IL-1 Inhibitors in the Treatment of Familial Mediterranean Fever: Treatment Indications and Clinical Features in a Large Real-World Cohort
Abstract
1. Introduction
2. Materials and Methods
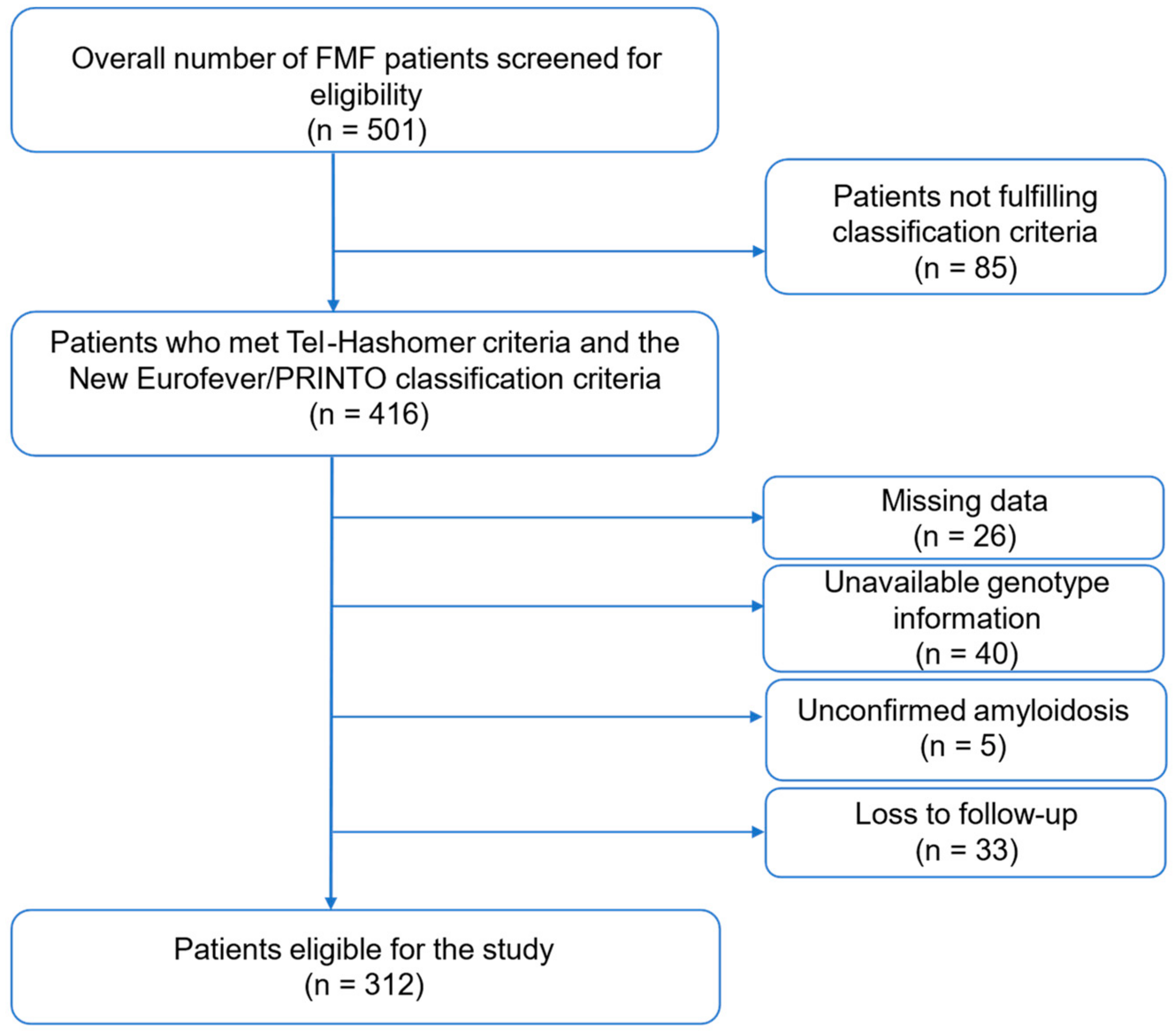
2.1. Patient Selection
2.2. Clinical and Genetic Information
2.3. Definition of Colchicine Resistance and Colchicine Intolerance
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Genetic Analysis
3.3. Indications for IL-1i Treatment
3.4. Comparing the Clinical and Genetic Profiles of Patients Who Required IL-1i with Those Who Did Not
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozen, S.; Bilginer, Y. A clinical guide to autoinflammatory diseases: Familial Mediterranean fever and next-of-kin. Nat. Rev. Rheumatol. 2014, 10, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, H.E.; Batu, E.D.; Özen, S. Familial Mediterranean fever: Current perspectives. J. Inflamm. Res. 2016, 9, 13–20. [Google Scholar] [CrossRef]
- Aksentijevich, I.; Kastner, D.L. Genetics of monogenic autoinflammatory diseases: Past successes, future challenges. Nat. Rev. Rheumatol. 2011, 7, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Ozen, S.; Demirkaya, E.; Erer, B.; Livneh, A.; Ben-Chetrit, E.; Giancane, G.; Ozdogan, H.; Abu, I.; Gattorno, M.; Hawkins, P.N.; et al. EULAR recommendations for the management of familial Mediterranean fever. Ann. Rheum. Dis. 2016, 75, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Goldfinger, S.E. Colchicine for familial Mediterranean fever. N. Engl. J. Med. 1972, 287, 1302. [Google Scholar] [CrossRef] [PubMed]
- Livneh, A.; Zemer, D.; Langevitz, P.; Shemer, J.; Sohar, E.; Pras, M. Colchicine in the treatment of AA and AL amyloidosis. Semin. Arthritis Rheum. 1993, 23, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Zemer, D.; Livneh, A.; Danon, Y.L.; Pras, M.; Sohar, E. Long-term colchicine treatment in children with familial Mediterranean fever. Arthritis Rheum. 1991, 34, 973–977. [Google Scholar] [CrossRef]
- Ozen, S.; Kone-Paut, I.; Gül, A. Colchicine resistance and intolerance in familial mediterranean fever: Definition, causes, and alternative treatments. Semin. Arthritis Rheum. 2017, 47, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hentgen, V.; Grateau, G.; Kone-Paut, I.; Livneh, A.; Padeh, S.; Rozenbaum, M.; Amselem, S.; Gershoni-Baruch, R.; Touitou, I.; Ben-Chetrit, E. Evidence-based recommendations for the practical management of Familial Mediterranean Fever. Semin. Arthritis Rheum. 2013, 43, 387–391. [Google Scholar] [CrossRef]
- Corsia, A.; Georgin-Lavialle, S.; Hentgen, V.; Hachulla, E.; Grateau, G.; Faye, A.; Quartier, P.; Rossi-Semerano, L.; Koné-Paut, I. A survey of resistance to colchicine treatment for French patients with familial Mediterranean fever. Orphanet J. Rare Dis. 2017, 12, 54. [Google Scholar] [CrossRef]
- Köhler, B.M.; Lorenz, H.M.; Blank, N. IL1-blocking therapy in colchicine-resistant familial Mediterranean fever. Eur. J. Rheumatol. 2018, 5, 230–234. [Google Scholar] [CrossRef]
- Hentgen, V.; Vinit, C.; Fayand, A.; Georgin-Lavialle, S. The Use of Interleukine-1 Inhibitors in Familial Mediterranean Fever Patients: A Narrative Review. Front. Immunol. 2020, 11, 971. [Google Scholar] [CrossRef]
- Mitroulis, I.; Papadopoulos, V.P.; Konstantinidis, T.; Ritis, K. Anakinra suppresses familial Mediterranean fever crises in a colchicine-resistant patient. Neth. J. Med. 2008, 66, 489–491. [Google Scholar]
- Kharouf, F.; Tsemach-Toren, T.; Ben-Chetrit, E. IL-1 inhibition in familial Mediterranean fever: Clinical outcomes and expectations. Clin. Exp. Rheumatol. 2022, 40, 1567–1574. [Google Scholar] [CrossRef]
- Agency, E.M. Rilonacept Regeneron (Previously Arcalyst). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/rilonacept-regeneron-previously-arcalyst (accessed on 23 May 2024).
- Giaglis, S.; Papadopoulos, V.; Kambas, K.; Doumas, M.; Tsironidou, V.; Rafail, S.; Kartalis, G.; Speletas, M.; Ritis, K. MEFV alterations and population genetics analysis in a large cohort of Greek patients with familial Mediterranean fever. Clin. Genet. 2007, 71, 458–467. [Google Scholar] [CrossRef]
- Lancieri, M.; Bustaffa, M.; Palmeri, S.; Prigione, I.; Penco, F.; Papa, R.; Volpi, S.; Caorsi, R.; Gattorno, M. An Update on Familial Mediterranean Fever. Int. J. Mol. Sci. 2023, 24, 9584. [Google Scholar] [CrossRef]
- Baglan, E.; Ozdel, S.; Bulbul, M. Do all colchicine preparations have the same effectiveness in patients with familial Mediterranean fever? Mod. Rheumatol. 2021, 31, 481–484. [Google Scholar] [CrossRef]
- Emmungil, H.; İlgen, U.; Turan, S.; Yaman, S.; Küçükşahin, O. Different pharmaceutical preparations of colchicine for Familial Mediterranean Fever: Are they the same? Rheumatol. Int. 2020, 40, 129–135. [Google Scholar] [CrossRef]
- Öner, N.; Çelikel, E.; Tekin, Z.E.; Güngörer, V.; Kurt, T.; Sezer, M.; Tekgöz, P.N.; Karagöl, C.; Coşkun, S.; Kaplan, M.M. Does Switching Colchicine Preparations Have a Role in The Treatment of Familial Mediterranean Fever? Res. Sq. 2022. preprint. [Google Scholar] [CrossRef]
- Varan, Ö.; Kucuk, H.; Babaoglu, H.; Guven, S.C.; Ozturk, M.A.; Haznedaroglu, S.; Goker, B.; Tufan, A. Efficacy and safety of interleukin-1 inhibitors in familial Mediterranean fever patients complicated with amyloidosis. Mod. Rheumatol. 2019, 29, 363–366. [Google Scholar] [CrossRef]
- Infevers. Infevers: An Online Database for Autoinflammatory Mutations. Available online: https://infevers.umai-montpellier.fr/web/search.php?n=1 (accessed on 11 August 2023).
- Lidar, M.; Yonath, H.; Shechter, N.; Sikron, F.; Sadetzki, S.; Langevitz, P.; Livneh, A.; Pras, E. Incomplete response to colchicine in M694V homozygote FMF patients. Autoimmun. Rev. 2012, 12, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, K.; Coşkuner, T.; Baglan, E.; Sönmez, H.E.; Yener, G.O.; Çakmak, F.; Demirkan, F.G.; Tanatar, A.; Karadag, S.G.; Ozdel, S.; et al. Real-Life Data from the Largest Pediatric Familial Mediterranean Fever Cohort. Front. Pediatr. 2021, 9, 805919. [Google Scholar] [CrossRef] [PubMed]
- Group, T.F.S. Familial Mediterranean fever (FMF) in Turkey: Results of a nationwide multicenter study. Medicine 2005, 84, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Touitou, I.; Lesage, S.; McDermott, M.; Cuisset, L.; Hoffman, H.; Dode, C.; Shoham, N.; Aganna, E.; Hugot, J.P.; Wise, C.; et al. Infevers: An evolving mutation database for auto-inflammatory syndromes. Hum. Mutat. 2004, 24, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Sarrauste de Menthière, C.; Terrière, S.; Pugnère, D.; Ruiz, M.; Demaille, J.; Touitou, I. INFEVERS: The Registry for FMF and hereditary inflammatory disorders mutations. Nucleic Acids Res. 2003, 31, 282–285. [Google Scholar] [CrossRef]
- Van Gijn, M.E.; Ceccherini, I.; Shinar, Y.; Carbo, E.C.; Slofstra, M.; Arostegui, J.I.; Sarrabay, G.; Rowczenio, D.; Omoyımnı, E.; Balci-Peynircioglu, B.; et al. New workflow for classification of genetic variants’ pathogenicity applied to hereditary recurrent fevers by the International Study Group for Systemic Autoinflammatory Diseases (INSAID). J. Med. Genet. 2018, 55, 530–537. [Google Scholar] [CrossRef]
- Özen, S.; Batu, E.D.; Demir, S. Familial Mediterranean Fever: Recent Developments in Pathogenesis and New Recommendations for Management. Front. Immunol. 2017, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Ben-Chetrit, E.; Aamar, S. About colchicine compliance, resistance and virulence. Clin. Exp. Rheumatol. 2009, 27, S1–S3. [Google Scholar] [PubMed]
- Livneh, A.; Langevitz, P. Diagnostic and treatment concerns in familial Mediterranean fever. Best Pract. Res. Clin. Rheumatol. 2000, 14, 477–498. [Google Scholar] [CrossRef]
- Varan, O.; Kucuk, H.; Babaoglu, H.; Tecer, D.; Atas, N.; Bilici Salman, R.; Satıs, H.; Ozturk, M.A.; Haznedaroglu, S.; Goker, B.; et al. Chronic inflammation in adult familial Mediterranean fever patients: Underlying causes and association with amyloidosis. Scand. J. Rheumatol. 2019, 48, 315–319. [Google Scholar] [CrossRef]
- Gafni, J.; Ravid, M.; Sohar, E. The role of amyloidosis in familial mediterranean fever. A population study. Isr. J. Med. Sci. 1968, 4, 995–999. [Google Scholar]
- Oner, A.; Erdoğan, O.; Demircin, G.; Bülbül, M.; Memiş, L. Efficacy of colchicine therapy in amyloid nephropathy of familial Mediterranean fever. Pediatr. Nephrol. 2003, 18, 521–526. [Google Scholar] [CrossRef]
- Livneh, A.; Zemer, D.; Langevitz, P.; Laor, A.; Sohar, E.; Pras, M. Colchicine treatment of AA amyloidosis of familial Mediterranean fever. An analysis of factors affecting outcome. Arthritis Rheum. 1994, 37, 1804–1811. [Google Scholar] [CrossRef]
- Bilginer, Y.; Akpolat, T.; Ozen, S. Renal amyloidosis in children. Pediatr. Nephrol. 2011, 26, 1215–1227. [Google Scholar] [CrossRef]
- Lane, T.; Wechalekar, A.D.; Gillmore, J.D.; Hawkins, P.N.; Lachmann, H.J. Safety and efficacy of empirical interleukin-1 inhibition using anakinra in AA amyloidosis of uncertain aetiology. Amyloid 2017, 24, 189–193. [Google Scholar] [CrossRef]
- Fradkin, A.; Yahav, J.; Zemer, D.; Jonas, A. Colchicine-induced lactose malabsorption in patients with familial Mediterranean fever. Isr. J. Med. Sci. 1995, 31, 616–620. [Google Scholar] [PubMed]
- Mor, A.; Gal, R.; Livneh, A. Abdominal and digestive system associations of familial Mediterranean fever. Am. J. Gastroenterol. 2003, 98, 2594–2604. [Google Scholar] [CrossRef]
- Batu, E.D.; Arici, Z.S.; Bilginer, Y.; Özen, S. Current therapeutic options for managing familial Mediterranean fever. Expert Opin. Orphan Drugs 2015, 3, 1063–1073. [Google Scholar] [CrossRef]
- Ben-Zvi, I.; Livneh, A. Chronic inflammation in FMF: Markers, risk factors, outcomes and therapy. Nat. Rev. Rheumatol. 2011, 7, 105–112. [Google Scholar] [CrossRef] [PubMed]
| Overall | Patients on Colchicine Monotherapy | Patients Requiring IL-1i | |||
|---|---|---|---|---|---|
| Number of participants, n | 312 | 275 | 37 | ||
| Age, mean (SD) | 34 (11.7) | 33.5 (11.4) | 40.5 (12.6) | ||
| Male/Female, n (%) | 115/196 (36.8/63.2) | 101/173 (36.7/62.9) | 14/23 (4.4/7.3) | ||
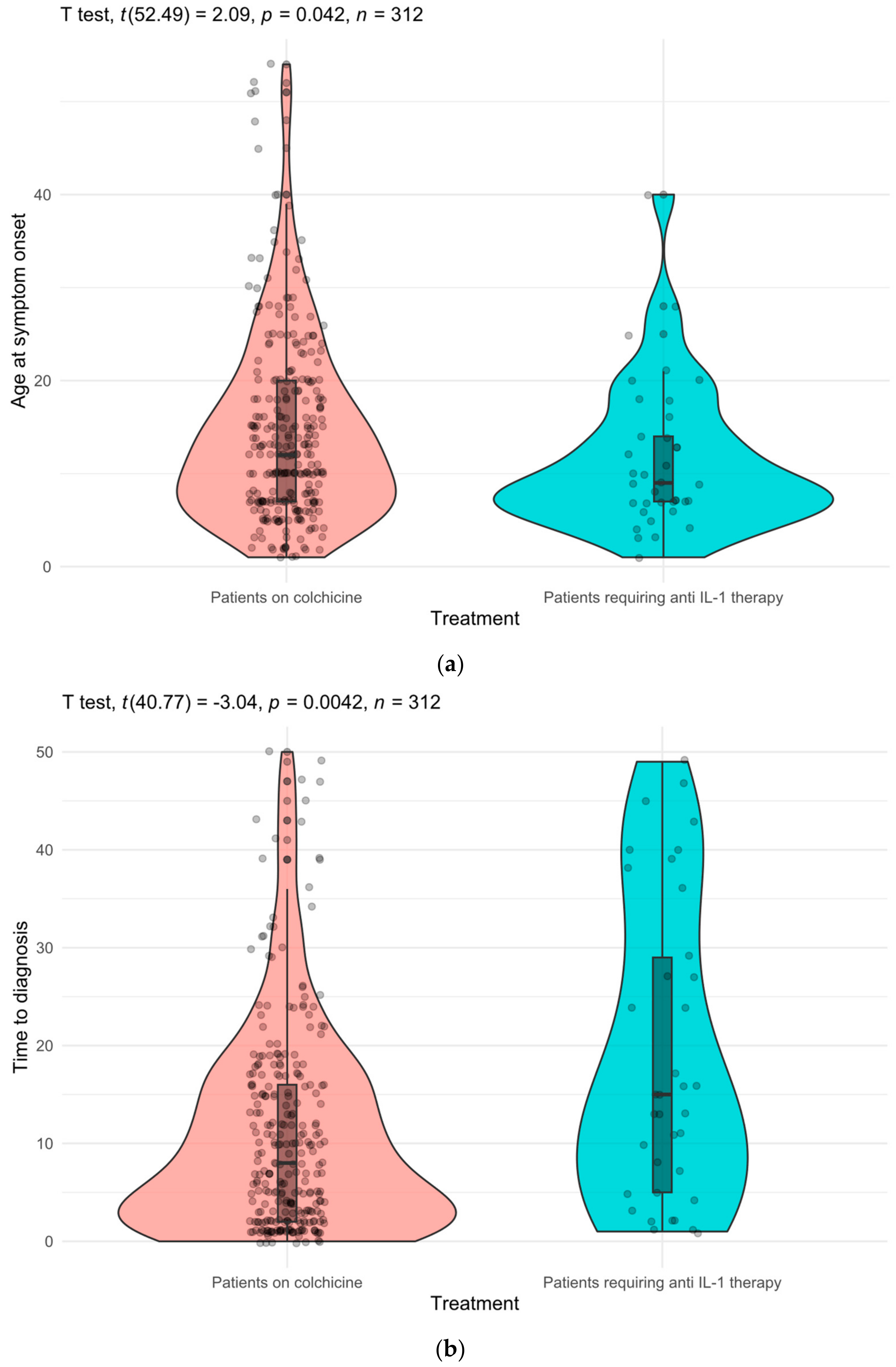
| Age at symptom onset, years, mean (SD) | 14.0 (9.71) | 14.5 (9.9) | 11.5 (7.97) | ||
| Age at diagnosis, years, mean (SD) | 26.0 (12.9) | 25.5 (12.3) | 30.4 (16.3) | ||
| Time between symptom onset and diagnosis, years, mean (SD) | 11.9 (11.6) | 11.0 (10.7) | 18.2 (15.6) | ||
| Family history of FMF, n (%) | 193 (61.8) | 170 (61.8) | 23 (62.1) | ||
| Family history of amyloidosis, n (%) | 21 (6.7) | 17 (6.1) | 4 (10.8) | ||
| Symptoms/manifestations n (%) | Abdominal pain | 292 (93.5) | 259 (94.1) | 33 (89.1) | |
| Fever | 246 (78.8) | 215 (78.1) | 31 (83.7) | ||
| Arthralgia | 180 (57.6) | 159 (57.8) | 21 (56.7) | ||
| Arthritis | 122 (39.1) | 105 (38.1) | 17 (45.9) | ||
| Pleuritic pain | 107 (34.2) | 99 (36.0) | 8 (21.6) | ||
| Erysipelas-like erythema | 67 (21.4) | 55 (20.0) | 12 (32.4) | ||
| Pericarditis | 2 (0.6) | 2 (0.7) | 0 (0.0) | ||
| MEFV variant n (%) | Exon 10 | M694V | 166 (53.2) | 143 (52.0) | 23 (62.1) |
| M680I | 54 (17.3) | 50 (18.1) | 4 (10.8) | ||
| V726A | 43 (13.7) | 41 (14.9) | 2 (5.4) | ||
| M694I | - | - | - | ||
| K695R | 1 (0.3) | 1 (0.3) | - | ||
| R761H | 1 (0.3) | 1 (0.3) | - | ||
| Exon 3 | P369S | 2 (0.6) | 1 (0.3) | 1 (2.7) | |
| Exon 2 | R202Q | 69 (22.1) | 62 (22.5) | 7 (18.9) | |
| E148Q | 44 (14.1) | 39 (14.1) | 5 (13.5) | ||
| E167D | 2 (0.6) | 2 (0.7) | - | ||
| Patients on colchicine monotherapy | 272 (87.1%) | ||
| Overall cohort (n = 312) | Patients requiring IL-1i * | 37 (11.8%) | |
| - | Colchicine resistance | 25 (8.0%) | |
| - | Amyloidosis | 16 (5.1%) | |
| - | Colchicine intolerance | 7 (2.2%) | |
| Non-compliant patients | 3 (0.9%) | ||
| Patients on Colchicine Monotherapy (n = 275) | Patients Requiring IL-1i (n = 37) | RR (95% CI) | p Value | |
|---|---|---|---|---|
| Homozygous M694V | 49 (17.8%) | 16 (43.2%) | 2.90 (1.60–5.22) | <0.001 |
| M694V mutation (homozygote, single or with other MEFV variants) | 143 (52.0%) | 23 (62.2%) | 1.44 (0.77–2.70) | 0.294 |
| M694V/V726A compound heterozygotes | 15 (5.5%) | 1 (2.7%) | 0.51 (0.08–3.51) | 0.704 |
| M694V/M680I compound heterozygotes | 18 (6.5%) | 2 (5.4%) | 0.83 (0.22–3.22) | >0.999 * |
| M680I/V726A compound heterozygotes | 2 (0.7%) | 0 (0%) | - § | >0.999 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalcin-Mutlu, M.; Icacan, O.C.; Yildirim, F.; Temiz, S.A.; Fagni, F.; Schett, G.; Tascilar, K.; Minopoulou, I.; Burul, G.; Bes, C. IL-1 Inhibitors in the Treatment of Familial Mediterranean Fever: Treatment Indications and Clinical Features in a Large Real-World Cohort. J. Clin. Med. 2024, 13, 3375. https://doi.org/10.3390/jcm13123375
Yalcin-Mutlu M, Icacan OC, Yildirim F, Temiz SA, Fagni F, Schett G, Tascilar K, Minopoulou I, Burul G, Bes C. IL-1 Inhibitors in the Treatment of Familial Mediterranean Fever: Treatment Indications and Clinical Features in a Large Real-World Cohort. Journal of Clinical Medicine. 2024; 13(12):3375. https://doi.org/10.3390/jcm13123375
Chicago/Turabian StyleYalcin-Mutlu, Melek, Ozan Cemal Icacan, Fatih Yildirim, Selahattin Alp Temiz, Filippo Fagni, Georg Schett, Koray Tascilar, Ioanna Minopoulou, Gokhan Burul, and Cemal Bes. 2024. "IL-1 Inhibitors in the Treatment of Familial Mediterranean Fever: Treatment Indications and Clinical Features in a Large Real-World Cohort" Journal of Clinical Medicine 13, no. 12: 3375. https://doi.org/10.3390/jcm13123375
APA StyleYalcin-Mutlu, M., Icacan, O. C., Yildirim, F., Temiz, S. A., Fagni, F., Schett, G., Tascilar, K., Minopoulou, I., Burul, G., & Bes, C. (2024). IL-1 Inhibitors in the Treatment of Familial Mediterranean Fever: Treatment Indications and Clinical Features in a Large Real-World Cohort. Journal of Clinical Medicine, 13(12), 3375. https://doi.org/10.3390/jcm13123375






