Ten-Year Outcomes in Patients Undergoing Simultaneous Coronary and Renal Angiography—Does Renal Artery Stenosis Matter?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Procedure Characteristics
2.4. Study Endpoints
2.5. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Periprocedural and Discharge Characteristics
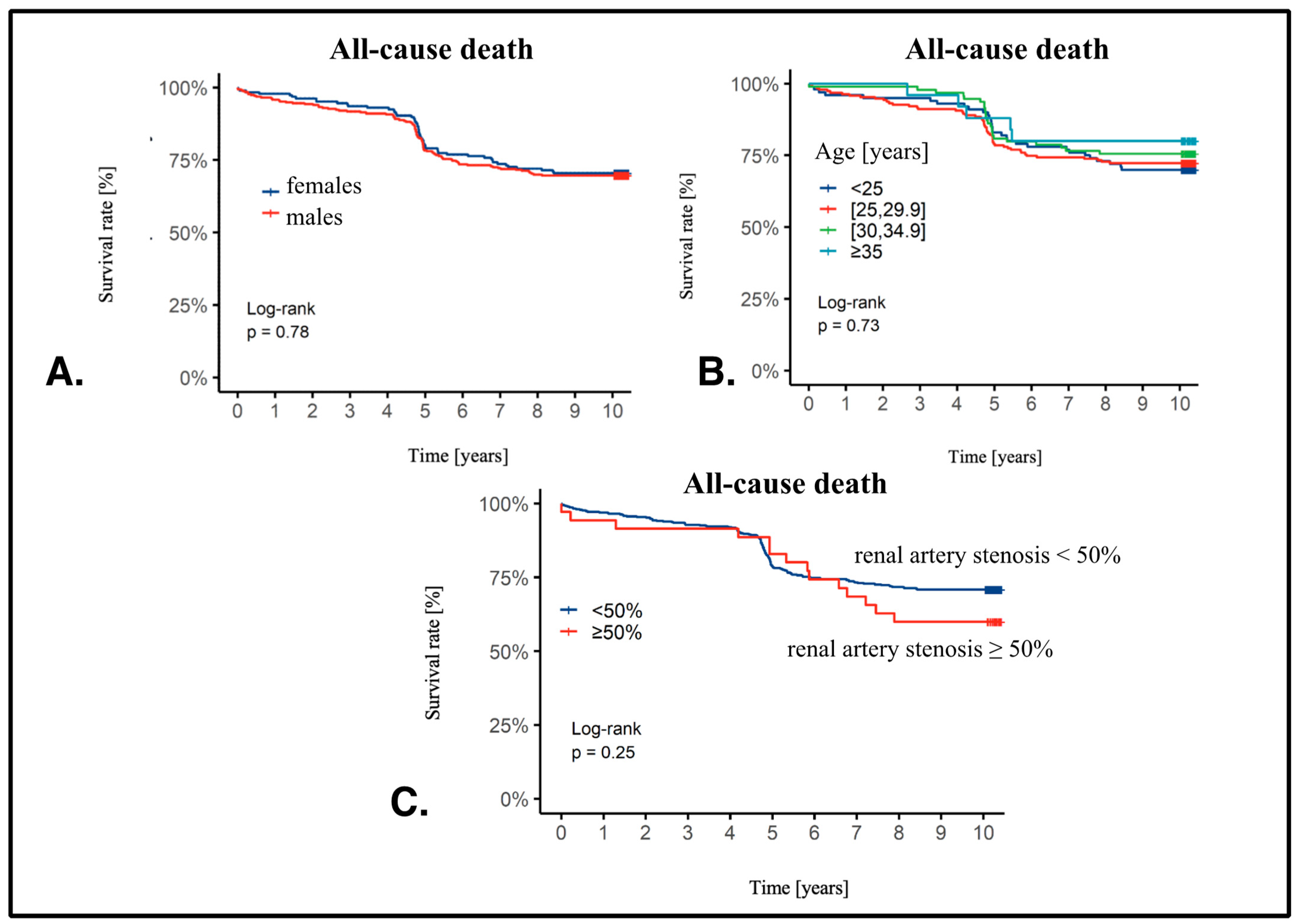
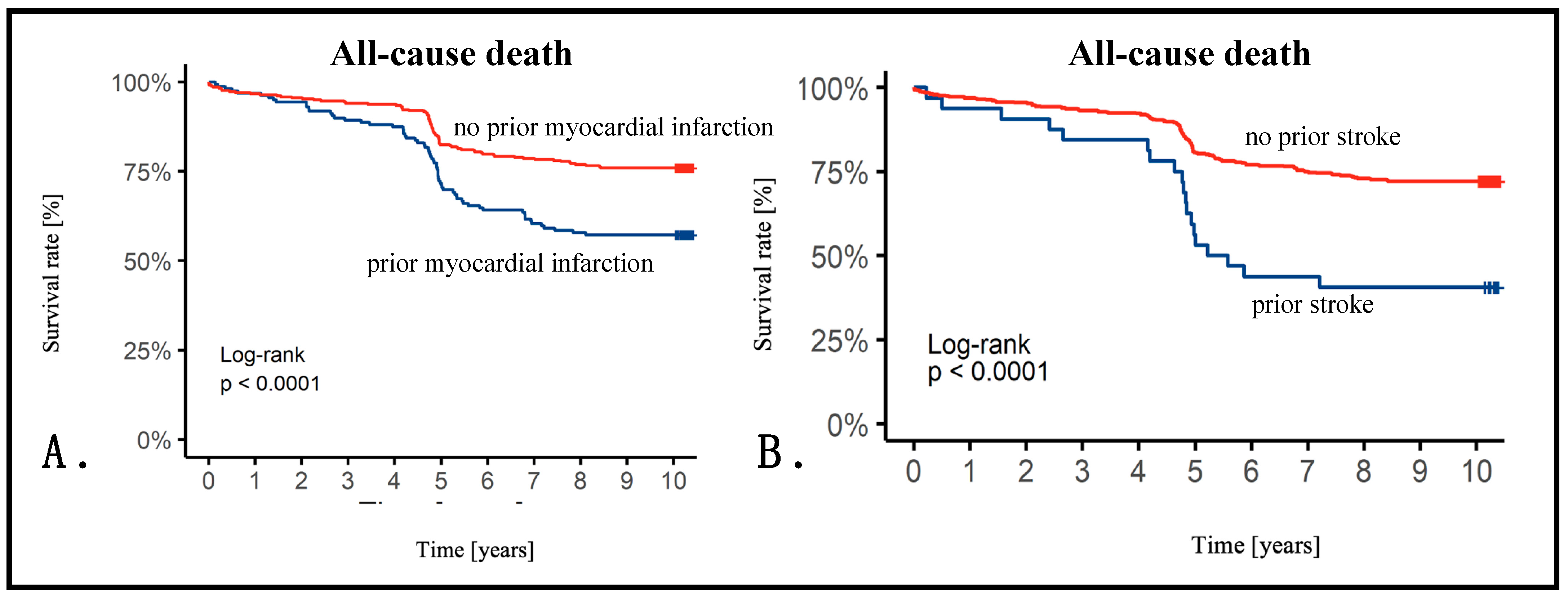
3.3. Ten-Year Follow-Up Data
3.4. Cox Analysis
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luca, A.C.; David, S.G.; David, A.G.; Tarca, V.; Paduret, I.A.; Mindru, D.E.; Rosu, S.T.; Rosu, E.V.; Adumitrachioaiei, H.; Bernic, J.; et al. Atherosclerosis from Newborn to Adult-Epidemiology, Pathological Aspects, and Risk Factors. Life 2023, 13, 2056. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Mallamaci, F.; Finocchiaro, P. Atherosclerotic renal artery stenosis: Epidemiology, cardiovascular outcomes, and clinical prediction rules. J. Am. Soc. Nephrol. 2002, 13 (Suppl. S3), S179–S183. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, S.; Nishida, N.; Uzu, T.; Takeji, M.; Nishimura, M.; Fujii, T.; Nakamura, S.; Inenaga, T.; Yutani, C.; Kimura, G. Prevalence of renal artery stenosis in autopsy patients with stroke. Stroke 2000, 31, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, P.T.; Kaiser, S.; Heinemann, L.; Frenzel, H.; Berger, M. Prevalence of renal artery stenosis in diabetes mellitus--an autopsy study. J. Intern. Med. 1991, 229, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Kalra, P.A.; Guo, H.; Kausz, A.T.; Gilbertson, D.T.; Liu, J.; Chen, S.C.; Ishani, A.; Collins, A.J.; Foley, R.N. Atherosclerotic renovascular disease in United States patients aged 67 years or older: Risk factors, revascularization, and prognosis. Kidney Int. 2005, 68, 293–301. [Google Scholar] [CrossRef]
- Salehi, N.; Firouzi, A.; Gholoobi, A.; Shakerian, F.; Sanati, H.R.; Ahmadabadi, M.N.; Moradi, M. relationship between distribution of coronary artery lesions and renal artery stenosis in patients undergoing simultaneous coronary and renal angiography. Clin. Med. Insights Cardiol. 2011, 5, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Dzielińska, Z.; Januszewicz, A.; Demkow, M.; Makowiecka-Cieśla, M.; Prejbisz, A.; Naruszewicz, M.; Nowicka, G.; Kadziela, J.; Zieliński, T.; Florczak, E.; et al. Cardiovascular risk factors in hypertensive patients with coronary artery disease and coexisting renal artery stenosis. J. Hypertens. 2007, 25, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Zamorano, J.; Habib, G.; Badano, L. The EACVI Textbook of Echocardiography; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Bil, J.; MoZeNska, O.; Segiet-SwiEcicka, A.; Gil, R.J. Revisiting the use of the provocative acetylcholine test in patients with chest pain and nonobstructive coronary arteries: A five-year follow-up of the AChPOL registry, with special focus on patients with MINOCA. Transl. Res. 2021, 231, 64–75. [Google Scholar] [CrossRef]
- Oliveira, M.D.; Navarro, E.C.; Branca, N.R.; Garcia, M.E.; Scarpa, M.C.; Caixeta, A. Coronary procedures via distal transradial access in older as compared with non-older patients: Insights from the DISTRACTION registry. J. Invasive Cardiol. 2023, 35. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schutt, K.; Muller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.C.; Cerci, R.; Matheson, M.B.; Magalhaes, T.; Kishi, S.; Brinker, J.; Clouse, M.E.; Rochitte, C.E.; Cox, C.; Lima, J.A.C.; et al. Predicting Significant Coronary Obstruction in a Population with Suspected Coronary Disease and Absence of Coronary Calcium: CORE-64/CORE320 Studies. Arq. Bras. Cardiol. 2023, 120, e20220183. [Google Scholar] [CrossRef]
- Neglia, D.; Liga, R.; Gimelli, A.; Podlesnikar, T.; Cvijic, M.; Pontone, G.; Miglioranza, M.H.; Guaricci, A.I.; Seitun, S.; Clemente, A.; et al. Use of cardiac imaging in chronic coronary syndromes: The EURECA Imaging registry. Eur. Heart J. 2023, 44, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Wara-Aswapati, S.; Kaewkes, D.; Chotmongkol, V.; Sawanyawisuth, K. Clinical predictive factors of coronary stenosis in patients with high-risk valvular heart disease who received diagnostic coronary angiography prior to cardiac valve surgery. Biomed. Rep. 2024, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Buller, P.; Kern, A.; Tyczynski, M.; Rosiak, W.; Figatowski, W.; Gil, R.J.; Bil, J. The Comparison of Predicting Factors and Outcomes of MINOCA and STEMI Patients in the 5-Year Follow-Up. J. Pers. Med. 2023, 13, 856. [Google Scholar] [CrossRef] [PubMed]
- Kern, A.; Stompor, T.; Kiewisz, J.; Krazinski, B.E.; Kiezun, J.; Kiezun, M.; Gorny, J.; Sienkiewicz, E.; Drozdowska, B.; Bil, J. Association of serum sclerostin levels with atherosclerosis severity in patients referred for invasive coronary angiography. Kardiol. Pol. 2020, 78, 1271–1273. [Google Scholar] [CrossRef]
- Kern, A.; Stompor, T.; Kiewisz, J.; Krazinski, B.E.; Kiezun, J.; Kiezun, M.; Gorny, J.; Sienkiewicz, E.; Gromadzinski, L.; Onichimowski, D.; et al. The Impact of Sclerostin Levels on Long-Term Prognosis in Patients Undergoing Coronary Angiography: A Personalized Approach with 9-Year Follow-Up. J. Pers. Med. 2021, 11, 186. [Google Scholar] [CrossRef]
- Goy, J.J.; Kaufmann, U.; Hurni, M.; Cook, S.; Versaci, F.; Ruchat, P.; Bertel, O.; Pieper, M.; Meier, B.; Chiarello, L.; et al. 10-year follow-up of a prospective randomized trial comparing bare-metal stenting with internal mammary artery grafting for proximal, isolated de novo left anterior coronary artery stenosis the SIMA (Stenting versus Internal Mammary Artery grafting) trial. J. Am. Coll. Cardiol. 2008, 52, 815–817. [Google Scholar] [CrossRef]
- Muller, O.; Mangiacapra, F.; Ntalianis, A.; Verhamme, K.M.; Trana, C.; Hamilos, M.; Bartunek, J.; Vanderheyden, M.; Wyffels, E.; Heyndrickx, G.R.; et al. Long-term follow-up after fractional flow reserve-guided treatment strategy in patients with an isolated proximal left anterior descending coronary artery stenosis. JACC Cardiovasc. Interv. 2011, 4, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, J.; Tanaka, N.; Shindo, N.; Ogawa, M.; Kimura, Y.; Sakoda, K.; Murata, N.; Hokama, Y.; Hoshino, K.; Ikeda, S.; et al. Seven-year clinical outcomes of patients with moderate coronary artery stenosis after deferral of revascularization based on gray-zone fractional flow reserve. Cardiovasc. Interv. Ther. 2015, 30, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Jovin, D.G.; Sumpio, B.E.; Greif, D.M. Manifestations of human atherosclerosis across vascular beds. JVS-Vasc. Insights 2024, 100089. [Google Scholar] [CrossRef]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef]
- Juillet, Y.; Blanchard, J.; Martin, J.J.; Dubois, C.; Fiessinger, J.N.; Cormier, J.M.; Housset, E. Diffusion of atherosclerosis in patients with arterial disease of the lower limbs (author’s transl). Ann. Med. Interne 1981, 132, 252–256. [Google Scholar]
- Uchiyama, S.; Goto, S.; Matsumoto, M.; Nagai, R.; Origasa, H.; Yamazaki, T.; Shigematsu, H.; Shimada, K.; Yamada, N.; Bhatt, D.L.; et al. Cardiovascular event rates in patients with cerebrovascular disease and atherothrombosis at other vascular locations: Results from 1-year outcomes in the Japanese REACH Registry. J. Neurol. Sci. 2009, 287, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Takx, R.A.; Zanen, P.; Leiner, T.; van der Graaf, Y.; de Jong, P.A.; the SMART Study Group. The interdependence between cardiovascular calcifications in different arterial beds and vascular risk factors in patients at high cardiovascular risk. Atherosclerosis 2015, 238, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Heldner, M.R.; Li, L.; Lovett, N.G.; Kubiak, M.M.; Lyons, S.; Rothwell, P.M.; Oxford Vascular, S. Long-Term Prognosis of Patients With Transient Ischemic Attack or Stroke and Symptomatic Vascular Disease in Multiple Arterial Beds. Stroke 2018, 49, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Bjorck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef]
- Dobrek, L. An Outline of Renal Artery Stenosis Pathophysiology-A Narrative Review. Life 2021, 11, 208. [Google Scholar] [CrossRef]
- Colbert, G.B.; Abra, G.; Lerma, E.V. Update and review of renal artery stenosis. Dis. Mon. 2021, 67, 101118. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006, 113, e463–e654. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.J.; Edwards, M.S.; Craven, T.E.; Cherr, G.S.; Jackson, S.A.; Appel, R.G.; Burke, G.L.; Dean, R.H. Prevalence of renovascular disease in the elderly: A population-based study. J. Vasc. Surg. 2002, 36, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Bageacu, S.; Cerisier, A.; Isaaz, K.; Nourissat, A.; Barral, X.; Favre, J.P. Incidental visceral and renal artery stenosis in patients undergoing coronary angiography. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Rihal, C.S.; Textor, S.C.; Breen, J.F.; McKusick, M.A.; Grill, D.E.; Hallett, J.W.; Holmes, D.R., Jr. Incidental renal artery stenosis among a prospective cohort of hypertensive patients undergoing coronary angiography. Mayo Clin. Proc. 2002, 77, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.J.; Murphy, T.P.; Cutlip, D.E.; Jamerson, K.; Henrich, W.; Reid, D.M.; Cohen, D.J.; Matsumoto, A.H.; Steffes, M.; Jaff, M.R.; et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N. Engl. J. Med. 2014, 370, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Van Jaarsveld, B.C.; Krijnen, P.; Pieterman, H.; Derkx, F.H.; Deinum, J.; Postma, C.T.; Dees, A.; Woittiez, A.J.; Bartelink, A.K.; Man in ‘t Veld, A.J.; et al. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N. Engl. J. Med. 2000, 342, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Shin, J.H.; Park, H.C.; Kim, S.G.; Choi, S.I. A prediction model for renal artery stenosis using carotid ultrasonography measurements in patients undergoing coronary angiography. BMC Nephrol. 2014, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Buller, C.E.; Nogareda, J.G.; Ramanathan, K.; Ricci, D.R.; Djurdjev, O.; Tinckam, K.J.; Penn, I.M.; Fox, R.S.; Stevens, L.A.; Duncan, J.A.; et al. The profile of cardiac patients with renal artery stenosis. J. Am. Coll. Cardiol. 2004, 43, 1606–1613. [Google Scholar] [CrossRef]
- Kihm, M.C.; Vogel, B.; Zeier, M.; Kihm, L.P. Renal Artery Stenosis—Are there Patients who Benefit from Intervention? Exp. Clin. Endocrinol. Diabetes 2016, 124, 342–349. [Google Scholar] [CrossRef]
- Burlacu, A.; Siriopol, D.; Voroneanu, L.; Nistor, I.; Hogas, S.; Nicolae, A.; Nedelciuc, I.; Tinica, G.; Covic, A. Atherosclerotic Renal Artery Stenosis Prevalence and Correlations in Acute Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Interventions: Data From Nonrandomized Single-Center Study (REN-ACS)-A Single Center, Prospective, Observational Study. J. Am. Heart Assoc. 2015, 4, e002379. [Google Scholar] [CrossRef] [PubMed]
- Caps, M.T.; Perissinotto, C.; Zierler, R.E.; Polissar, N.L.; Bergelin, R.O.; Tullis, M.J.; Cantwell-Gab, K.; Davidson, R.C.; Strandness, D.E., Jr. Prospective study of atherosclerotic disease progression in the renal artery. Circulation 1998, 98, 2866–2872. [Google Scholar] [CrossRef] [PubMed]
- Jennings, C.G.; Houston, J.G.; Severn, A.; Bell, S.; Mackenzie, I.S.; Macdonald, T.M. Renal artery stenosis-when to screen, what to stent? Curr. Atheroscler. Rep. 2014, 16, 416. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.O.; Higgins, R.M.; Walters, H.L.; Mackie, A.D.; Drury, P.L.; Hendry, B.M.; Scoble, J.E. Presentation, clinical features and outcome in different patterns of atherosclerotic renovascular disease. QJM 1994, 87, 413–421. [Google Scholar] [PubMed]
- Conlon, P.J.; Little, M.A.; Pieper, K.; Mark, D.B. Severity of renal vascular disease predicts mortality in patients undergoing coronary angiography. Kidney Int. 2001, 60, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, L.U.; Napolitano, B.; Bellucci, A.G.; Vernace, M.; Wilkes, B.M.; Mossey, R.T. Renal vascular disease causing end-stage renal disease, incidence, clinical correlates, and outcomes: A 20-year clinical experience. Am. J. Kidney Dis. 1994, 24, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.A.; Pavlinac, P.; Wright, C.M. The differential associations between HDL, non-HDL and total cholesterols and atherosclerotic calcium deposits in multiple vascular beds. Atherosclerosis 2007, 194, E87–E94. [Google Scholar] [CrossRef]
- Dregoesc, M.I.; Bolboaca, S.D.; Doroltan, P.M.; Istrate, M.; Marc, M.C.; Iancu, A.C. Long-Term Mortality After Renal Artery Stenting in Patients With Severe Atherosclerotic Renal Artery Stenosis and High-Risk Clinical Manifestations. Am. J. Hypertens. 2021, 34, 880–887. [Google Scholar] [CrossRef]
- Zachrisson, K.; Elverfors, S.; Jensen, G.; Hellstrom, M.; Svensson, M.; Herlitz, H.; Falkenberg, M. Long-term outcome of stenting for atherosclerotic renal artery stenosis and the effect of angiographic restenosis. Acta Radiol. 2018, 59, 1438–1445. [Google Scholar] [CrossRef]

| Parameter | Study Population N = 492 (%) | Patients with RAS < 50% N = 457 (%) | Patients with RAS ≥ 50% N = 35 (%) | p |
|---|---|---|---|---|
| Females | 183 (37.2%) | 163 (35.7%) | 20 (57.1%) | 0.011 |
| Age (years) | 64.4 ± 9.9 | 64.0 ± 9.7 | 69.1 ± 10.4 | 0.005 |
| BMI (kg/m2) | 27.9 ± 4.3 | 28.0 ± 4.3 | 26.9 ± 3.2 | 0.307 |
| Arterial hypertension | 367 (74.6%) | 339 (74.2%) | 28 (80.0%) | 0.446 |
| Dyslipidemia | 226 (45.9%) | 212 (46.4%) | 14 (40.0%) | 0.465 |
| Diabetes | 128 (26.0%) | 114 (24.9%) | 14 (40.0%) | 0.049 |
| Obesity | 147 (29.9%) | 135 (29.5%) | 12 (34.3%) | 0.554 |
| Previous MI | 157 (31.9%) | 144 (31.5%) | 13 (37.1%) | 0.491 |
| Previous stroke | 32 (6.5%) | 24 (5.3%) | 8 (22.9%) | <0.001 |
| Peripheral artery disease | 25 (5.1%) | 23 (5.0%) | 2 (5.7%) | 0.696 |
| Chronic kidney disease | 48 (9.8%) | 38 (8.3%) | 10 (28.6%) | <0.001 |
| Chronic kidney disease treated with hemodialysis | 4 (0.8%) | 2 (0.4%) | 2 (5.7%) | 0.027 |
| Previous CABG | 21 (4.3%) | 17 (3.7%) | 4 (11.4%) | 0.054 |
| Previous PCI | 105 (21.3%) | 96 (21.0%) | 9 (25.7%) | 0.512 |
| Echocardiography results | ||||
| LV ejection fraction (%) | 52.5 ± 11.5 | 52.7 ± 11.5 | 51.3 ± 11,9 | 0.475 |
| LVEDd [mm] | 49 [46–54] | 49 [46–54.5] | 48.5 [42.2–51.8] | 0.135 |
| IVSd [mm] | 10 [8–12] | 10 [8–12.7] | 10 [8–12] | 0.868 |
| RVSP [mmHg] | 39 [32.8–45.8] | 39 [35–46] | 26 [22–30] | 0.202 |
| TAPSE [mm] | 17.5 [13.2–21.0] | 18 [14–21] | 17 [13–19] | 0.381 |
| Parameter | Study Population N = 492 (%) | Patients with RAS < 50% N = 457 (%) | Patients with RAS ≥ 50% N = 35 (%) | p |
|---|---|---|---|---|
| Erythrocytes [1012/L] | 4.7 [4.3–5.0] | 4.7 [4.3–5.0] | 4.4 [4.2–4.8] | 0.026 |
| Hemoglobin [g/dL] | 14 [13–14.9] | 14 [13–14.9] | 13 [12.6–14.5] | 0.014 |
| Glucose [mg/dL] | 103 [93–126] | 103 [93–126] | 113 [93.5–139] | 0.316 |
| Creatinine [mg/dL] | 0.9 [0.8–1.0] | 0.9 [0.8–1.0] | 1.1 [0.8–1.4] | 0.003 |
| eGFR [mL/min] | 81 [67–93] | 82 [68–93] | 65 [52.2–79.2] | <0.001 |
| K+ [mmol/L] | 4.3 [4.1–4.7] | 4.3 [4.1–4.7] | 4.3 [4.1–4.6] | 0.711 |
| Na+ [mmol/L] | 141 [139–143] | 141 [139–143] | 140 [138–142] | 0.298 |
| Total cholesterol [mg/dL] | 178 [149.8–211] | 177 [149–210.8] | 179.5 [153.2–224] | 0.508 |
| LDL [mg/dL] | 101.5 [76–135.2] | 101 [76–135] | 111 [77.5–141] | 0.573 |
| HDL [mg/dL] | 51 [42–62] | 51 [42–62] | 46 [40–57.5] | 0.186 |
| Triglycerides [mg/dL] | 116 [86–161] | 115 [85.8–161] | 124 [88.5–158.5] | 0.704 |
| TSH [μIU/mL] | 1.3 [0.8–2.1] | 1.3 [0.8–2.1] | 1.0 [0.6–1.5] | 0.276 |
| NT-proBNP [pg/mL] | 1307 [627–4334] | 1148 [638–4295] | 1699 [934–8368] | 0.605 |
| hs-CRP [mg/L] | 0.2 [0.1–0.5] | 0.2 [0.1–0.5] | 0.4 [0.2–0.8] | 0.040 |
| Uric acid [mg/dL] | 8.4 [8.3–8.6] | 8.4 [8.3–8.6] | - | - |
| Max. TnT [μg/L] | 0.7 [0.1–1.4] | 0.6 [0.1–1.1] | 1.0 [0.2–1.6] | 0.320 |
| Max. CK-MB [IU/mL] | 23 [16.2–42] | 23 [17–42] | 13 [10–24] | 0.171 |
| Parameter | Study Population N = 492 (%) | Patients with RAS < 50% N = 457 (%) | Patients with RAS ≥ 50% N = 35 (%) | p |
|---|---|---|---|---|
| Coronary angiography indications | ||||
| Planned coronary artery disease diagnostics | 253 (51.4%) | 238 (52.1%) | 15 (42.9%) | 0.016 |
| Acute coronary syndrome | 186 (37.8%) | 169 (36.9%) | 17 (48.6%) | |
| Heart failure diagnostics | 8 (1.6%) | 7 (1.5%) | 1 (2.9%) | |
| Pacemaker/ICD qualification | 14 (2.8%) | 14 (3.1%) | 0 | |
| Cardiovascular surgery (heart valve defect, ascending aorta aneurysm) | 28 (5.7%) | 26 (5.7%) | 2 (5.7%) | |
| Others | 3 (0.6%) | 3 (0.7%) | 0 | |
| Coronary angiography results | ||||
| No significant atherosclerotic lesions * | 128 (26.0%) | 122 (26.7%) | 6 (17.1%) | 0.009 |
| One-vessel disease | 140 (38.1%) | 136 (29.8%) | 4 (11.4%) | |
| Two-vessel disease | 138 (37.6%) | 124 (27.1%) | 14 (40.0%) | |
| Three-vessel disease | 64 (17.4%) | 56 (12.3%) | 8 (22.9%) | |
| Left main stem | 22 (6.0%) | 19 (4.2%) | 3 (8.6%) | |
| Qualification for revascularization | ||||
| Pharmacological treatment | 191 (38.8%) | 8 (24.2%) | 8 (24.2%) | 0.023 |
| PCI | 211 (46.1%) | 19 (57.6%) | 19 (57.6%) | |
| CABG | 90 (19.7%) | 8 (24.2%) | 8 (24.2%) | |
| Location of lesions treated by PCI (n = 238) | ||||
| Left main stem | 1 (0.4%) | 1 (0.5%) | 0 | 0.628 |
| Left anterior descending artery/ diagonal branches | 90 (37.8%) | 80 (41.7%) | 10 (52.6%) | |
| Left circumflex artery/marginal branches | 59 (24.8%) | 55 (28.6%) | 4 (21.1%) | |
| Intermediate artery | 4 (1.7%) | 4 (2.1%) | 0 | |
| Right coronary artery | 82 (34.5%) | 75 (39.1%) | 7 (36.8%) | |
| Venous graft | 2 (0.8%) | 2 (1.0%) | 0 | |
| Coronary bifurcation | 3 (0.6%) | 3 (1.6%) | 0 | 0.65 |
| Number of implanted bare metal stents | ||||
| 1 | 120 (82.8%) | 109 (81.9%) | 11 (91.7%) | 0.758 |
| 2 | 21 (14.5%) | 20 (15.1%) | 1 (8.3%) | |
| 3 | 4 (2.8%) | 4 (3.0%) | 0 | |
| Number of implanted drug-eluting stents | ||||
| 1 | 77 (89.5%) | 70 (90.9%) | 7 (77.8%) | 0.153 |
| 2 | 8 (9.3%) | 6 (7.8%) | 2 (22.2%) | |
| 3 | 1 (1.2%) | 1 (1.3%) | 0 | |
| TIMI after PCI (n = 211) | N = 192 | N = 19 | ||
| 0 | 11 (5.2%) | 11 (5.7%) | 0 | 0.723 |
| 1 | 3 (1.4%) | 3 (1.6%) | 0 | |
| 2 | 1 (0.5%) | 1 (0.5%) | 0 | |
| 3 | 196 (92.9%) | 177 (92.2%) | 19 (100%) | |
| Periprocedural complications (PCI) (n = 211) | N = 192 | N = 19 | ||
| No reflow/slow reflow | 9 (4.3%) | 9 (4.7%) | 0 | 0.998 |
| Stent thrombosis | 1 (0.5%) | 1 (0.5%) | 0 | 1 |
| Parameter | Study Population N = 492 (%) | Patients with RAS < 50% N = 457 (%) | Patients with RAS ≥ 50% N = 35 (%) | p |
|---|---|---|---|---|
| Acetylsalicylic acid | 444 (90.2%) | 410 (89.7%) | 34 (97.1%) | 0.999 |
| Clopidogrel | 304 (61.8%) | 280 (61.3%) | 24 (68.6%) | 0.091 |
| ACE inhibitor | 427 (86.8%) | 399 (87.3%) | 28 (80.0%) | 0.170 |
| Angiotensin antagonist | 21 (4.3%) | 20 (4.4%) | 1 (2.9%) | 0.290 |
| Beta-blocker | 455 (92.5%) | 426 (93.2%) | 29 (82.9%) | 0.999 |
| Ca-blocker | 124 (25.2%) | 110 (24.1%) | 14 (40.0%) | 0.090 |
| Statin | 460 (93.5%) | 429 (93.9%) | 31 (88.6%) | 0.476 |
| Fibrate | 17 (3.5%) | 17 (3.7%) | 0 | 0.999 |
| Loop diuretic | 90 (18.3%) | 77 (16.8%) | 13 (37.1%) | <0.001 |
| Thiazide | 51 (10.4%) | 49 (10.7%) | 2 (5.7%) | 0.713 |
| Mineralocorticoid receptor antagonist | 66 (13.4%) | 62 (13.6%) | 4 (11.4%) | 0.785 |
| Vitamin K antagonist | 36 (7.3%) | 30 (6.6%) | 6 (17.1%) | 0.006 |
| Oral hypoglycemic drugs | 87 (17.7%) | 79 (17.3%) | 8 (22.9%) | 0.384 |
| Insulin | 45 (9.1%) | 40 (8.8%) | 5 (14.3%) | 0.579 |
| Endpoint | Study Population N = 492 (%) | Patients with RAS < 50% N = 457 (%) | Patients with RAS ≥ 50% N = 35 (%) | p |
|---|---|---|---|---|
| Death | 147 (29.9%) | 133 (29.2%) | 14 (40.0%) | 0.177 |
| MI | 58 (11.8%) | 54 (11.8%) | 4 (11.4%) | >0.999 |
| Stroke | 24 (4.9%) | 21 (4.6%) | 3 (8.6%) | 0.24 |
| CABG | 37 (4.9%) | 36 (7.9%) | 1 (2.9%) | 0.502 |
| PCI | 82 (16.7%) | 77 (16.9%) | 5 (14.3%) | 0.691 |
| Study Population N = 492 (%) | |||
|---|---|---|---|
| Parameter | HR | 95% CI | p-Value |
| Age: | |||
| 30–55 years | — | — | — |
| 55–60 years | 2.12 | 0.92, 4.86 | 0.076 |
| 60–65 years | 1.24 | 0.48, 3.16 | 0.7 |
| 65–75 years | 2.88 | 1.31, 6.34 | 0.008 |
| 75–90 years | 8.07 | 3.65, 17.8 | <0.001 |
| Diabetes | 1.59 | 1.05–2.42 | 0.028 |
| Previous myocardial infarction | 1.64 | 1.09–2.44 | 0.017 |
| Chronic kidney disease | 2.22 | 1.33–3.70 | 0.002 |
| Coronary angiography indications: | |||
| CAD | — | — | — |
| NSTEMI | 0.93 | 0.53, 1.61 | 0.8 |
| STEMI | 1.73 | 0.99, 3.02 | 0.052 |
| Unstable angina | 0.37 | 0.15, 0.93 | 0.034 |
| Left ventricular ejection fraction | |||
| ≤40 | — | — | — |
| [40, 50] | 0.53 | 0.32, 0.87 | 0.011 |
| [50, 60] | 0.51 | 0.29, 0.90 | 0.020 |
| >60 | 0.43 | 0.23, 0.78 | 0.006 |
| LDL cholesterol | |||
| ≤100 | — | — | — |
| [100, 129] | 0.61 | 0.35, 1.06 | 0.080 |
| [129, 159] | 0.77 | 0.46, 1.29 | 0.3 |
| [159, 465] | 0.96 | 0.51, 1.81 | >0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kern, A.; Stompór, T.; Bojko, K.; Sienkiewicz, E.; Pawlak, S.; Pawlak, D.; Poskrobko, G.; Andrasz, E.; Gromadziński, L.; Jalali, R.; et al. Ten-Year Outcomes in Patients Undergoing Simultaneous Coronary and Renal Angiography—Does Renal Artery Stenosis Matter? J. Clin. Med. 2024, 13, 3374. https://doi.org/10.3390/jcm13123374
Kern A, Stompór T, Bojko K, Sienkiewicz E, Pawlak S, Pawlak D, Poskrobko G, Andrasz E, Gromadziński L, Jalali R, et al. Ten-Year Outcomes in Patients Undergoing Simultaneous Coronary and Renal Angiography—Does Renal Artery Stenosis Matter? Journal of Clinical Medicine. 2024; 13(12):3374. https://doi.org/10.3390/jcm13123374
Chicago/Turabian StyleKern, Adam, Tomasz Stompór, Krystian Bojko, Ewa Sienkiewicz, Sebastian Pawlak, Dariusz Pawlak, Grzegorz Poskrobko, Ewa Andrasz, Leszek Gromadziński, Rakesh Jalali, and et al. 2024. "Ten-Year Outcomes in Patients Undergoing Simultaneous Coronary and Renal Angiography—Does Renal Artery Stenosis Matter?" Journal of Clinical Medicine 13, no. 12: 3374. https://doi.org/10.3390/jcm13123374
APA StyleKern, A., Stompór, T., Bojko, K., Sienkiewicz, E., Pawlak, S., Pawlak, D., Poskrobko, G., Andrasz, E., Gromadziński, L., Jalali, R., Onichimowski, D., Piwko, G., Zalewski, A., & Bil, J. (2024). Ten-Year Outcomes in Patients Undergoing Simultaneous Coronary and Renal Angiography—Does Renal Artery Stenosis Matter? Journal of Clinical Medicine, 13(12), 3374. https://doi.org/10.3390/jcm13123374






