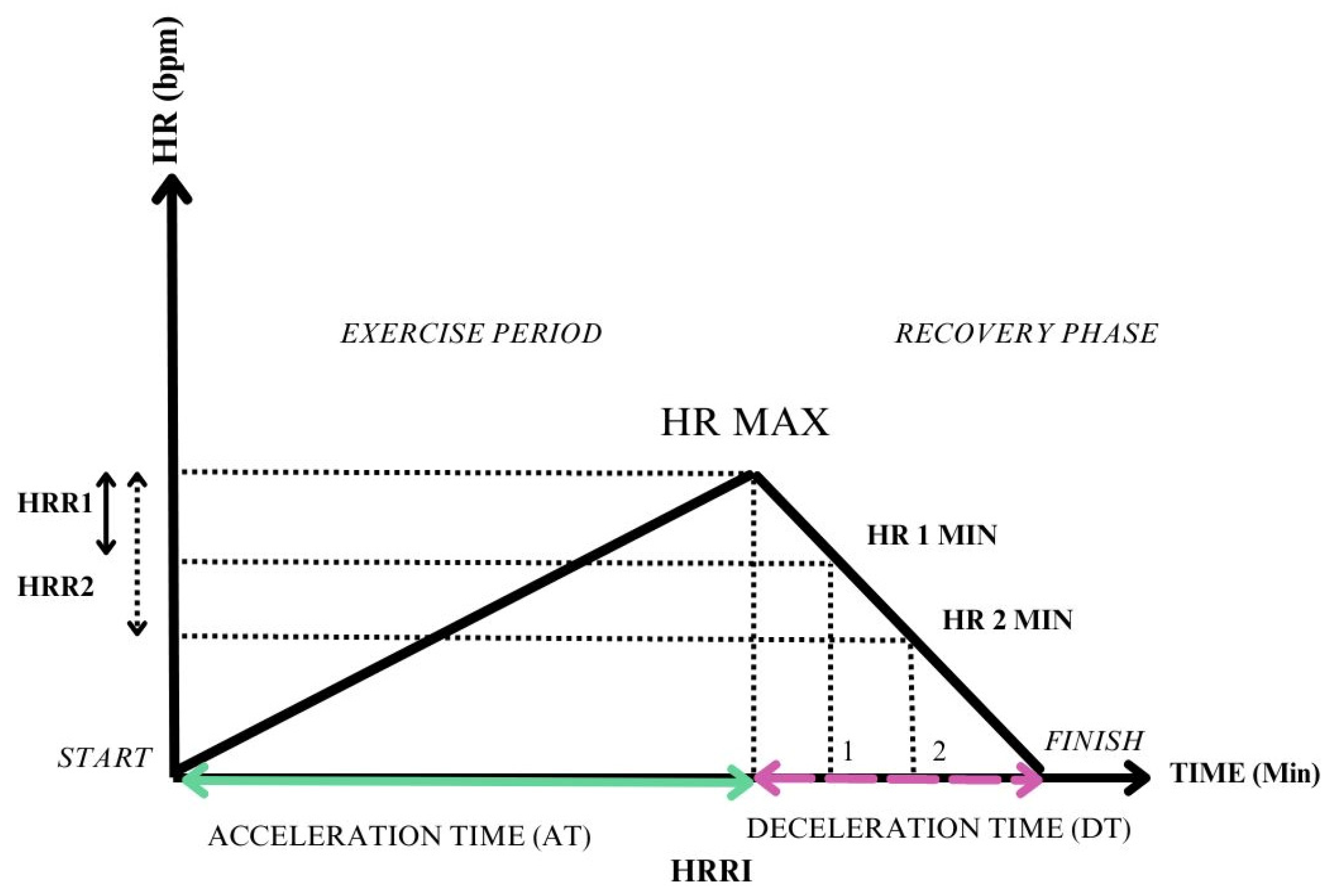
3.1. Difference between Peak HR and HR at a Certain Moment of Recovery
3.2. A Ratio between Different Phases of the Effort
- Heart rate recovery index—measured as the ratio between heart rate acceleration time (AT) and heart rate deceleration time (DT) [35].
3.3. A Delay in the Maximum Heart Rate
- Delay of peak HR: HRR assessment 6 months after heart transplantation reflects the cardiac denervation and the loss of vagal tone, which would normally induce HR drop after exercise [32].
4. Clinical Applications of HRR
5. Methodology
6. HRR in Heart Failure: Bedside Studies
| Study (Year) | Patients Enrolled (n) | HF Population | Purpose of the Study | Exercise Test Methodology | Beta-Blocker Treatment | HRR Evaluation Method and Cut-Off | Conclusions |
|---|---|---|---|---|---|---|---|
| Hossri et al. (2024) [24] | 106 | HFpEF and HFmrEF with concomitant CAD | Benefits of CPMR in HF patients with CAD | Treadmill CPET using an incremental maximal protocol, followed later by a submaximal constant load protocol at 80% of the initial test; recovery period of 6 min | 87% of the patients. Dosage N/A. | HRR1 was evaluated at the first recovery min; Cut-off N/A | 12 weeks of CPMR were associated with improved NYHA class, significant exercise test performance, an increased HRR, and an enhanced QOL. |
| Irfanullah et al. (2023) [47] | 39 | HFpEF, HFmrEF, HFrEF | The effects of cycle ergometer training on heart rate recovery and mindfulness in patients with NYHA Class I and II heart failure | 6 MWT on a 400–700 m distance. | All patients received either beta-blockers or CCB. Dosage N/A. | HRR1; HRR2 = HRmax-HR at 1; 2 min of recovery; Cut-off N/A | HRR1 and HRR2 improved after 6 weeks of cycle-ergometer training, as well as the MAAS. |
| Carneiro et al. (2021) [34] | 2066 | Participants without HF | Incidence of HF and its type (HFpEF and HFrEF) during the follow-up period (16.8 years) | Submaximal treadmill exercise test using the Bruce protocol; recovery in a supine position. | N/A | HRR3 = HRmax-HR at 3 min of recovery; Cut-off N/A | Slower HRR3 is associated with a higher risk of developing heart failure, particularly HFrEF. |
| Hajdusek et al. (2017) [33] | 103 | 78 advanced HFrEF and 25 healthy controls, assessed for device implantation or transplant eligibility | Evaluation of HRR and MCR as outcome determinants in HF, during a follow-up of ~3.4 years | Symptom limited using a bicycle CPET, with a 25 W increase/3 min. | 97% of HF patients. Daily low-middle doses (12.5–50 mg Carvedilol; 2.5–200 mg Metoprolol and 2.5–10 mg Bisoprolol) | The difference between HRmax and HR at 150 s of recovery; Cut-off N/A | MCR slope correlates with distinct clinical variables compared to HRR. In heart failure patients, the MCR slope offers significant prognostic value beyond HRR. |
| Yaylalı et al. (2015) [31] | 41 | HFrEF and HFmrEF | Correlation between exercise training and HRR improvement, before and after entering a training program | Symptom limited bicycle CPET with 10 W/min followed by 3 min active cool-down | 51.2% of patients. Dosage N/A | HRR1; HRR2 = HRmax-HR at 1; 2 min of recovery; N.V. Preestablished HRR1 > 12 bpm; HRR2 > 22 bpm | The training enhanced only HRR2, with IT showing a greater impact on the HRR2 improvement. Both HRR (1 and 2) in those with an abnormal HRR baseline have improved after exercise. |
| Imamura et al. (2015) [32] | 21 | Heart transplant patients | Impact of post-heart transplantation parasympathetic reinnervation | Symptom limited Cycle-ergometer CPET using a 10 W/min incremental protocol, followed by a 5 min passive recovery in a seated position. | 19% of patients. Dosage N/A. | HRR2-the difference between peak HR and HR after 2 min of recovery (measured 2 years after transplant) Delay of peak HR-the delay from the peak HR after the recovery time initiation (measured 6 months after transplant). Cut-off N/A | Parasympathetic reinnervation coincides with enhanced post-exercise recovery and heart failure-specific quality of life during the 2 years following heart transplantation. |
| Lindemberg et al. (2014) [26] | 161. 154 completed the test | 126 patients with HFrEF and 35 healthy individuals. | Correlations between HRR1 and 6 MWD | 6 MWT followed by passive (seated) recovery. | 70.58% of HF patients. Carvedilol mean dose 30 ± 29 mg. | HRR1 = HRmax-HR at 1 min of recovery; N.V. Preestablished HRR1 > 12 bpm | HF patients who received beta-blockers had better exercise tolerance than those without receiving beta-blocker medication, even though they had altered HRR. |
| Cahalin et al. (2014) [30] | 240 | 200 patients with HFrEF and 40 patients with HFpEF | Correlations between HRR measured post 6 MWT and CPET and predictors of abnormal HRR. | 6 MWT followed by a passive recovery. A symptom-limited bicycle CPET lasting 8–10 min. 1 min active cool-down period. | 60% of the entire study group. Dosage N/A | HRR1 = HRmax-HR at 1 min of recovery; N.V. Preestablished HRR1 > 12 bpm | The 6 MWT and CPET were correlated concerning HRR, HR Reserve, and peak HR. Predictors of abnormal HRR were found to be Peak HR, EOV and E/e’ ratio. |
7. Echocardiographic Correlations
8. Discussion
9. Further Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewar, A.; Kass, L.; Stephens, R.C.M.; Tetlow, N.; Desai, T. Heart Rate Recovery Assessed by Cardiopulmonary Exercise Testing in Patients with Cardiovascular Disease: Relationship with Prognosis. Int. J. Environ. Res. Public Health 2023, 20, 4678. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, T.; Wu, J.; Zhu, P.; Zhang, M.; Zheng, W.; Gu, Y. Heart rate recovery in hypertensive patients: Relationship with blood pressure control. J. Hum. Hypertens. 2017, 31, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.A.; Manolis, T.A.; Manolis, A.S. Neurohumoral Activation in Heart Failure. Int. J. Mol. Sci. 2023, 24, 15472. [Google Scholar] [CrossRef] [PubMed]
- Fecchio, R.Y.; Brito, L.; Leicht, A.S.; Forjaz, C.L.M.; Peçanha, T. Reproducibility of post-exercise heart rate recovery indices: A systematic review. Auton. Neurosci. 2019, 221, 102582. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, X.; Sun, Z.; Li, L.; Zuegel, M.; Steinacker, J.M.; Schumann, U.; Yin, J.; Jin, X.; Shan, Z.; et al. Heart Rate Recovery and Risk of Cardiovascular Events and All-Cause Mortality: A Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2017, 6, e005505. [Google Scholar] [CrossRef] [PubMed]
- Peçanha, T.; Silva-Júnior, N.D.; Forjaz, C.L.D.M. Heart rate recovery: Autonomic determinants, methods of assessment and association with mortality and cardiovascular diseases. Clin. Physiol. Funct. Imaging 2014, 34, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Okutucu, S.; Karakulak, U.N.; Aytemir, K.; Oto, A. Heart rate recovery: A practical clinical indicator of abnormal cardiac autonomic function. Expert Rev. Cardiovasc. Ther. 2011, 9, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Sato, H.; Hori, M.; Kusuoka, H.; Ozaki, H.; Yokoyama, H.; Takeda, H.; Inoue, M.; Kamada, T. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J. Am. Coll. Cardiol. 1994, 24, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Severino, P.; D’amato, A.; Prosperi, S.; Cas, A.D.; Mattioli, A.V.; Cevese, A.; Novo, G.; Prat, M.; Pedrinelli, R.; Raddino, R.; et al. Do the Current Guidelines for Heart Failure Diagnosis and Treatment Fit with Clinical Complexity? J. Clin. Med. 2022, 11, 857. [Google Scholar] [CrossRef]
- Gronda, E.; Dusi, V.; D’elia, E.; Iacoviello, M.; Benvenuto, E.; Vanoli, E. Sympathetic activation in heart failure. Eur. Heart J. Suppl. 2022, 24, E4–E11. [Google Scholar] [CrossRef] [PubMed]
- Borovac, J.A.; D’Amario, D.; Bozic, J.; Glavas, D. Sympathetic nervous system activation and heart failure: Current state of evidence and the pathophysiology in the light of novel biomarkers. World J. Cardiol. 2020, 12, 373–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Anderson, A.S. The Sympathetic Nervous System and Heart Failure. Cardiol. Clin. 2014, 32, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.U.; Nearing, B.D.; Mittal, S.; Premchand, R.K.; Libbus, I.; DiCarlo, L.A.; Amurthur, B.; KenKnight, B.H.; Anand, I.S.; Verrier, R.L. Autonomic regulation therapy in chronic heart failure with preserved/mildly reduced ejection fraction: ANTHEM-HFpEF study results. Int. J. Cardiol. 2023, 381, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Peçanha, T.; de Brito, L.C.; Fecchio, R.Y.; de Sousa, P.N.; Junior, N.D.d.S.; de Abreu, A.P.; da Silva, G.V.; Mion-Junior, D.; Forjaz, C.L.d.M. Metaboreflex activation delays heart rate recovery after aerobic exercise in never-treated hypertensive men. J. Physiol. 2016, 594, 6211–6223. [Google Scholar] [CrossRef] [PubMed]
- Roberto, S.; Mulliri, G.; Milia, R.; Solinas, R.; Pinna, V.; Sainas, G.; Piepoli, M.F.; Crisafulli, A. Hemodynamic response to muscle reflex is abnormal in patients with heart failure with preserved ejection fraction. J. Appl. Physiol. 2017, 122, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, Y.; Nyberg, M. Cardiovascular Adaptations to Exercise Training. In Comprehensive Physiology; Wiley: Hoboken, NY, USA, 2015; pp. 1–32. [Google Scholar] [CrossRef]
- Gourine, A.V.; Ackland, G.L. Cardiac vagus and exercise. Physiology 2019, 34, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Kannankeril, P.J.; Le, F.K.; Kadish, A.H.; Goldberger, J.J. Parasympathetic Effects on Heart Rate Recovery after Exercise. J. Investig. Med. 2004, 52, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Facioli, T.P.; Philbois, S.V.; Gastaldi, A.C.; Almeida, D.S.; Maida, K.D.; Rodrigues, J.A.L.; Sanchez-Delgado, J.C.; Souza, H.C.D. Study of heart rate recovery and cardiovascular autonomic modulation in healthy participants after submaximal exercise. Sci. Rep. 2021, 11, 3620. [Google Scholar] [CrossRef]
- Roberto, S.; Milia, R.; Doneddu, A.; Pinna, V.; Palazzolo, G.; Serra, S.; Orrù, A.; Kakhak, S.A.H.; Ghiani, G.; Mulliri, G.; et al. Hemodynamic abnormalities during muscle metaboreflex activation in patients with type 2 diabetes mellitus. J. Appl. Physiol. 2019, 126, 444–453. [Google Scholar] [CrossRef]
- Smarz, K.; Tysarowski, M.; Zaborska, B.; Pilichowska-Paszkiet, E.; Sikora-Frac, M.; Budaj, A.; Jaxa-Chamiec, T. Chronotropic incompetence limits aerobic exercise capacity in patients taking beta-blockers: Real-life observation of consecutive patients. Healthcare 2021, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.R.; Foody, J.M.; Blackstone, E.H.; Lauer, M.S. Heart Rate Recovery after Submaximal Exercise Testing as a Predictor of Mortality in a Cardiovascularly Healthy Cohort. Ann. Intern. Med. 2000, 132, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Hossri, C.A.C.; Araujo, F.; Baldi, B.; Otterstetter, R.; Uemoto, V.; Carvalho, C.; Mastrocola, L.; Albuquerque, A. Association among cardiopulmonary and metabolic rehabilitation, arrhythmias, and myocardial ischemia responses of patients with HFpEF or HFmrEF. Braz. J. Med. Biol. Res. 2024, 57, e13174. [Google Scholar] [CrossRef]
- Andrade, G.N.; Rodrigues, T.; Takada, J.; Braga, L.; Umeda, I.; Nascimento, J.; Pereira-Filho, H.; Grupi, C.; Salemi, V.; Jacob-Filho, W.; et al. Prolonged heart rate recovery time after 6-minute walk test is an independent risk factor for cardiac events in heart failure: A prospective cohort study. Physiotherapy 2022, 114, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lindemberg, S.; Chermont, S.; Quintão, M.; Derossi, M.; Guilhon, S.; Bernardez, S.; Marchese, L.; Martins, W.; Nóbrega, A.C.L.; Mesquita, E.T. Heart Rate Recovery in the First Minute at the Six-Minute Walk Test in Patients with Heart Failure. Arq. Bras. Cardiol. 2014, 102, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Miyamoto, T.; Mori, Y.; Harada, T.; Tasaki, H. Heart rate recovery is useful for evaluating the recovery of exercise tolerance in patients with heart failure and atrial fibrillation. Heart Vessels 2021, 36, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, G.W.P.; dos Santos, M.R.; de Souza, F.R.; da Costa, M.J.A.; von Haehling, S.; Takayama, L.; Pereira, R.M.R.; Negrão, C.E.; Anker, S.D.; Alves, M.J.d.N.N. Sympatho-Vagal Imbalance is Associated with Sarcopenia in Male Patients with Heart Failure. Arq. Bras. Cardiol. 2019, 112, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.C.; Lee, H.S.; Choi, S.-W.; Han, S.-W.; Ryu, K.-H.; Shin, E.-C.; Kang, S.-M. Post-exercise heart rate recovery independently predicts clinical outcome in patients with acute decompensated heart failure. PLoS ONE 2016, 11, e0154534. [Google Scholar] [CrossRef] [PubMed]
- Cahalin, L.P.; Arena, R.; Labate, V.; Bandera, F.; Guazzi, M. Predictors of abnormal heart rate recovery in patients with heart failure reduced and preserved ejection fraction. Eur. J. Prev. Cardiol. 2014, 21, 906–914. [Google Scholar] [CrossRef]
- Yaylali, Y.T.; Fındıkoglu, G.; Yurtdas, M.; Konukcu, S.; Senol, H. The effects of baseline heart rate recovery normality and exercise training protocol on heart rate recovery in patients with heart failure. Anatol. J. Cardiol. 2015, 15, 727–734. [Google Scholar] [CrossRef]
- Imamura, T.; Kinugawa, K.; Okada, I.; Kato, N.; Fujino, T.; Inaba, T.; Maki, H.; Hatano, M.; Kinoshita, O.; Nawata, K.; et al. Parasympathetic Reinnervation Accompanied by Improved Post-Exercise Heart Rate Recovery and Quality of Life in Heart Transplant Recipients. Int. Heart J. 2015, 56, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Hajdusek, P.; Kotrc, M.; Kautzner, J.; Melenovsky, V.; Benesova, E.; Jarolim, P.; Benes, J. Heart rate response to exercise in heart failure patients: The prognostic role of metabolic–chronotropic relation and heart rate recovery. Int. J. Cardiol. 2017, 228, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, H.A.; Song, R.J.; Lee, J.; Schwartz, B.; Vasan, R.S.; Xanthakis, V. Association of Blood Pressure and Heart Rate Responses to Submaximal Exercise with Incident Heart Failure: The Framingham Heart Study. J. Am. Heart Assoc. 2021, 10, e019460. [Google Scholar] [CrossRef] [PubMed]
- Cozlac, A.-R.; Petrescu, L.; Crisan, S.; Luca, C.T.; Vacarescu, C.; Streian, C.G.; Lazar, M.-A.; Gurgu, A.; Dragomir, A.; Goanta, E.V.; et al. A Novel and Simple Exercise Test Parameter to Assess Responsiveness to Cardiac Resynchronization Therapy. Diagnostics 2020, 10, 920. [Google Scholar] [CrossRef] [PubMed]
- Peçanha, T.; Bartels, R.; Brito, L.C.; Paula-Ribeiro, M.; Oliveira, R.S.; Goldberger, J.J. Methods of assessment of the post-exercise cardiac autonomic recovery: A methodological review. Int. J. Cardiol. 2017, 227, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Sydó, N.; Sydó, T.; Merkely, B.; Carta, K.G.; Murphy, J.G.; Lopez-Jimenez, F.; Allison, T.G. Impaired Heart Rate Response to Exercise in Diabetes and Its Long-term Significance. Mayo Clin. Proc. 2016, 91, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Alihanoglu, Y.I.; Yildiz, B.S.; Kilic, I.D.; Uludag, B.; Demirci, E.E.; Zungur, M.; Evrengul, H.; Kaftan, A.H. Impaired systolic blood pressure recovery and heart rate recovery after graded exercise in patients with metabolic syndrome. Medicine 2015, 94, e428. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.T.; Ammann, P.; Schoch, O.D.; Rickli, H.; Korte, W.; Hürny, C.; Myers, J.; Münzer, T. Determinants of postexercise heart rate recovery in patients with the obstructive sleep apnea syndrome. Chest 2010, 137, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Shim, C.Y. Stress Testing in Heart Failure with Preserved Ejection Fraction. Heart Fail Clin. 2021, 17, 435–445. [Google Scholar] [CrossRef]
- Vukomanovic, V.; Suzic-Lazic, J.; Celic, V.; Cuspidi, C.; Grassi, G.; Galderisi, M.; Djukic, V.; Tadic, M. Is there association between left atrial function and functional capacity in patients with uncomplicated type 2 diabetes? Int. J. Cardiovasc. Imaging 2020, 36, 15–22. [Google Scholar] [CrossRef]
- Tadic, M.; Suzic-Lazic, J.; Vukomanovic, V.; Cuspidi, C.; Ilic, S.; Celic, V. Functional capacity and left ventricular diastolic function in patients with type 2 diabetes. Acta Diabetol. 2021, 58, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Lin, L.Y.; Chuang, K.J. N terminal prohormone of brain natriuretic peptide is associated with improved heart rate recovery after treadmill exercise test. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023, 18, 200203. [Google Scholar] [CrossRef] [PubMed]
- van de Vegte, Y.J.; van der Harst, P.; Verweij, N. Heart rate recovery 10 seconds after cessation of exercise predicts death. J. Am. Heart Assoc. 2018, 7, e008341. [Google Scholar] [CrossRef] [PubMed]
- Giannitsi, S.; Bougiakli, M.; Bechlioulis, A.; Kotsia, A.; Michalis, L.K.; Naka, K.K. 6-minute walking test: A useful tool in the management of heart failure patients. Ther. Adv. Cardiovasc. Dis. 2019, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhu, J.; Shen, T.; Song, Y.; Tao, L.; Xu, S.; Zhao, W.; Gao, W. Comparison Between Treadmill and Bicycle Ergometer Exercises in Terms of Safety of Cardiopulmonary Exercise Testing in Patients with Coronary Heart Disease. Front. Cardiovasc. Med. 2022, 9, 864637. [Google Scholar] [CrossRef]
- Irfanullah, M.W.; Yaqoob, N.; Shah, S.; Tariq, I.; Chaudhary, F.; Ibrahim, U.; Huma, Z.E.; Khan, N. Effects of Cycle Ergometer Training on Heart Rate Recovery and Mind Fullness in NYHA class I, II Heart Failure Patients. Pak. J. Med. Health Sci. 2023, 17, 10–13. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [CrossRef]
- Maldonado-Martín, S.; Brubaker, P.H.; Ozemek, C.; Jayo-Montoya, J.A.; Becton, J.T.; Kitzman, D.W. Impact of β-Blockers on Heart Rate and Oxygen Uptake During Exercise and Recovery in Older Patients with Heart Failure With Preserved Ejection Fraction. J. Cardiopulm. Rehabil. Prev. 2020, 40, 174. [Google Scholar] [CrossRef]
- Skaluba, S.J.; Litwin, S.E. Doppler-derived left ventricular filling pressures and the regulation of heart rate recovery after exercise in patients with suspected coronary artery disease. Am. J. Cardiol. 2005, 95, 832–837. [Google Scholar] [CrossRef]
- Gharacholou, S.M.; Scott, C.G.; Borlaug, B.A.; Kane, G.C.; McCully, R.B.; Oh, J.K.; Pellikka, P.A. Relationship between diastolic function and heart rate recovery after symptom-limited exercise. J. Card. Fail. 2012, 18, 34–40. [Google Scholar] [CrossRef]
- Mashayekhi, B.; Mohseni-Badalabadi, R.; Hosseinsabet, A.; Ahmadian, T. Correlation between Heart rate recovery and Left Atrial phasic functions evaluated by 2D speckle-tracking Echocardiography after Acute Myocardial infarction. BMC Cardiovasc. Disord. 2023, 23, 164. [Google Scholar] [CrossRef] [PubMed]
- Arshi, B.; Geurts, S.; Tilly, M.J.; Berg, M.v.D.; Kors, J.A.; Rizopoulos, D.; Ikram, M.A.; Kavousi, M. Heart rate variability is associated with left ventricular systolic, diastolic function and incident heart failure in the general population. BMC Med. 2022, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Ma, H.-P.; Hung, C.-S.; Huang, S.-H.; Chuang, B.-L.; Lin, C.; Lo, M.-T.; Peng, C.-K.; Lin, Y.-H. Usefulness of heart rhythm complexity in heart failure detection and diagnosis. Sci. Rep. 2020, 10, 14916. [Google Scholar] [CrossRef]
- Lahiri, M.K.; Kannankeril, P.J.; Goldberger, J.J. Assessment of Autonomic Function in Cardiovascular Disease. Physiological Basis and Prognostic Implications. J. Am. Coll. Cardiol. 2008, 51, 1725–1733. [Google Scholar] [CrossRef]
- Julario, R.; Mulia, E.P.B.; Rachmi, D.A.; A’yun, M.Q.; Septianda, I.; Dewi, I.P.; Juwita, R.R.; Dharmadjati, B.B. Evaluation of heart rate variability using 24-hour Holter electrocardiography in hypertensive patients. J. Arrhythm. 2021, 37, 157–164. [Google Scholar] [CrossRef]
- Bechke, E.; Kliszczewicz, B.; McLester, C.; Tillman, M.; Esco, M.; Lopez, R. An examination of single day vs. multi-day heart rate variability and its relationship to heart rate recovery following maximal aerobic exercise in females. Sci. Rep. 2020, 10, 14760. [Google Scholar] [CrossRef]
- Vacarescu, C.; Luca, C.-T.; Feier, H.; Gaiță, D.; Crișan, S.; Negru, A.-G.; Iurciuc, S.; Goanță, E.-V.; Mornos, C.; Lazăr, M.-A.; et al. Betablockers and Ivabradine Titration According to Exercise Test in LV Only Fusion CRT Pacing. Diagnostics 2022, 12, 1096. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef]
- Sardu, C.; Massetti, M.M.; Rambaldi, P.; Gatta, G.; Cappabianca, S.; Sasso, F.C.; Santamaria, M.; Volpicelli, M.; Ducceschi, V.; Signoriello, G.; et al. SGLT2-inhibitors reduce the cardiac autonomic neuropathy dysfunction and vaso-vagal syncope recurrence in patients with type 2 diabetes mellitus: The SCAN study. Metabolism 2022, 137, 155243. [Google Scholar] [CrossRef] [PubMed]
- van Bilsen, M.; Patel, H.C.; Bauersachs, J.; Böhm, M.; Borggrefe, M.; Brutsaert, D.; Coats, A.J.; de Boer, R.A.; de Keulenaer, G.W.; Filippatos, G.S.; et al. The autonomic nervous system as a therapeutic target in heart failure: A scientific position statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2017, 19, 1361–1378. [Google Scholar] [CrossRef]
- Franssen, W.M.A.; Beyens, M.; Al Hatawe, T.; Frederix, I.; Verboven, K.; Dendale, P.; Eijnde, B.O.; Massa, G.; Hansen, D. Cardiac function in adolescents with obesity: Cardiometabolic risk factors and impact on physical fitness. Int. J. Obes. 2019, 43, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Ackland, G.L.; Minto, G.; Clark, M.; Whittle, J.; Stephens, R.C.; Owen, T.; Prabhu, P.; del Arroyo, A.G. Autonomic regulation of systemic inflammation in humans: A multi-center, blinded observational cohort study. Brain Behav. Immun. 2018, 67, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Teodoru, M.; Negrea, M.O.; Cozgarea, A.; Cozma, D.; Boicean, A. Enhancing Pulmonary Embolism Mortality Risk Stratification Using Machine Learning: The Role of the Neutrophil-to-Lymphocyte Ratio. J. Clin. Med. 2024, 13, 1191. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020, 17, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Rachieru, C.; Luca, C.T.; Văcărescu, C.; Petrescu, L.; Cirin, L.; Cozma, D. Future Perspectives to Improve CHA2 DS2 VASc Score: The Role of Left Atrium Remodelling, Inflammation and Genetics in Anticoagulation of Atrial Fibrillation. Clin. Interv. Aging 2023, 18, 1737–1748. [Google Scholar] [CrossRef]
- da Fonseca, R.X.; da Cruz, C.J.G.; Soares, E.M.K.V.K.; Garcia, G.L.; Porto, L.G.G.; Molina, G.E. Post-exercise heart rate recovery and its speed are associated with resting-reactivity cardiovagal modulation in healthy women. Sci. Rep. 2024, 14, 5526. [Google Scholar] [CrossRef]
| Study (Year) | Patients Enrolled (n) | HF Population | Purpose of the Study | Exercise Test Methodology | Beta-Blocker Treatment | HRR Evaluation Method and Cut-Off | Conclusions |
|---|---|---|---|---|---|---|---|
| Andrade et al. (2022) [25] | 76 | HFrEF | Correlation of all-cause mortality and HRR1 and HRR2 during a 2-year follow-up | 6 MWT followed by passive supine recovery. | 97% of patients. Dosage N/A. | HRR1; HRR2 = HRmax-HR at 1; 2 min of recovery; Preestablished N.V. HRR1 > 12 bpm; HRR2 > 22 bpm | Decreased HRR1 and HRR2 are associated with increased mortality. |
| Tanaka et al. (2021) [27] | 84 | HFrEF with AF | Correlation of HRR and exercise capacity of HFrEF and AF patients before and after the rehabilitation program | Cycle ergometer CPET using a ramp protocol of 10 W/min until exhaustion, with an active 1 min recovery, followed by a 4 min passive recovery. | 90% of patients. Dosage N/A | HRR1 = HRmax-HR at 1 min of recovery. For AF patients, HR was determined by averaging the last ten beats at each point; Cut-off N/A | Improved HRR is associated with improved exercise capacity in patients with HFrEF and AF after completing the cardiac rehabilitation program. |
| Cozlac et al. (2020) [35] | 109 | HFrEF patients following CRT implantation | Correlation of HRRI and CRT responsiveness | Cycle ergometer using the Bruce Protocol with a 25 W increase/2 min. | 82.8% of the patients. Dosage N/A. | HRRI = The ratio between HR AT and DT. The cut-off for CRT response predictability was 1.51. | HRRI was significantly higher in CRT responders vs. non-responders. |
| Fonseca et al. (2019) [28] | 116 | HFrEF | Association of sarcopenia and autonomic regulation | Symptom limited cycle ergometer CPET using a ramp protocol 5–10 W/min. Active recovery for 2 min, followed by 4 min of passive recovery. | 100% of sarcopenic and 94% of non-sarcopenic patients. Dosage N/A. | HRR1; HRR2 = HRmax-HR at 1; 2 min of recovery; Cut-off N/A | Sarcopenia is associated with decreased HRR1 and HRR2 in HF patients. |
| Youn et al. (2016) [29] | 107 | Recovered acute decompensated HFrEF (Eligible for discharge) | Correlation between HRR and pro-inflammatory states with clinical outcomes | Treadmill CPET using a modified Bruce Protocol. Passive recovery in seated position. | Total of 33.3% in the CV-events group and 68.8% in the no-CV-events group. Dosage N/A. | HRR1; HRR2 = HRmax-HR at 1; 2 min of recovery; Cut off HRR1 < 13, HRR2 < 27 | Impaired HRR is associated with an exaggerated pro-inflammatory response and independently predicts clinical outcomes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozgarea, A.; Cozma, D.; Teodoru, M.; Lazăr-Höcher, A.-I.; Cirin, L.; Faur-Grigori, A.-A.; Lazăr, M.-A.; Crișan, S.; Gaiță, D.; Luca, C.-T.; et al. Heart Rate Recovery: Up to Date in Heart Failure—A Literature Review. J. Clin. Med. 2024, 13, 3328. https://doi.org/10.3390/jcm13113328
Cozgarea A, Cozma D, Teodoru M, Lazăr-Höcher A-I, Cirin L, Faur-Grigori A-A, Lazăr M-A, Crișan S, Gaiță D, Luca C-T, et al. Heart Rate Recovery: Up to Date in Heart Failure—A Literature Review. Journal of Clinical Medicine. 2024; 13(11):3328. https://doi.org/10.3390/jcm13113328
Chicago/Turabian StyleCozgarea, Andreea, Dragoș Cozma, Minodora Teodoru, Alexandra-Iulia Lazăr-Höcher, Liviu Cirin, Adelina-Andreea Faur-Grigori, Mihai-Andrei Lazăr, Simina Crișan, Dan Gaiță, Constantin-Tudor Luca, and et al. 2024. "Heart Rate Recovery: Up to Date in Heart Failure—A Literature Review" Journal of Clinical Medicine 13, no. 11: 3328. https://doi.org/10.3390/jcm13113328
APA StyleCozgarea, A., Cozma, D., Teodoru, M., Lazăr-Höcher, A.-I., Cirin, L., Faur-Grigori, A.-A., Lazăr, M.-A., Crișan, S., Gaiță, D., Luca, C.-T., & Văcărescu, C. (2024). Heart Rate Recovery: Up to Date in Heart Failure—A Literature Review. Journal of Clinical Medicine, 13(11), 3328. https://doi.org/10.3390/jcm13113328






