A Case Report Demonstrating Preservation of Vestibular Receptor Function after Transcochlear Removal of an Intracochlear Schwannoma with Extension to the Fundus of the Internal Auditory Canal
Abstract
1. Introduction
2. Patient Information and Clinical Findings
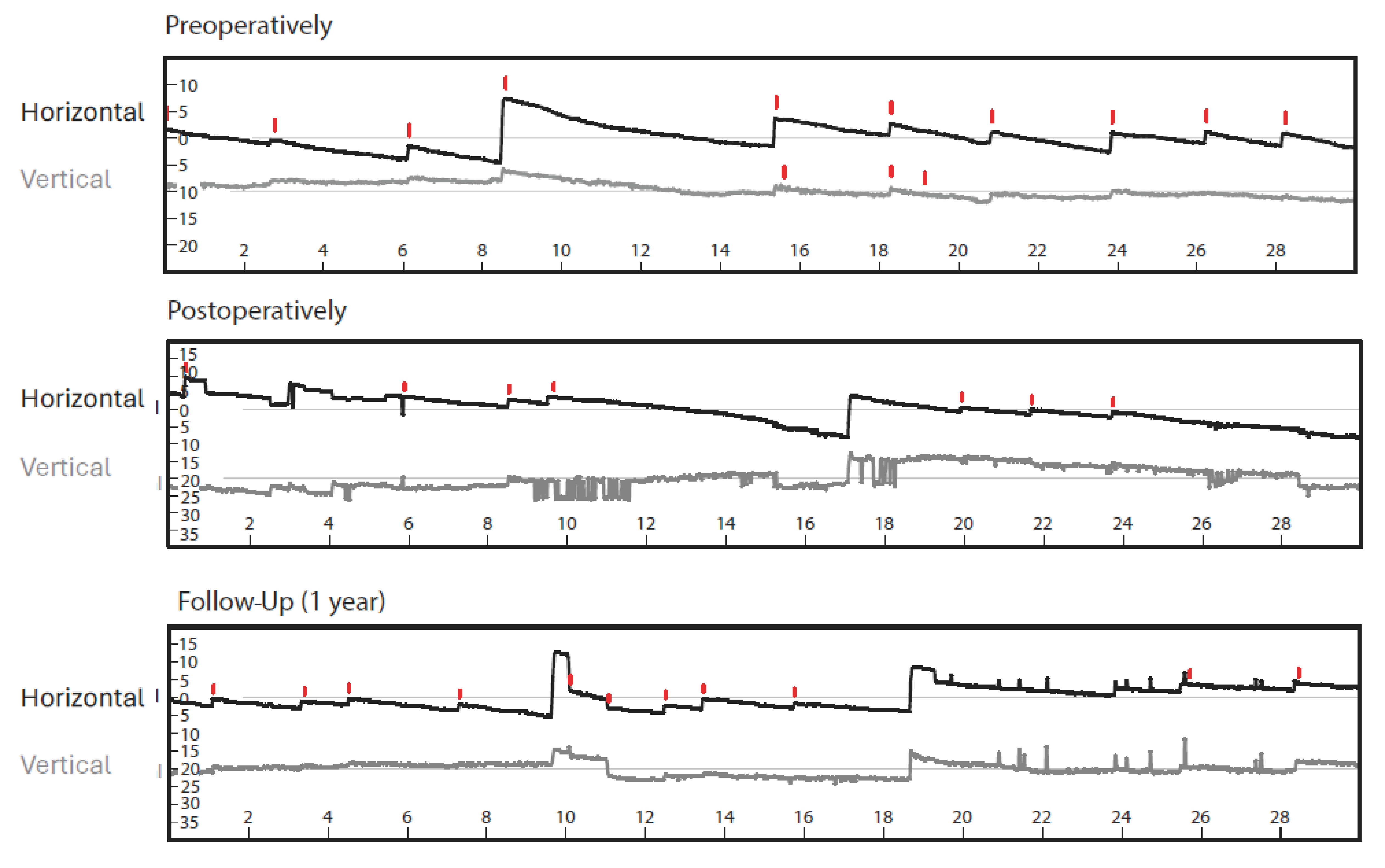
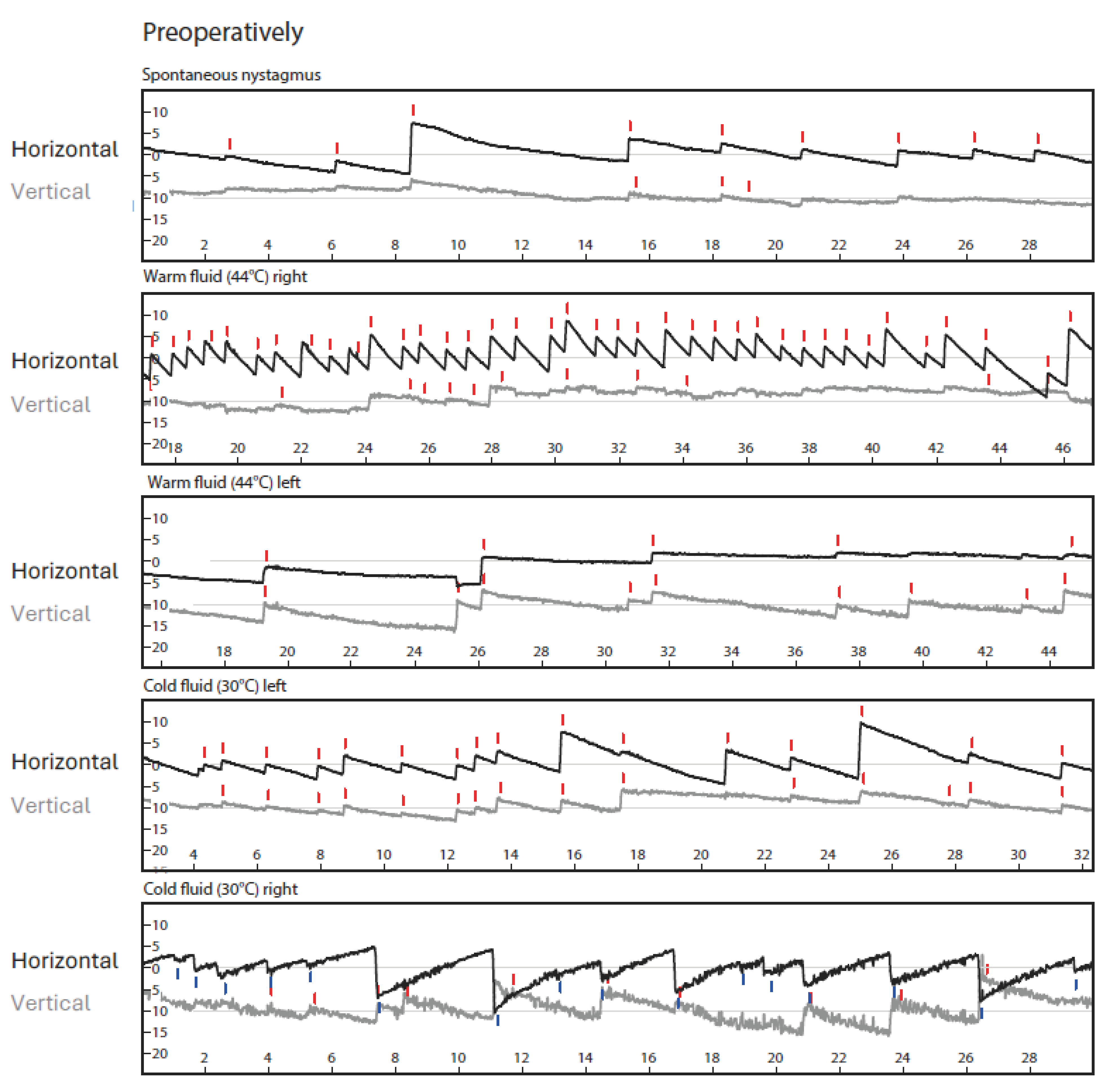
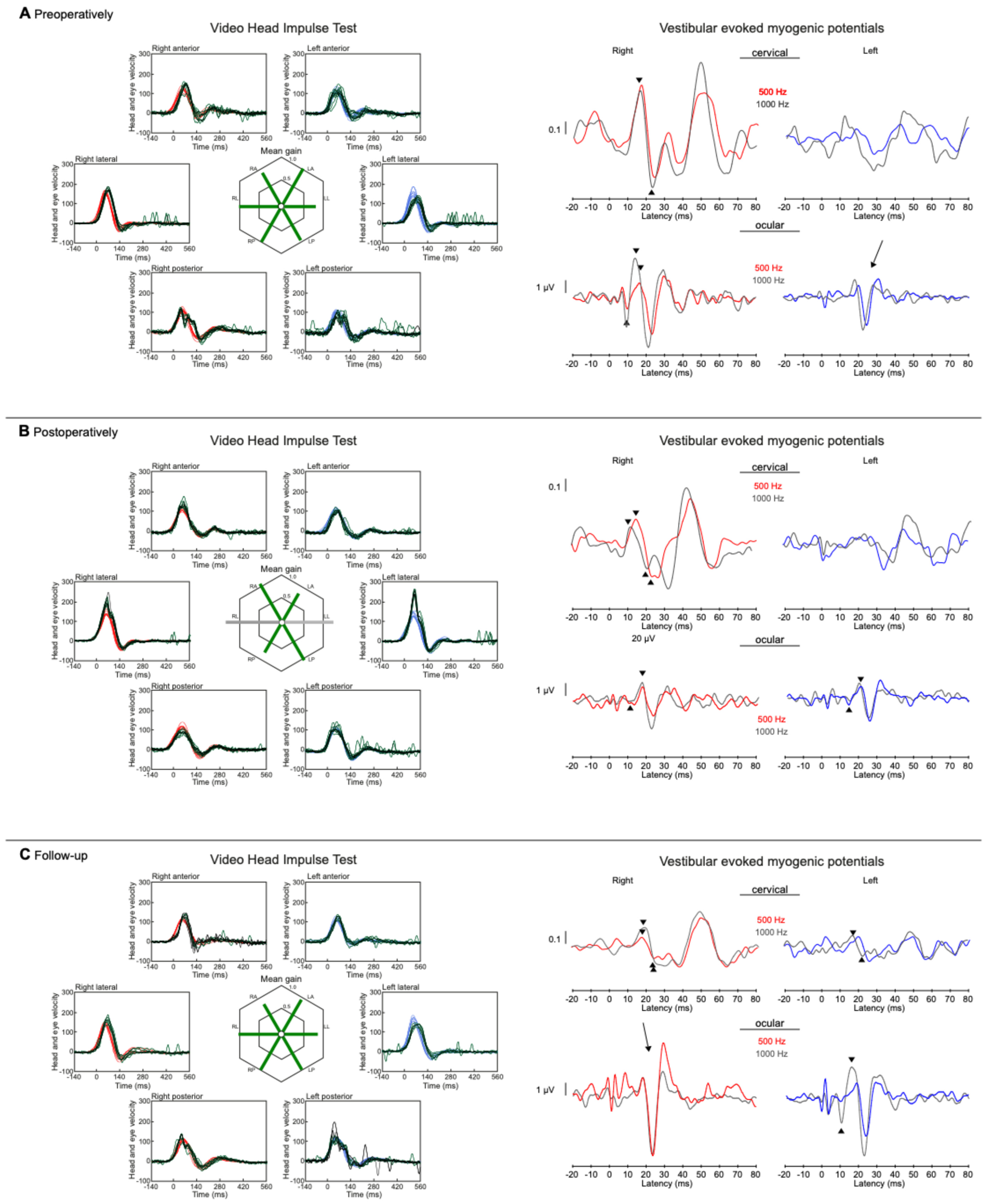
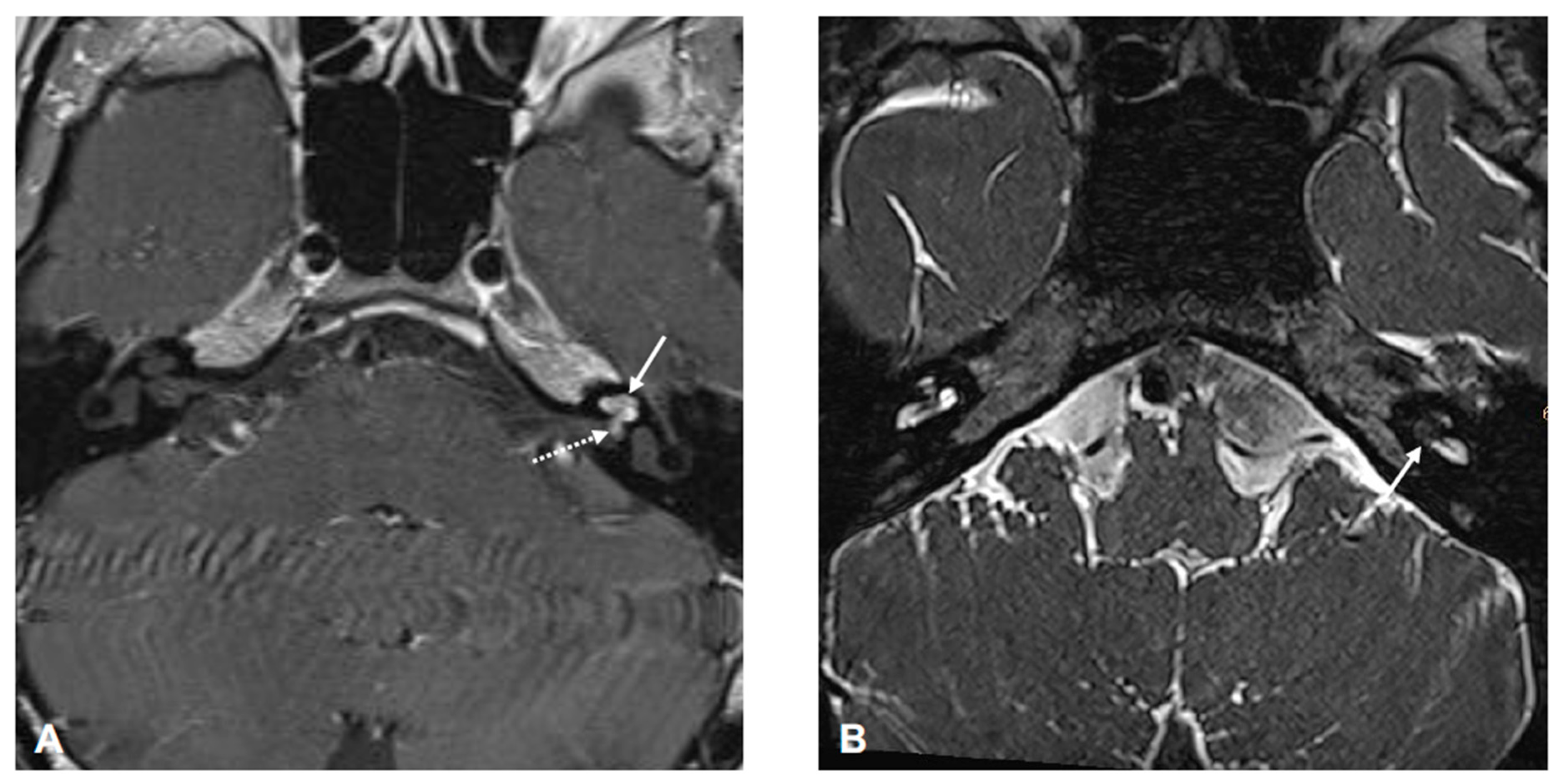
3. Diagnostic Assessment and Interpretation
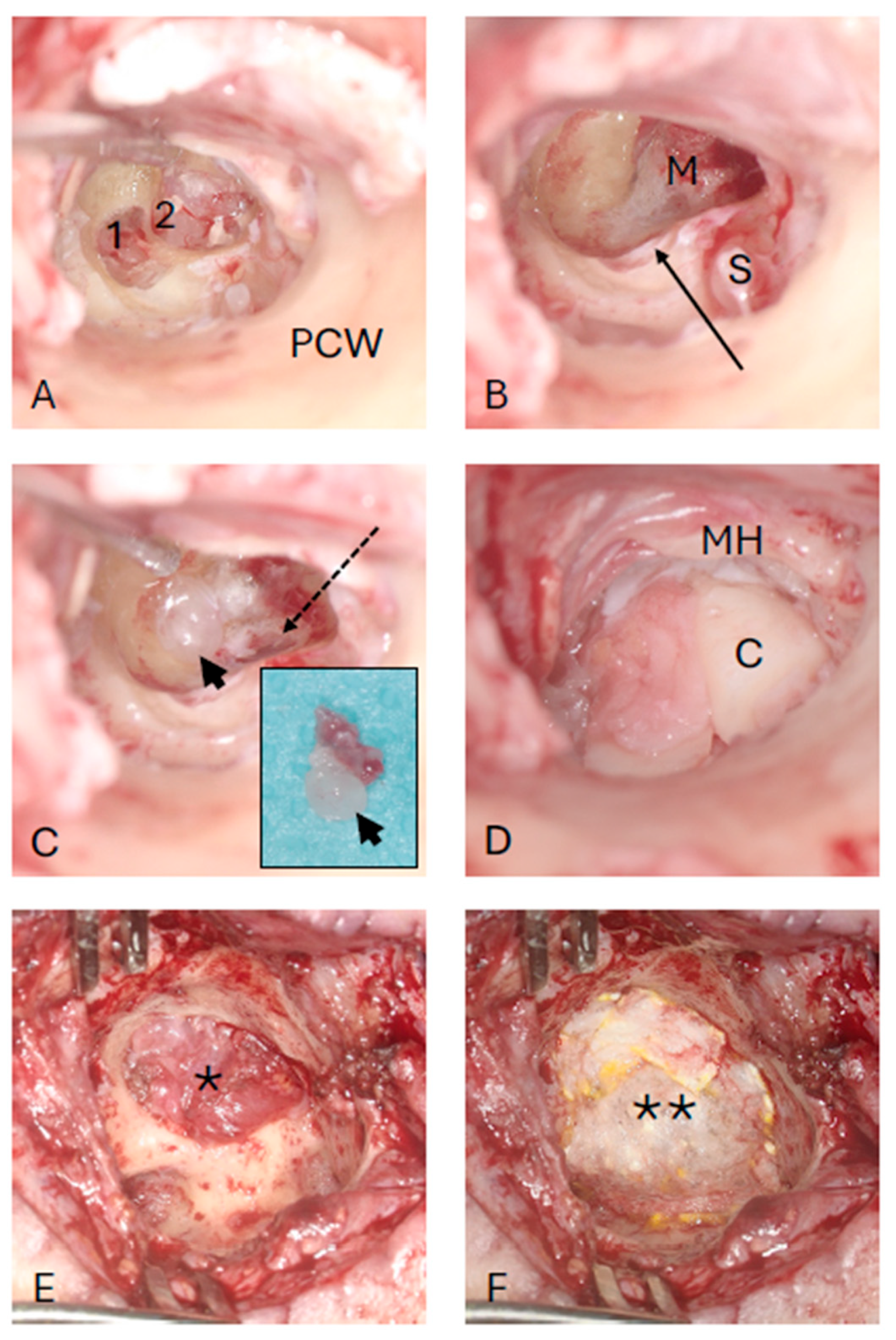
4. Intervention
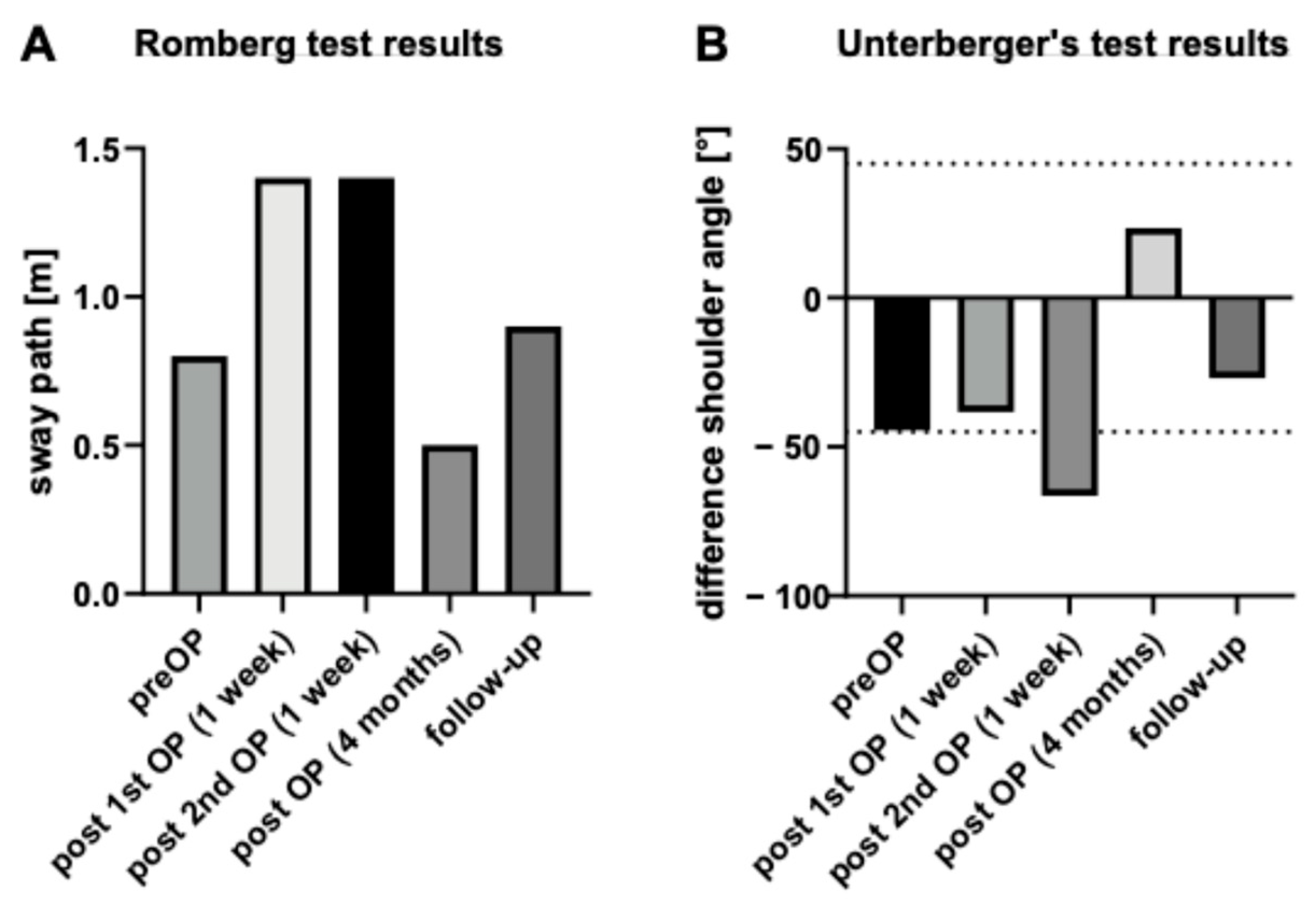
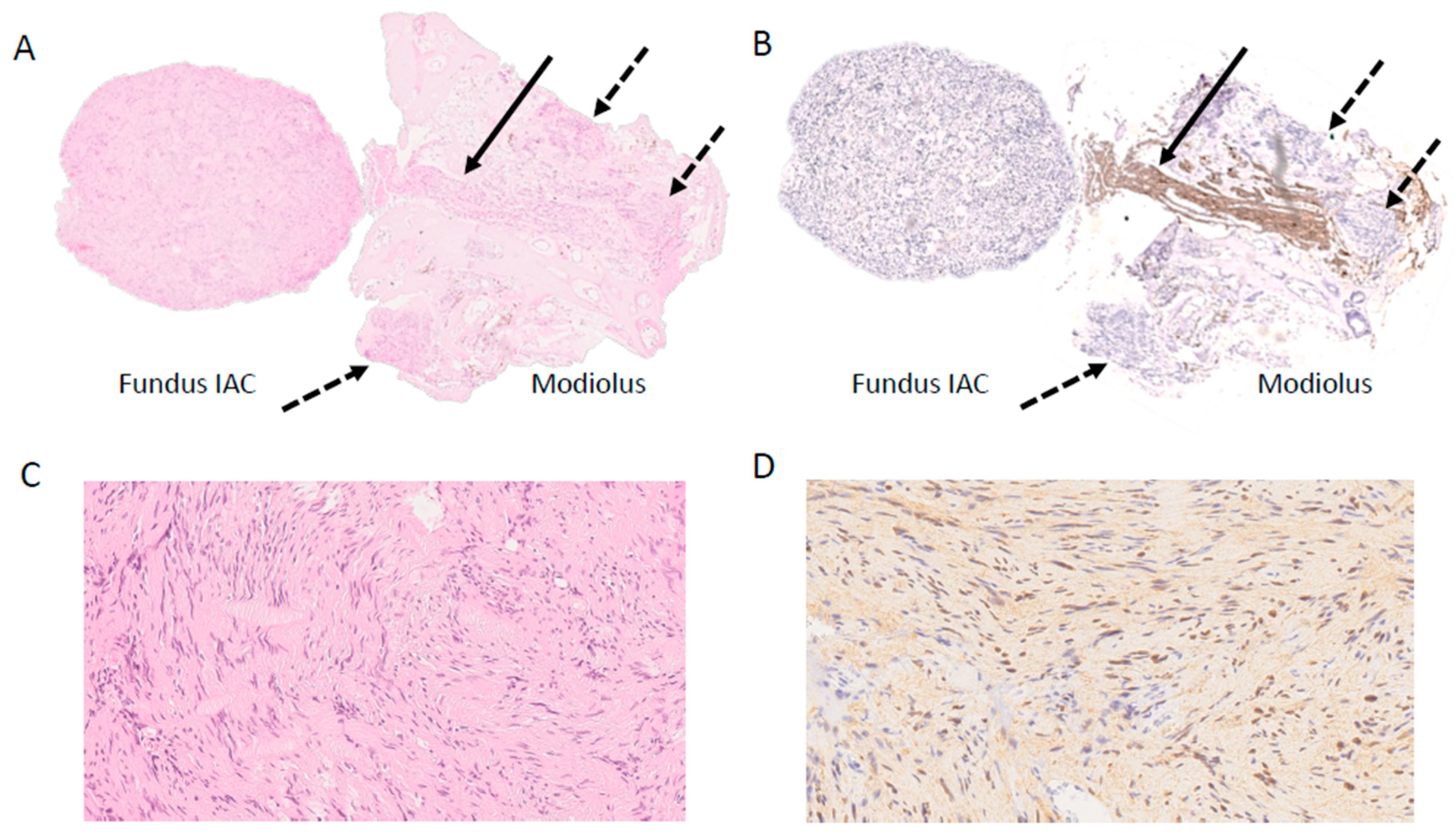
5. Follow-Up and Outcomes
6. Patient Perspective
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlson, M.L.; Link, M.J. Vestibular Schwannomas. Reply. N. Engl. J. Med. 2021, 385, 381–382. [Google Scholar] [PubMed]
- Sass, H.C.R.; Miyazaki, H.; West, N.; Hansen, S.; Moller, M.N.; Caye-Thomasen, P. Extended Retrolabyrinthine Approach: Results of Hearing Preservation Surgery Using a New System for Continuous Near Real-time Neuromonitoring in Patients with Growing Vestibular Schwannomas. Otol. Neurotol. 2019, 40 (Suppl. S1), S72–S79. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, D.; Carner, M.; Soloperto, D.; Bianconi, L.; Sacchetto, A.; Sacchetto, L.; Masotto, B.; Presutti, L. Expanded Transcanal Transpromontorial Approach: A Novel Surgical Technique for Cerebellopontine Angle Vestibular Schwannoma Removal. Otolaryngol. Head. Neck Surg. 2018, 158, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Palmisciano, P.; Doyle, E.J., 3rd; Hoz, S.S.; Cass, D.; Samy, R.N.; Andaluz, N.; Zuccarello, M. Transcanal Transpromontorial Approaches to the Internal Auditory Canal: A Systematic Review. Laryngoscope 2023, 133, 2856–2867. [Google Scholar] [CrossRef]
- Dallari, V.; Apa, E.; Monzani, D.; Genovese, E.; Marchioni, D.; Soloperto, D.; Sacchetto, L. Cochlear Implantation Following Transcanal Infrapromontorial Approach for Vestibular Schwannoma: A Case Series. Audiol. Res. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Reddy, P.; Yan, F.; Liu, Y.F.; McRackan, T.R.; Rizk, H.G. Hearing Preservation in Patients who Undergo Labyrinthectomy and Translabyrinthine Procedures: A Case Report and Systematic Review. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 741–747. [Google Scholar] [CrossRef]
- Tringali, S.; Ferber-Viart, C.; Gallego, S.; Dubreuil, C. Hearing preservation after translabyrinthine approach performed to remove a large vestibular schwannoma. Eur. Arch. Otorhinolaryngol. 2009, 266, 147–150. [Google Scholar] [CrossRef]
- Iannacone, F.P.; Visconti, F.; Zanoletti, E. How Cochlear Implant Rehabilitation Impacts the Therapeutic Strategy for Vestibular Schwannoma. Audiol. Res. 2023, 13, 116–129. [Google Scholar] [CrossRef]
- Plontke, S.K.; Lloyd, S.K.W.; Freeman, S.R.M.; Kösling, S.; Arnoldner, C.; Biggs, N.; Borsetto, D.; Gubbels, S.; Hess-Erga, J.; Koo, J.W.; et al. Revised classification of inner ear schwannomas. Otol. Neurotol. 2024, in press. [Google Scholar]
- Gosselin, E.; Maniakas, A.; Saliba, I. Meta-analysis on the clinical outcomes in patients with intralabyrinthine schwannomas: Conservative management vs. microsurgery. Eur. Arch. Otorhinolaryngol. 2016, 273, 1357–1367. [Google Scholar] [CrossRef]
- Choudhury, B.; Carlson, M.L.; Jethanamest, D. Intralabyrinthine Schwannomas: Disease Presentation, Tumor Management, and Hearing Rehabilitation. J. Neurol. Surg. B Skull Base 2019, 80, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gjini, E.K.; Kooper-Johnson, S.; Cooper, M.I.; Gallant, C.; Noonan, K.Y. Cochlear Implant Outcomes in Patients with Intralabyrinthine Schwannoma: A Scoping Review. Laryngoscope 2024. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.L.; Neff, B.A.; Sladen, D.P.; Link, M.J.; Driscoll, C.L. Cochlear Implantation in Patients with Intracochlear and Intralabyrinthine Schwannomas. Otol. Neurotol. 2016, 37, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Plontke, S.K.; Caye-Thomasen, P.; Strauss, C.; Kosling, S.; Gotze, G.; Siebolts, U.; Vordermark, D.; Wagner, L.; Frohlich, L.; Rahne, T. Management of transmodiolar and transmacular cochleovestibular schwannomas with and without cochlear implantation. HNO 2021, 69 (Suppl. S1), 7–19. [Google Scholar] [CrossRef]
- Plontke, S.K.; Rahne, T.; Curthoys, I.S.; Hakansson, B.; Frohlich, L. A case series shows independent vestibular labyrinthine function after major surgical trauma to the human cochlea. Commun. Med. 2021, 1, 37. [Google Scholar] [CrossRef]
- Eitutis, S.T.; Jansen, T.; Borsetto, D.; Scoffings, D.J.; Tam, Y.C.; Panova, T.; Tysome, J.R.; Donnelly, N.P.; Axon, P.R.; Bance, M.L. Cochlear Implantation in NF2 Patients without Intracochlear Schwannoma Removal. Otol. Neurotol. 2021, 42, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Plontke, S.K. An Improved Technique of Subtotal Cochleoectomy for Removal of Intracochlear Schwannoma and Single-stage Cochlear Implantation. Otol. Neurotol. 2020, 41, e891. [Google Scholar] [CrossRef]
- Plontke, S.K.; Frohlich, L.; Wagner, L.; Kosling, S.; Gotze, G.; Siebolts, U.; Liebau, A.; Rahne, T. How Much Cochlea Do You Need for Cochlear Implantation? Otol. Neurotol. 2020, 41, 694–703. [Google Scholar] [CrossRef]
- Dubernard, X.; Somers, T.; Veros, K.; Vincent, C.; Franco-Vidal, V.; Deguine, O.; Bordure, P.; Linder, T.; Lescanne, E.; Ayache, D.; et al. Clinical presentation of intralabyrinthine schwannomas: A multicenter study of 110 cases. Otol. Neurotol. 2014, 35, 1641–1649. [Google Scholar] [CrossRef]
- Slattery, E.L.; Babu, S.C.; Chole, R.A.; Zappia, J.J. Intralabyrinthine schwannomas mimic cochleovestibular disease: Symptoms from tumor mass effect in the labyrinth. Otol. Neurotol. 2015, 36, 167–171. [Google Scholar] [CrossRef]
- Hannigan, I.P.; Welgampola, M.S.; Watson, S.R.D. Dissociation of caloric and head impulse tests: A marker of Meniere’s disease. J. Neurol. 2021, 268, 431–439. [Google Scholar] [CrossRef] [PubMed]
- McGarvie, L.A.; Curthoys, I.S.; MacDougall, H.G.; Halmagyi, G.M. What does the head impulse test versus caloric dissociation reveal about vestibular dysfunction in Meniere’s disease? Ann. N. Y. Acad. Sci. 2015, 1343, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Migliaccio, A.A.; Della Santina, C.C.; Minor, L.B.; Carey, J.P. Search-coil head-thrust and caloric tests in Meniere’s disease. Acta Otolaryngol. 2005, 125, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Liu, B. Dissociation of Caloric and Video Head Impulse Tests in Patients with Delayed Endolymphatic Hydrops. Front. Neurol. 2020, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Blodow, A.; Helbig, R.; Wichmann, N.; Wenzel, A.; Walther, L.E.; Bloching, M.B. Video head impulse test or caloric irrigation? Contemporary diagnostic tests for vestibular schwannoma. HNO 2013, 61, 781–785. [Google Scholar]
- Machner, B.; Gottschalk, S.; Sander, T.; Helmchen, C.; Rambold, H. Intralabyrinthine schwannoma affecting the low but not high frequency function of the vestibulo-ocular reflex: Implications for the clinical diagnosis of chronic peripheral vestibular deficits. J. Neurol. Neurosurg. Psychiatry 2007, 78, 772–774. [Google Scholar] [CrossRef]
- Jerin, C.; Krause, E.; Ertl-Wagner, B.; Gurkov, R. Clinical features of delayed endolymphatic hydrops and intralabyrinthine schwannoma: An imaging-confirmed comparative case series. English version. HNO 2017, 65 (Suppl. S1), 41–45. [Google Scholar] [CrossRef]
- Venkatasamy, A.; Bretz, P.; Karol, A.; Karch-Georges, A.; Charpiot, A.; Veillon, F. MRI of endolymphatic hydrops in patients with intralabyrinthine schwannomas: A case-controlled study using non-enhanced T2-weighted images at 3 T. Eur. Arch. Otorhinolaryngol. 2021, 278, 1821–1827. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Dai, C.; Wang, W. Endolymphatic Hydrops in Patients with Intralabyrinthine Schwannomas. Front. Surg. 2020, 7, 623078. [Google Scholar] [CrossRef]
- Massager, N.; Drogba, L.; Delbrouck, C.; Benmebarek, N.; Desmedt, F.; Devriendt, D. Gamma knife radiosurgery for intralabyrinthine schwannomas. J. Radiosurg. SBRT 2011, 1, 237–245. [Google Scholar]
- Tuleasca, C.; George, M.; Schiappacasse, L.; Patin, D.; Fenu, J.; Maire, R.; Levivier, M. Gamma Knife radiosurgery for intravestibular and intracochlear schwannomas. Acta Neurochir. 2019, 161, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Schart-Moren, N.; Rajan, G.; Shaw, J.; Rohani, S.A.; Atturo, F.; Ladak, H.M.; Rask-Andersen, H.; Agrawal, S. Vestibular Organ and Cochlear Implantation-A Synchrotron and Micro-CT Study. Front. Neurol. 2021, 12, 663722. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Curthoys, I.S.; Plontke, S.K.; Menzel, M.; Mukherjee, P.; Wong, C.; Laitman, J.T. Insights into Inner Ear Function and Disease Through Novel Visualization of the Ductus Reuniens, a Seminal Communication Between Hearing and Balance Mechanisms. J. Assoc. Res. Otolaryngol. 2022, 23, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Laborai, A.; Ghiselli, S.; Cuda, D. Cochlear Implant in Patients with Intralabyrinthine Schwannoma without Tumor Removal. Audiol. Res. 2022, 12, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, H.; Chen, B.; Yuan, Y.; Wang, W.; Ren, D. Full Endoscopic Resection of Intralabyrinthine Schwannomas: A Case Series. Ear Nose Throat J. 2023, 1455613231176170. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.K.; Patel, N. Endoscope-assisted Partial Cochlectomy for Intracochlear Schwannoma with Simultaneous Cochlear Implantation: A Case Report. Otol. Neurotol. 2020, 41, 334–338. [Google Scholar] [CrossRef]
- Marchioni, D.; De Rossi, S.; Soloperto, D.; Presutti, L.; Sacchetto, L.; Rubini, A. Intralabyrinthine schwannomas: A new surgical treatment. Eur. Arch. Otorhinolaryngol. 2018, 275, 1095–1102. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plontke, S.K.; Iannacone, F.P.; Siebolts, U.; Ludwig-Kraus, B.; Kösling, S.; Wagner, L. A Case Report Demonstrating Preservation of Vestibular Receptor Function after Transcochlear Removal of an Intracochlear Schwannoma with Extension to the Fundus of the Internal Auditory Canal. J. Clin. Med. 2024, 13, 3373. https://doi.org/10.3390/jcm13123373
Plontke SK, Iannacone FP, Siebolts U, Ludwig-Kraus B, Kösling S, Wagner L. A Case Report Demonstrating Preservation of Vestibular Receptor Function after Transcochlear Removal of an Intracochlear Schwannoma with Extension to the Fundus of the Internal Auditory Canal. Journal of Clinical Medicine. 2024; 13(12):3373. https://doi.org/10.3390/jcm13123373
Chicago/Turabian StylePlontke, Stefan K., Francesco P. Iannacone, Udo Siebolts, Beatrice Ludwig-Kraus, Sabrina Kösling, and Luise Wagner. 2024. "A Case Report Demonstrating Preservation of Vestibular Receptor Function after Transcochlear Removal of an Intracochlear Schwannoma with Extension to the Fundus of the Internal Auditory Canal" Journal of Clinical Medicine 13, no. 12: 3373. https://doi.org/10.3390/jcm13123373
APA StylePlontke, S. K., Iannacone, F. P., Siebolts, U., Ludwig-Kraus, B., Kösling, S., & Wagner, L. (2024). A Case Report Demonstrating Preservation of Vestibular Receptor Function after Transcochlear Removal of an Intracochlear Schwannoma with Extension to the Fundus of the Internal Auditory Canal. Journal of Clinical Medicine, 13(12), 3373. https://doi.org/10.3390/jcm13123373





