Switching from a Fixed Monthly Aflibercept Regimen to Bi-Monthly Brolucizumab in Refractory Cases of Neovascular Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Study Design and Patients
2.3. Treatment Protocol
2.4. Intervention and Procedure
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Visual Outcomes after Intravitreal Brolucizumab Injection
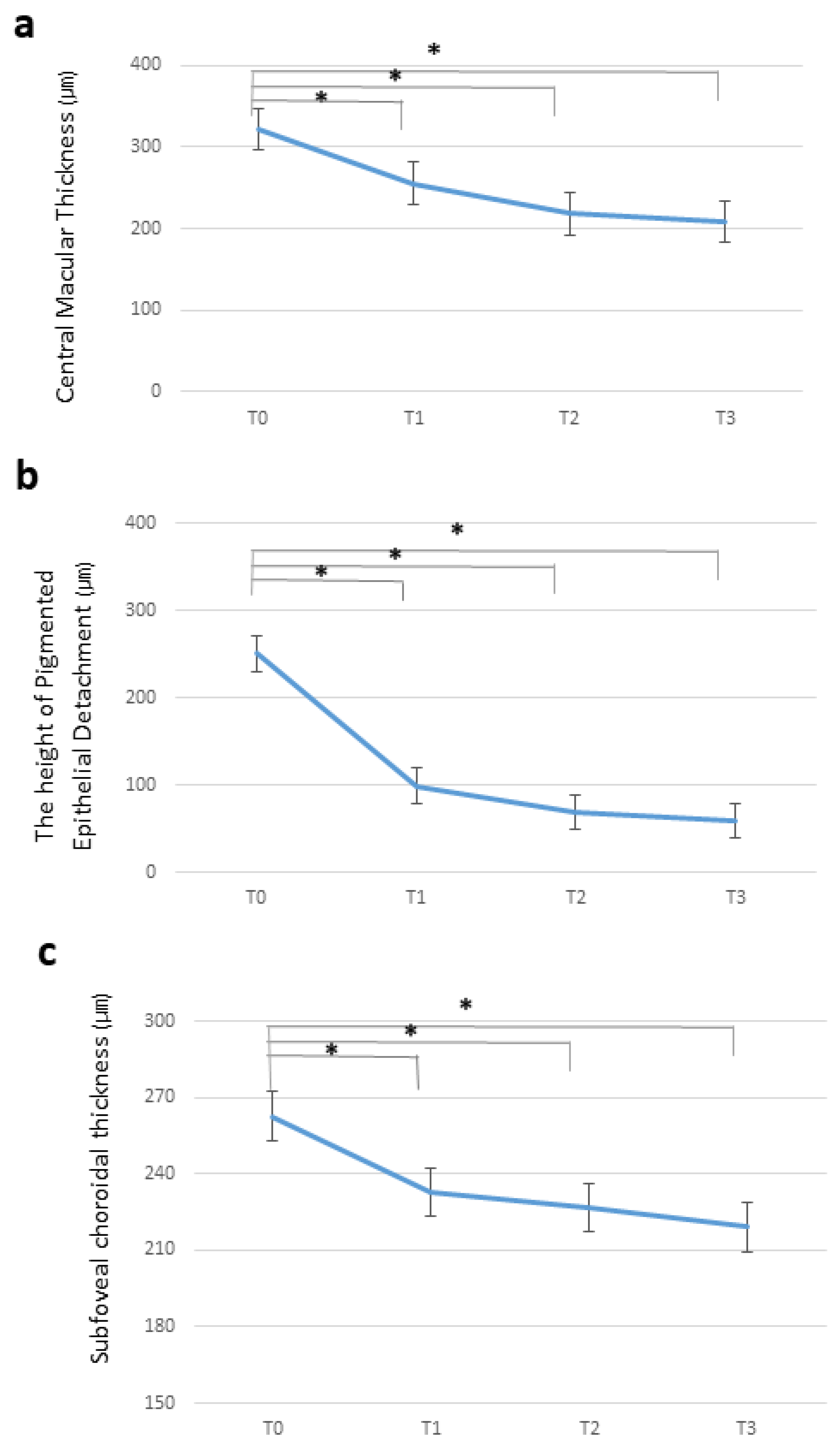
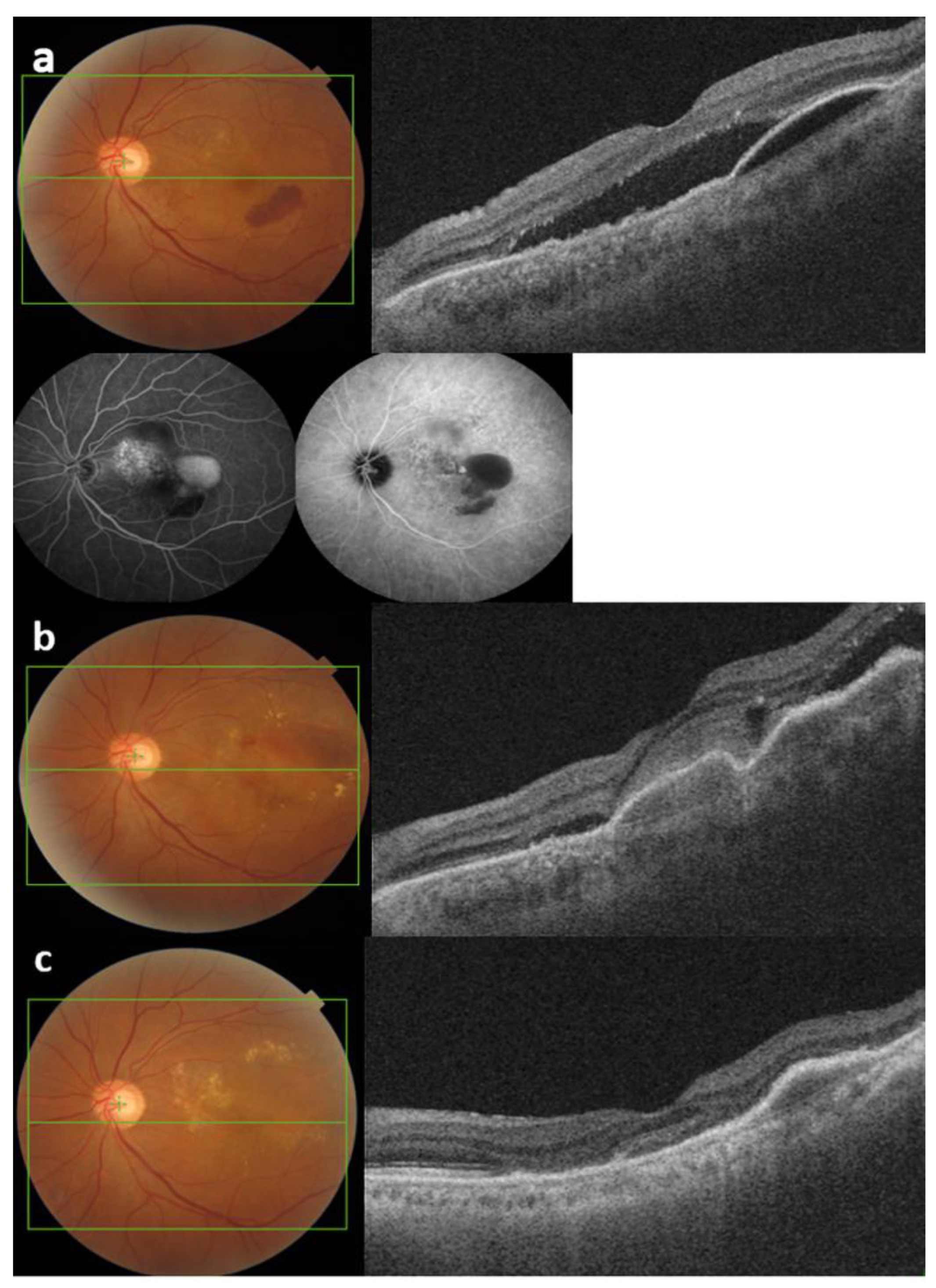
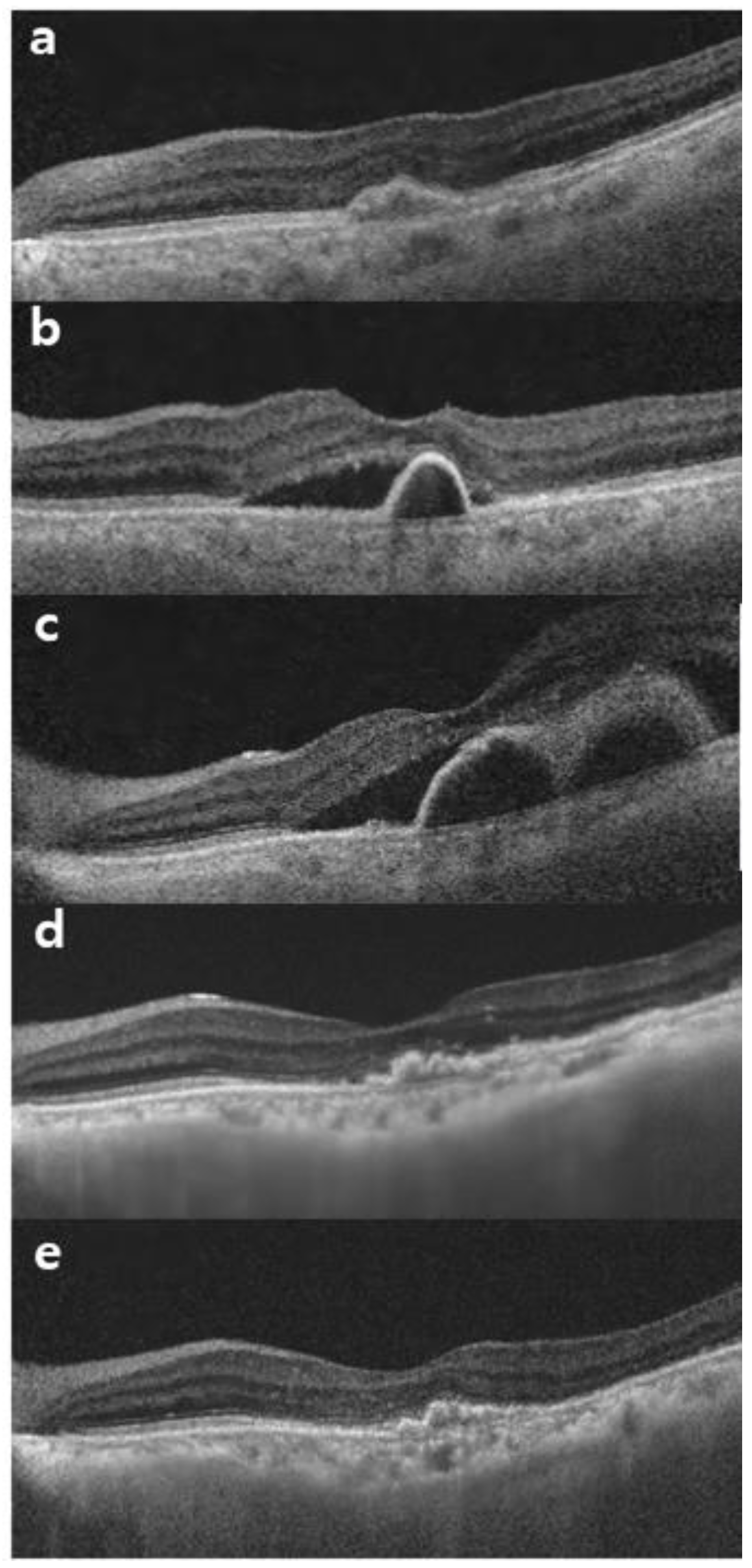
3.3. Optical Coherence Tomography Measurements after Intravitreal Brolucizumab Injection
3.3.1. Central Macular Thickness
3.3.2. Pigment Epithelial Detachment Height
3.3.3. Subfoveal Choroidal Thickness
3.3.4. Intraocular Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, R.; Klein, B.E.; Tomany, S.C.; Meuer, S.M.; Huang, G.-H. Ten-year incidence and progression of age-related maculopathy: The Beaver Dam eye study. Ophthalmology 2002, 109, 1767–1779. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Smith, W.; Attebo, K.; Wang, J.J. Prevalence of Age-related Maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology 1995, 102, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.F.; Paloma, J.B. Therapeutic anti-VEGF in age-related macular degeneration: Ranibizumab and Bevacizumab controversy. Br. J. Ophthalmol. 2008, 92, 866–867. [Google Scholar] [PubMed]
- Querques, G.; Capuano, V.; Frascio, P.; Bandello, F.; Souied, E.H. Emerging Therapeutic Options in Age-Related Macular Degeneration. Ophthalmic Res. 2015, 53, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Amoaku, W.M.; Chakravarthy, U.; Gale, R.; Gavin, M.; Ghanchi, F.; Gibson, J.; Harding, S.; Johnston, R.L.; Kelly, S.P.; Lotery, A.; et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye 2015, 29, 1397–1398. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, D.; Deonarain, D.M.; Gould, J.; Sothivannan, A.; Phillips, M.R.; Sarohia, G.S.; Sivaprasad, S.; Wykoff, C.C.; Cheung, C.M.G.; Sarraf, D.; et al. Efficacy, safety, and treatment burden of treat-and-extend versus alternative anti-VEGF regimens for nAMD: A systematic review and meta-analysis. Eye 2023, 37, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Boyer, D.S.; Heier, J.S.; Brown, D.M.; Francom, S.F.; Ianchulev, T.; Rubio, R.G. A Phase IIIb Study to Evaluate the Safety of Ranibizumab in Subjects with Neovascular Age-related Macular Degeneration. Ophthalmology 2009, 116, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska, B.; Sabsabi, M.; Ziemssen, F. Importance of Treatment Duration: Unmasking Barriers and Discovering the Reasons for Undertreatment of Anti-VEGF Agents in Neovascular Age-Related Macular Degeneration. Clin. Ophthalmol. 2021, 15, 4317–4326. [Google Scholar] [CrossRef]
- Mones, J.; Singh, R.P.; Bandello, F.; Souied, E.; Liu, X.; Gale, R. Undertreatment of Neovascular Age-Related Macular Degeneration after 10 Years of Anti-Vascular Endothelial Growth Factor Therapy in the Real World: The Need for A Change of Mindset. Ophthalmologica 2020, 243, 1–8. [Google Scholar] [CrossRef]
- Desideri, L.F.; Traverso, C.E.; Nicolò, M. Brolucizumab: A novel anti-VEGF humanized single-chain antibody fragment for treating w-AMD. Expert Opin. Biol. Ther. 2021, 21, 553–561. [Google Scholar] [CrossRef]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.-F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-related Macular Degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Pearce, I.; Amoaku, W.; Bailey, C.; Downey, L.; Gale, R.; Ghanchi, F.; Hamilton, R.; Mahmood, S.; Menon, G.; Nosek, J.; et al. The changing landscape for the management of patients with neovascular AMD: Brolucizumab in clinical practice. Eye 2022, 36, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Bodaghi, B.; Khanani, A.M.; Khoramnia, R.; Pavesio, C.; Nguyen, Q.D. Gains in the current understanding of managing neovascular AMD with brolucizumab. J. Ophthalmic Inflamm. Infect. 2023, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.U.; Singh, R.P.; Koh, A.; Ogura, Y.; Weissgerber, G.; Gedif, K.; Jaffe, G.J.; Tadayoni, R.; Schmidt-Erfurth, U.; Holz, F.G. HAWK and HARRIER: Ninety-Six-Week Outcomes from the Phase 3 Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2021, 128, 89–99. [Google Scholar] [CrossRef]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Bulirsch, L.M.; Saßmannshausen, M.; Nadal, J.; Liegl, R.; Thiele, S.; Holz, F.G. Short-term real-world outcomes following intravitreal brolucizumab for neovascular AMD: SHIFT study. Br. J. Ophthalmol. 2021, 106, 1288–1294. [Google Scholar] [CrossRef]
- Viggiano, P.; Grassi, M.O.; Boscia, G.; Pignataro, M.; Petruzzella, G.; Borrelli, E.; Molfetta, T.; Alessio, G.; Boscia, F. Short-Term Morphofunctional Changes in Previously Treated Neovascular AMD Eyes Switched to Brolucizumab. J. Clin. Med. 2022, 11, 5517. [Google Scholar] [CrossRef]
- Rübsam, A.; Rau, S.; Pilger, D.; Zeitz, O.; Joussen, A.M. Early OCT Angiography Changes of Macular Neovascularization in Patients with Exudative AMD Treated with Brolucizumab in a Real-World Setting. J. Ophthalmol. 2022, 2022, 2659714. [Google Scholar] [CrossRef]
- Haensli, C.; Pfister, I.B.; Garweg, J.G. Switching to Brolucizumab in Neovascular Age-Related Macular Degeneration Incompletely Responsive to Ranibizumab or Aflibercept: Real-Life 6 Month Outcomes. J. Clin. Med. 2021, 10, 2666. [Google Scholar] [CrossRef]
- Kikushima, W.; Sakurada, Y.; Fukuda, Y.; Matsubara, M.; Kotoda, Y.; Sugiyama, A.; Kashiwagi, K. A Treat-and-Extend Regimen of Intravitreal Brolucizumab for Exudative Age-Related Macular Degeneration Refractory to Aflibercept: A 12-Month Result. Pharmaceuticals 2023, 16, 562. [Google Scholar] [CrossRef]
- Lanzetta, P.; Loewenstein, A.; The Vision Academy Steering Committee. Fundamental principles of an anti-VEGF treatment regimen: Optimal application of intravitreal anti–vascular endothelial growth factor therapy of macular diseases. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Berta, A.; Larsen, M.; Macfadden, W.; Feller, C.; Mones, J. Treat-and-Extend versus Monthly Regimen in Neovascular Age-Related Macular Degeneration Results with Ranibizumab from the TREND Study. Ophthalmology 2018, 125, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Saitta, A.; D’eliseo, L.A.; D’eliseo, D. Efficacy and safety of brolucizumab for serous drusenoid pigment epithelium detachment non-responder to bevacizumab and aflibercept. Eur. J. Ophthalmol. 2023, 33, NP109–NP112. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Takeuchi, J.; Nakano, Y.; Horiguchi, E.; Taki, Y.; Ito, Y.; Terasaki, H.; Nishiguchi, K.M.; Kataoka, K. Switching from aflibercept to brolucizumab for the treatment of refractory neovascular age-related macular degeneration. Jpn. J. Ophthalmol. 2022, 66, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Eghøj, M.S.; Sørensen, T.L. Tachyphylaxis during treatment of exudative age-related macular degeneration with ranibizumab. Br. J. Ophthalmol. 2012, 96, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Forooghian, F.; Cukras, C.; Meyerle, C.B.; Chew, E.Y.; Wong, W.T. Tachyphylaxis after Intravitreal Bevacizumab for Exudative Age-Related Macular Degeneration. Retina 2009, 29, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Hara, C.; Wakabayashi, T.; Fukushima, Y.; Sayanagi, K.; Kawasaki, R.; Sato, S.; Sakaguchi, H.; Nishida, K. Tachyphylaxis during treatment of exudative age-related macular degeneration with aflibercept. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Schmitz-Valckenberg, S.; Wolf, A.; Agostini, H.; Lorenz, K.; Pielen, A.; Feltgen, N.; Guthoff, R.; Quiering, C.; Clemens, A.; et al. A randomized, open-label, multicenter study of switching to brolucizumab with or without a loading dose for patients with suboptimal anatomically controlled neovascular age-related macular degeneration—The FALCON study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 2695–2702. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Jaffe, G.J.; Cheung, C.M.G.; Kokame, G.T.; Iida, T.; Takahashi, K.; Lee, W.K.; Chang, A.A.; Monés, J.; D’souza, D.; et al. Efficacy and safety of brolucizumab versus aflibercept in eyes with polypoidal choroidal vasculopathy in Japanese participants of HAWK. Br. J. Ophthalmol. 2022, 106, 994–999. [Google Scholar] [CrossRef]
- Reitan, G.; Haugen, I.B.K.; Andersen, K.; Bragadottir, R.; Bindesbøll, C. Through the Eyes of Patients: Understanding Treatment Burden of Intravitreal Anti-VEGF Injections for nAMD Patients in Norway. Clin. Ophthalmol. 2023, 17, 1465–1474. [Google Scholar] [CrossRef]
- Chopra, R.; Preston, G.C.; Keenan, T.D.L.; Mulholland, P.; Patel, P.J.; Balaskas, K.; Hamilton, R.D.; Keane, P.A. Intravitreal injections: Past trends and future projections within a UK tertiary hospital. Eye 2021, 36, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Regillo, C.; Singh, R.; Hamilton, R.; Gedif, K.; Best, C.; Koh, A.; Holz, F.G. Fluid Control in Neovascular Age-Related Macular Degeneration with Brolucizumab: An Analysis of the HAWK and HARRIER Phase 3 Trials. Ophthalmologica 2022, 245, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, R.; Hata, M.; Tanaka, A.; Tsuchikawa, M.; Ueda-Arakawa, N.; Tamura, H.; Miyata, M.; Takahashi, A.; Kido, A.; Muraoka, Y.; et al. Therapeutic effects of faricimab on aflibercept-refractory age-related macular degeneration. Sci. Rep. 2023, 13, 21128. [Google Scholar] [CrossRef]
- Wang, R.; McClard, C.K.; Laswell, S.; Mahmoudzadeh, R.; Salabati, M.; Ammar, M.; Vannavong, J.; Aziz, A.A.; Ewald, A.; Calvanese, A.V.; et al. Quantifying burden of intravitreal injections: Questionnaire assessment of life impact of treatment by intravitreal injections (QUALITII). BMJ Open Ophthalmol. 2022, 7, e001188. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Suzuki, M.; Uchida, A.; Kurihara, T.; Kamoshita, M.; Minami, S.; Shinoda, H.; Tsubota, K.; Ozawa, Y. Non-responsiveness to intravitreal aflibercept treatment in neovascular age-related macular degeneration: Implications of serous pigment epithelial detachment. Sci. Rep. 2016, 6, 29619. [Google Scholar] [CrossRef]
- Marín, B.P.; Paniagua, N.M.G.; Gómez-Baldó, L.; Gallego-Pinazo, R. Burden of disease assessment in patients with neovascular age-related macular degeneration in Spain: Results of the AMD-MANAGE study. Eur. J. Ophthalmol. 2021, 32, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Miotto, S.; Zemella, N.; Gusson, E.; Panozzo, G.; Saviano, S.; Scarpa, G.; Boschi, G.; Piermarocchi, S.; on behalf of the Italian GAT (Gruppo Angiografico Triveneto) Study Group. Morphologic Criteria of Lesion Activity in Neovascular Age-Related Macular Degeneration: A Consensus Article. J. Ocul. Pharmacol. Ther. 2018, 34, 298–308. [Google Scholar] [CrossRef]
- Bae, K.W.; Kim, D.I.; Hwang, D.D.-J. The effect of intravitreal brolucizumab on choroidal thickness in patients with neovascular age-related macular degeneration. Sci. Rep. 2022, 12, 19855. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hoshino, J.; Nakamura, K.; Akiyama, H. Two-year outcomes of treat-and-extend regimen with intravitreal brolucizumab for treatment-naive neovascular age-related macular degeneration with type 1 macular neovascularization. Sci. Rep. 2023, 13, 3249. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hoshino, J.; Mukai, R.; Nakamura, K.; Akiyama, H. One-year results of treat-and-extend regimen with intravitreal brolucizumab for treatment-naïve neovascular age-related macular degeneration with type 1 macular neovascularization. Sci. Rep. 2022, 12, 8195. [Google Scholar] [CrossRef]
- Chung, S.E.; Kang, S.W.; Lee, J.H.; Kim, Y.T. Choroidal Thickness in Polypoidal Choroidal Vasculopathy and Exudative Age-related Macular Degeneration. Ophthalmology 2011, 118, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Koizumi, H.; Yamagishi, T.; Kinoshita, S. Subfoveal Choroidal Thickness after Ranibizumab Therapy for Neovascular Age-related Macular Degeneration: 12-Month Results. Ophthalmology 2012, 119, 1621–1627. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Das, A.; Do, D.V.; Dugel, P.U.; Gomes, A.; Holz, F.G.; Koh, A.; Pan, C.K.; Sepah, Y.J.; Patel, N.; et al. Brolucizumab: Evolution through Preclinical and Clinical Studies and the Implications for the Management of Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Sakurada, Y.; Matsubara, M.; Kotoda, Y.; Kasai, Y.; Sugiyama, A.; Kashiwagi, K. Comparison of one-year outcomes between as-needed brolucizumab and aflibercept for polypoidal choroidal vasculopathy. Jpn. J. Ophthalmol. 2023, 67, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Hoshino, J.; Mukai, R.; Nakamura, K.; Akiyama, H. Short-term outcomes of intravitreal brolucizumab for treatment-naïve neovascular age-related macular degeneration with type 1 choroidal neovascularization including polypoidal choroidal vasculopathy. Sci. Rep. 2021, 11, 6759. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, H.; Kano, M.; Yamamoto, A.; Saito, M.; Maruko, I.; Sekiryu, T.; Okada, A.A.; Iida, T. Subfoveal Choroidal Thickness during Aflibercept Therapy for Neovascular Age-Related Macular Degeneration: Twelve-Month Results. Ophthalmology 2016, 123, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Sadda, S.R.; Abdelfattah, N.S.; Lei, J.; Shi, Y.; Marion, K.M.; Morgenthien, E.; Gune, S.; Balasubramanian, S. Spectral-Domain OCT Analysis of Risk Factors for Macular Atrophy Development in the HARBOR Study for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 1360–1370. [Google Scholar] [CrossRef]
- Haug, S.J.; Hien, D.L.; Uludag, G.; Ngoc, T.T.T.; Lajevardi, S.; Halim, M.S.; Sepah, Y.J.; Do, D.V.; Khanani, A.M. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am. J. Ophthalmol. Case Rep. 2020, 18, 100680. [Google Scholar] [CrossRef] [PubMed]
- Maruko, I.; Okada, A.A.; Iida, T.; Hasegawa, T.; Izumi, T.; Kawai, M.; Maruko, R.; Nakayama, M.; Yamamoto, A.; Koizumi, H.; et al. Brolucizumab-related intraocular inflammation in Japanese patients with age-related macular degeneration: A short-term multicenter study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 2857–2859. [Google Scholar] [CrossRef]
- Kim, D.J.; Jin, K.W.; Han, J.M.; Lee, S.H.; Park, Y.S.; Lee, J.Y.; Lee, E.K.; Lee, J.S.; Kim, S.T.; Shin, M.H.; et al. Short-Term Safety and Efficacy of Intravitreal Brolucizumab Injections for Neovascular Age-Related Macular Degeneration: A Multicenter Retrospective Real-World Study. Ophthalmologica 2023, 246, 192–202. [Google Scholar] [CrossRef]

| Variables | Patients |
|---|---|
| Age (years) | 72.12 ± 8.23 |
| Sex, male (%) | 19 (59.3%) |
| Number of injections before the switch | 17.45 ± 8.93 |
| Best-corrected visual acuity (logMAR) | 0.52 ± 0.23 |
| Central macular thickness (μm) | 321.27 ± 91.89 |
| Subfoveal choroidal thickness (μm) | 262.76 ± 79.73 |
| Pigment epithelial detachment height (μm) | 251.00 ± 165.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Kang, J.E.; Park, Y.G. Switching from a Fixed Monthly Aflibercept Regimen to Bi-Monthly Brolucizumab in Refractory Cases of Neovascular Age-Related Macular Degeneration. J. Clin. Med. 2024, 13, 3434. https://doi.org/10.3390/jcm13123434
Kim M, Kang JE, Park YG. Switching from a Fixed Monthly Aflibercept Regimen to Bi-Monthly Brolucizumab in Refractory Cases of Neovascular Age-Related Macular Degeneration. Journal of Clinical Medicine. 2024; 13(12):3434. https://doi.org/10.3390/jcm13123434
Chicago/Turabian StyleKim, Minhee, Ji Eon Kang, and Young Gun Park. 2024. "Switching from a Fixed Monthly Aflibercept Regimen to Bi-Monthly Brolucizumab in Refractory Cases of Neovascular Age-Related Macular Degeneration" Journal of Clinical Medicine 13, no. 12: 3434. https://doi.org/10.3390/jcm13123434
APA StyleKim, M., Kang, J. E., & Park, Y. G. (2024). Switching from a Fixed Monthly Aflibercept Regimen to Bi-Monthly Brolucizumab in Refractory Cases of Neovascular Age-Related Macular Degeneration. Journal of Clinical Medicine, 13(12), 3434. https://doi.org/10.3390/jcm13123434





