Association between the Anatomical Location of Glioblastoma and Its Evaluation with Clinical Considerations: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Electronic Search
2.4. Study Selection
2.5. Data Collection Process
2.6. Assessment of the Methodological Quality of the Included Studies
2.7. Statistical Methods
3. Results
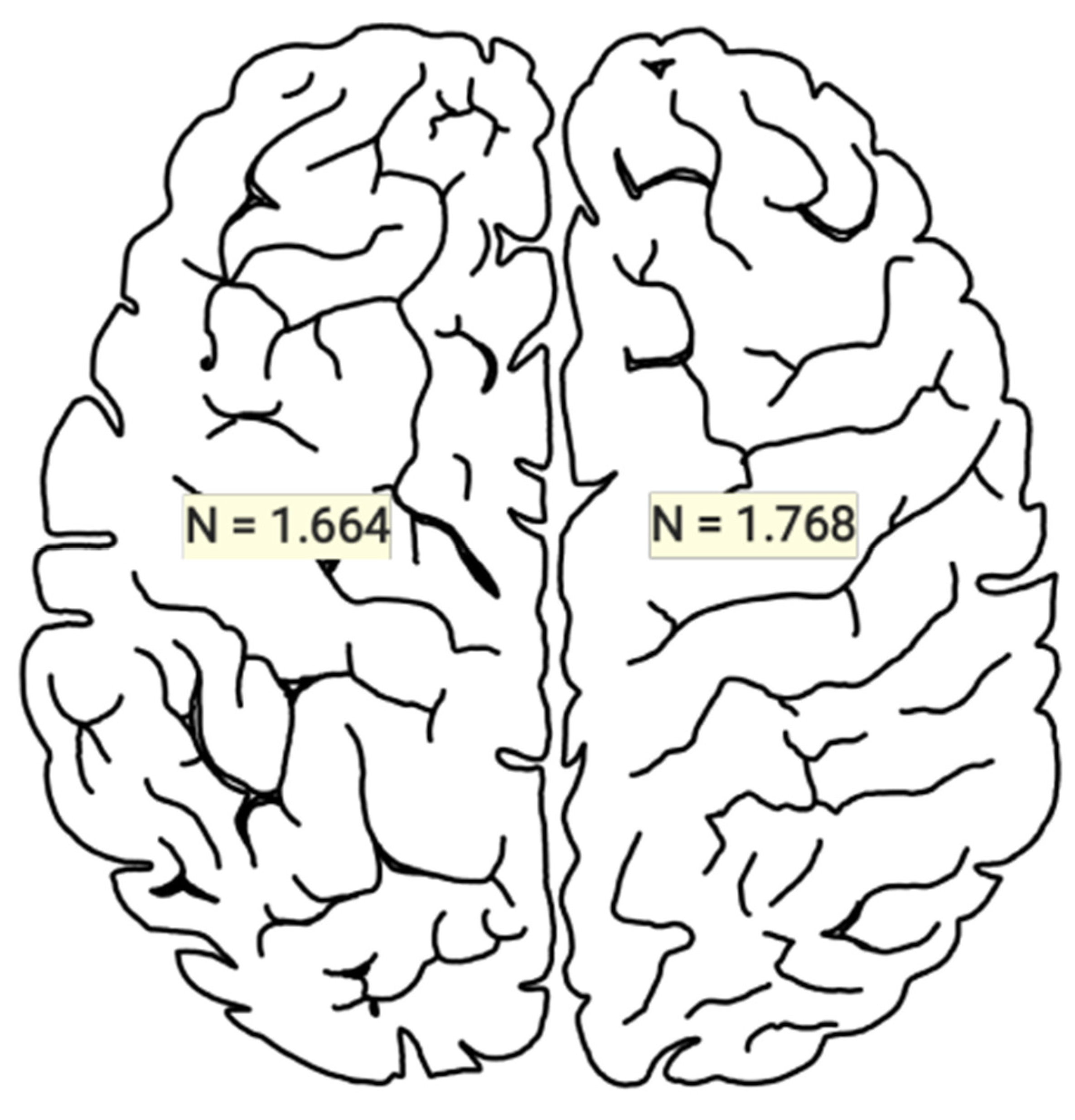
3.1. Statistical Results
3.2. Risk of Bias of Included Studies
3.3. Clinical Implications
4. Discussion
5. Limitations
6. Conclusions
Funding
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Grech, N.; Dalli, T.; Mizzi, S.; Meilak, L.; Calleja, N.; Zrinzo, A. Rising Incidence of Glioblastoma Multiforme in a Well-Defined Population. Cureus 2020, 12, e8195. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Verdugo, E.; Puerto, I.; Medina, M.Á. An update on the molecular biology of glioblastoma, with clinical implications and progress in its treatment. Cancer Commun. 2022, 42, 1083–1111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Angom, R.S.; Nakka, N.M.R.; Bhattacharya, S. Advances in Glioblastoma Therapy: An Update on Current Approaches. Brain Sci. 2023, 13, 1536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wlodarczyk, A.; Grot, D.; Stoczynska-Fidelus, E.; Rieske, P. Gaps and Doubts in Search to Recognize Glioblastoma Cellular Origin and Tumor Initiating Cells. J. Oncol. 2020, 2020, 6783627. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Park, J.; Jang, D.S.; Kim, H.J.; Lee, J.H.; Jo, E.; Choi, R.J.; Shim, J.-K.; Moon, J.H.; Kim, E.-H.; et al. Glioblastoma Cellular Origin and the Firework Pattern of Cancer Genesis from the Subventricular Zone. J. Korean Neurosurg. Soc. 2020, 63, 26–33. [Google Scholar] [CrossRef]
- Montemurro, N.; Pahwa, B.; Tayal, A.; Shukla, A.; De Jesus Encarnacion, M.; Ramirez, I.; Nurmukhametov, R.; Chavda, V.; De Carlo, A. Macrophages in Recurrent Glioblastoma as a Prognostic Factor in the Synergistic System of the Tumor Microenvironment. Neurol. Int. 2023, 15, 595–608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71, Erratum in Rev. Esp. Cardiol. 2022, 75, 192. [Google Scholar]
- Hashiguchi, M.; Tanaka, K.; Nagashima, H.; Fujita, Y.; Tanaka, H.; Kohta, M.; Nakai, T.; Uozumi, Y.; Maeyama, M.; Somiya, Y.; et al. Glutamic Acid and Total Creatine as Predictive Markers for Epilepsy in Glioblastoma by Using Magnetic Resonance Spectroscopy Before Surgery. World Neurosurg. 2022, 160, e501–e510. [Google Scholar] [CrossRef] [PubMed]
- Drabycz, S.; Roldán, G.; de Robles, P.; Adler, D.; McIntyre, J.B.; Magliocco, A.M.; Cairncross, J.G.; Mitchell, J.R. An analysis of image texture, tumor location, and MGMT promoter methylation in glioblastoma using magnetic resonance imaging. Neuroimage 2010, 49, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.C.; Tai, M.H.; Li, C.F.; Chen, T.Y.; Chen, J.H.; Shu, G.; Kuo, Y.T.; Lee, Y.C. Differentiation between Glioblastoma Multiforme and Primary Cerebral Lymphoma: Additional Benefits of Quantitative Diffusion-Weighted MR Imaging. PLoS ONE 2016, 11, e0162565. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abecassis, I.J.; Cordy, B.; Durfy, S.; Andre, J.B.; Levitt, M.R.; Ellenbogen, R.G.; Silbergeld, D.L.; Ko, A.L. Evaluating angioarchitectural characteristics of glial and metastatic brain tumors with conventional magnetic resonance imaging. J. Clin. Neurosci. 2020, 76, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.F.; Barbosa, M.; Amaral, L.L.; Mendonça, R.A.; Lima, S.S. Estudo através da ressonância magnética de 67 casos de glioblastoma multiforme e a ocorrência de metástases [Magnetic resonance imaging in 67 cases of glioblastoma multiforme and occurrence of metastases]. Arq Neuropsiquiatr. 2004, 62, 695–700. (In Portuguese) [Google Scholar] [CrossRef] [PubMed]
- Stark, A.M.; Maslehaty, H.; Hugo, H.H.; Mahvash, M.; Mehdorn, H.M. Glioblastoma of the cerebellum and brainstem. J. Clin. Neurosci. 2010, 17, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fan, X.; Zhang, C.; Wang, Z.; Li, S.; Wang, Y.; Qiu, X.; Jiang, T. MR imaging based fractal analysis for differentiating primary CNS lymphoma and glioblastoma. Eur. Radiol. 2019, 29, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shi, Q.; Li, S.; Zhao, Y.; Huang, H. Clinical characteristics of glioblastoma with metastatic spinal dissemination. Ann. Palliat. Med. 2022, 11, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Jaskólski, D.J.; Fortuniak, J.; Majos, A.; Gajewicz, W.; Papierz, W.; Liberski, P.P.; Sikorska, B.; Stefańczyk, L. Magnetic resonance spectroscopy in intracranial tumours of glial origin. Neurol. Neurochir. Pol. 2013, 47, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Yoon, M.J.; Park, J.E.; Choi, E.J.; Lee, J.; Kim, H.S. Radiomics in peritumoral non-enhancing regions: Fractional anisotropy and cerebral blood volume improve prediction of local progression and overall survival in patients with glioblastoma. Neuroradiology 2019, 61, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, J.; Ono, T.; Takahashi, M.; Oda, M.; Shimizu, H. Differentiating between Primary Central Nervous System Lymphoma and Glioblastoma: The Diagnostic Value of Combining 18F-fluorodeoxyglucose Positron Emission Tomography with Arterial Spin Labeling. Neurol. Med. Chir. 2021, 61, 367–375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamashita, K.; Hatae, R.; Hiwatashi, A.; Togao, O.; Kikuchi, K.; Momosaka, D.; Yamashita, Y.; Kuga, D.; Hata, N.; Yoshimoto, K.; et al. Predicting TERT promoter mutation using MR images in patients with wild-type IDH1 glioblastoma. Diagn. Interv. Imaging 2019, 100, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, T.; Numaguchi, Y.; Rothman, M.I.; Zoarski, G.H.; Morikawa, M.; Zagardo, M.T.; Kristt, D.A. Posterior fossa glioblastoma multiforme: MR findings. Am. J. Neuroradiol. 1995, 16, 583–589. [Google Scholar] [PubMed] [PubMed Central]
- Awad, A.W.; Karsy, M.; Sanai, N.; Spetzler, R.; Zhang, Y.; Xu, Y.; Mahan, M.A. Impact of removed tumor volume and location on patient outcome in glioblastoma. J. Neurooncol. 2017, 135, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Quan, G.; Wang, T.; Ren, J.L.; Xue, X.; Wang, W.; Wu, Y.; Li, X.; Yuan, T. Prognostic and predictive impact of abnormal signal volume evolution early after chemoradiotherapy in glioblastoma. J. Neurooncol. 2023, 162, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Onuma, K.; Ishikawa, E.; Matsuda, M.; Hirata, K.; Osuka, S.; Yamamoto, T.; Masumoto, T.; Zaboronok, A.; Matsumura, A. Clinical characteristics and neuroimaging findings in 12 cases of recurrent glioblastoma with communicating hydrocephalus. Neurol. Med. Chir. 2013, 53, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Chen, H.; Sun, G.; Liu, J.; Zhang, M.; Lin, H.; Sun, C.; Ma, X. Combined use of multimodal techniques for the resection of glioblastoma involving corpus callosum. Acta Neurochir. 2022, 164, 689–702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, L.; Li, T.; Li, Y.; Zhang, J.; Li, S.; Zhu, L.; Qin, J.; Tang, L.; Zeng, Z. Combining amide proton transfer-weighted and arterial spin labeling imaging to differentiate solitary brain metastases from glioblastomas. Magn. Reson. Imaging 2023, 102, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Wach, J.; Hamed, M.; Schuss, P.; Güresir, E.; Herrlinger, U.; Vatter, H.; Schneider, M. Impact of initial midline shift in glioblastoma on survival. Neurosurg. Rev. 2021, 44, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Steidl, E.; Filipski, K.; Hattingen, E.; Steinbach, J.P.; Maurer, G.D. Longitudinal study on MRI and neuropathological findings: Neither DSC-perfusion derived rCBVmax nor vessel densities correlate between newly diagnosed and progressive glioblastoma. PLoS ONE 2023, 18, e0274400. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, M.; Lee, S.K.; Chang, J.H.; Kang, S.G.; Kim, E.H.; Kim, S.H.; Song, M.K.; Ma, B.G.; Ahn, S.S. Elderly patients with newly diagnosed glioblastoma: Can preoperative imaging descriptors improve the predictive power of a survival model? J. Neurooncol. 2017, 134, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Ideguchi, M.; Kajiwara, K.; Goto, H.; Sugimoto, K.; Nomura, S.; Ikeda, E.; Suzuki, M. MRI findings and pathological features in early-stage glioblastoma. J. Neurooncol. 2015, 123, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gao, T.; Niu, X.; Xing, X.; Yang, Y.; Liu, Y.; Mao, Q. Clinical Characteristics and Prognostic Analysis of Glioma in Human Immunodeficiency Virus-Infected Patients. World Neurosurg. 2018, 114, e218–e223. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.M.J.; Robe, P.A.J.T.; Eijgelaar, R.S.; Witte, M.G.; Visser, M.; de Munck, J.C.; Broekman, M.L.D.; Seute, T.; Hendrikse, J.; Noske, D.P.; et al. Comparing Glioblastoma Surgery Decisions Between Teams Using Brain Maps of Tumor Locations, Biopsies, and Resections. JCO Clin. Cancer Inform. 2019, 3, 1–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomas, J.G.; Rao, G.; Kew, Y.; Prabhu, S.S. Laser interstitial thermal therapy for newly diagnosed and recurrent glioblastoma. Neurosurg. Focus 2016, 41, E12. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Morikawa, M.; Ishimaru, H.; Ideguchi, R.; Uetani, M.; Hiu, T.; Matsuo, T.; Miyoshi, M. Amide proton transfer MRI differentiates between progressive multifocal leukoencephalopathy and malignant brain tumors: A pilot study. BMC Med. Imaging 2022, 22, 227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xing, Z.; Zhang, H.; She, D.; Lin, Y.; Zhou, X.; Zeng, Z.; Cao, D. IDH genotypes differentiation in glioblastomas using DWI and DSC-PWI in the enhancing and peri-enhancing region. Acta Radiol. 2019, 60, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Smedley, N.F.; Ellingson, B.M.; Cloughesy, T.F.; Hsu, W. Longitudinal Patterns in Clinical and Imaging Measurements Predict Residual Survival in Glioblastoma Patients. Sci. Rep. 2018, 8, 14429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miquelini, L.A.; Pérez Akly, M.S.; Funes, J.A.; Besada, C.H. Usefulness of the apparent diffusion coefficient for the evaluation of the white matter to differentiate between glioblastoma and brain metastases. Radiologia 2016, 58, 207–213. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yan, L.F.; Wang, X.B.; Sun, Y.Z.; Zhang, X.; Liu, Z.C.; Nan, H.Y.; Hu, Y.C.; Yang, Y.; Zhang, J.; et al. Structural and advanced imaging in predicting MGMT promoter methylation of primary glioblastoma: A region of interest based analysis. BMC Cancer 2018, 18, 215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mathew, B.S.; Kaliyath, S.B.; Krishnan, J.; Bhasi, S. Impact of subventricular zone irradiation on outcome of patients with glioblastoma. J. Cancer Res. Ther. 2018, 14, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Rui, Q.; Wang, Y.; Heo, H.Y.; Zou, T.; Yu, H.; Zhang, Y.; Wang, X.; Du, Y.; Wen, X.; et al. Discriminating MGMT promoter methylation status in patients with glioblastoma employing amide proton transfer-weighted MRI metrics. Eur. Radiol. 2018, 28, 2115–2123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.Y.; Sun, C.R.; He, M.; Yin, L.C.; Du, H.G.; Zhang, J.M. Correlation between Tumor Location and Clinical Properties of Glioblastomas in Frontal and Temporal Lobes. World Neurosurg. 2018, 112, e407–e414. [Google Scholar] [CrossRef] [PubMed]
- Utsuki, S.; Oka, H.; Suzuki, S.; Shimizu, S.; Tanizaki, Y.; Kondo, K.; Tanaka, S.; Kawano, N.; Fujii, K. Pathological and clinical features of cystic and noncystic glioblastomas. Brain Tumor Pathol. 2006, 23, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Qi, C.; Liu, X.; Wang, Y.; Liu, S.; Li, S.; Wang, L.; Wang, Y. Regional specificity of matrix metalloproteinase-9 expression in the brain: Voxel-level mapping in primary glioblastomas. Clin. Radiol. 2018, 73, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.G.; Price, S.J.; Suckling, J. Connectome analysis for pre-operative brain mapping in neurosurgery. Br. J. Neurosurg. 2016, 30, 506–517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Fan, X.; Zhang, C.; Zhang, T.; Peng, X.; Li, S.; Wang, L.; Ma, J.; Jiang, T. Anatomical specificity of O6-methylguanine DNA methyltransferase protein expression in glioblastomas. J. Neurooncol. 2014, 120, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Smets, T.; Lawson, T.M.; Grandin, C.; Jankovski, A.; Raftopoulos, C. Immediate post-operative MRI suggestive of the site and timing of glioblastoma recurrence after gross total resection: A retrospective longitudinal preliminary study. Eur. Radiol. 2013, 23, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Eoli, M.; Menghi, F.; Bruzzone, M.G.; De Simone, T.; Valletta, L.; Pollo, B.; Bissola, L.; Silvani, A.; Bianchessi, D.; D’Incerti, L.; et al. Methylation of O6-methylguanine DNA methyltransferase and loss of heterozygosity on 19q and/or 17p are overlapping features of secondary glioblastomas with prolonged survival. Clin. Cancer Res. 2007, 13, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Ideguchi, M.; Kimura, T.; Kajiwara, K.; Imoto, H.; Sadahiro, H.; Ishii, A.; Kawano, H.; Ikeda, E.; Suzuki, M. Epithelioid/rhabdoid glioblastoma: A highly aggressive subtype of glioblastoma. Brain Tumor Pathol. 2016, 33, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Seidel, C.; Dörner, N.; Osswald, M.; Wick, A.; Platten, M.; Bendszus, M.; Wick, W. Does age matter?—A MRI study on peritumoral edema in newly diagnosed primary glioblastoma. BMC Cancer 2011, 11, 127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, H.R.; Jeon, H.; Park, C.K.; Park, S.H.; Choi, S.H. Radiogenomics Profiling for Glioblastoma-related Immune Cells Reveals CD49d Expression Correlation with MRI parameters and Prognosis. Sci. Rep. 2018, 8, 16022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olar, A.; Raghunathan, A.; Albarracin, C.T.; Aldape, K.D.; Cahill, D.P., 3rd; Powell, S.Z.; Goodman, J.C.; Fuller, G.N. Absence of IDH1-R132H mutation predicts rapid progression of nonenhancing diffuse glioma in older adults. Ann. Diagn. Pathol. 2012, 16, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.Y.M.; Ho, J.M.K.; Tse, T.P.K.; Lam, S.W.; Mak, C.H.K.; Chan, D.T.M.; Lee, M.W.Y.; Wong, S.T.; Chan, K.Y.; Poon, W.S. Determining a cut-off residual tumor volume threshold for patients with newly diagnosed glioblastoma treated with temozolomide chemoradiotherapy: A multicenter cohort study. J. Clin. Neurosci. 2019, 63, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Joseph, N.M.; Perry, A.; Barajas, R.F., Jr.; Cha, S. Apparent diffusion coefficient in glioblastoma with PNET-like components, a GBM variant. J. Neurooncol. 2014, 119, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Wang, S.; Coban, G.; Kural, F.; Chawla, S.; O’Rourke, D.M.; Poptani, H. Detection of occult neoplastic infiltration in the corpus callosum and prediction of overall survival in patients with glioblastoma using diffusion tensor imaging. Eur. J. Radiol. 2019, 112, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Mangla, R.; Singh, G.; Ziegelitz, D.; Milano, M.T.; Korones, D.N.; Zhong, J.; Ekholm, S.E. Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology. 2010, 256, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Adeberg, S.; Bostel, T.; König, L.; Welzel, T.; Debus, J.; Combs, S.E. A comparison of long-term survivors and short-term survivors with glioblastoma, subventricular zone involvement: A predictive factor for survival? Radiat. Oncol. 2014, 9, 95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wright, J.; Chugh, J.; Wright, C.H.; Alonso, F.; Hdeib, A.; Gittleman, H.; Barnholtz-Sloan, J.; Sloan, A.E. Laser interstitial thermal therapy followed by minimal-access transsulcal resection for the treatment of large and difficult to access brain tumors. Neurosurg. Focus 2016, 41, E14. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, I.; Saito, R.; Osada, Y.; Kanamori, M.; Sonoda, Y.; Kumabe, T.; Mugikura, S.; Watanabe, M.; Tominaga, T. Incidence of initial spinal metastasis in glioblastoma patients and the importance of spinal screening using MRI. J. Neurooncol. 2019, 141, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Tykocinski, E.S.; Grant, R.A.; Kapoor, G.S.; Krejza, J.; Bohman, L.E.; Gocke, T.A.; Chawla, S.; Halpern, C.H.; Lopinto, J.; Melhem, E.R.; et al. Use of magnetic perfusion-weighted imaging to determine epidermal growth factor receptor variant III expression in glioblastoma. Neuro Oncol. 2012, 14, 613–623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanas, V.G.; Zacharaki, E.I.; Thomas, G.A.; Zinn, P.O.; Megalooikonomou, V.; Colen, R.R. Learning MRI-based classification models for MGMT methylation status prediction in glioblastoma. Comput. Methods Programs Biomed. 2017, 140, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Reimer, C.; Deike, K.; Graf, M.; Reimer, P.; Wiestler, B.; Floca, R.O.; Kickingereder, P.; Schlemmer, H.P.; Wick, W.; Bendszus, M.; et al. Differentiation of pseudoprogression and real progression in glioblastoma using ADC parametric response maps. PLoS ONE 2017, 12, e0174620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiguet-Jiglaire, C.; Boissonneau, S.; Denicolai, E.; Hein, V.; Lasseur, R.; Garcia, J.; Romain, S.; Appay, R.; Graillon, T.; Mason, W.; et al. Plasmatic MMP9 released from tumor-infiltrating neutrophils is predictive for bevacizumab efficacy in glioblastoma patients: An AVAglio ancillary study. Acta Neuropathol. Commun. 2022, 10, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Senders, J.T.; Cho, L.D.; Calvachi, P.; McNulty, J.J.; Ashby, J.L.; Schulte, I.S.; Almekkawi, A.K.; Mehrtash, A.; Gormley, W.B.; Smith, T.R.; et al. Automating Clinical Chart Review: An Open-Source Natural Language Processing Pipeline Developed on Free-Text Radiology Reports From Patients With Glioblastoma. JCO Clin. Cancer Inform. 2020, 4, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, H.Z.; Cui, Y.Y.; Zhang, Z.Z.; Ma, X.D. The Associations Between Preoperative Conventional MRI Features and Genetic Biomarkers Status in Newly Diagnosed GBMs: A Clinical Summary and Prognostic Analysis. Turk. Neurosurg. 2021, 31, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, L.; Tan, Y.; Jiang, Y.; Lu, H. Observation of the delineation of the target volume of radiotherapy in adult-type diffuse gliomas after temozolomide-based chemoradiotherapy: Analysis of recurrence patterns and predictive factors. Radiat. Oncol. 2023, 18, 16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, O.; Biran, I.; Steiner, I. Cerebrospinal fluid oligoclonal IgG bands in patients with spinal arteriovenous malformation and structural central nervous system lesions. Arch. Neurol. 2000, 57, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Morioka, T.; Suzuki, S.; Takeshita, I.; Fukui, M. Thalamic gliomas: A clinicopathologic analysis of 20 cases with reference to patient age. Acta Neurochir. 1997, 139, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Fan, X.; Li, S.; Wang, R.; Wang, L.; Ma, J.; Jiang, T.; Ma, W. Age-associated brain regions in gliomas: A volumetric analysis. J. Neurooncol. 2015, 123, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Simonet Redondo, M.; Auger Acosta, C.; Rovira-Gols, A.; Toledo Argany, M.; Sarria Estrada, S.; Rovira Cañellas, A. Neuroimaging findings in the initial phase of development of high grade cortical astrocytomas. Radiologia 2012, 54, 424–431, English, Spanish. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, L.; Choi, S.H.; Yoo, R.E.; Kang, K.M.; Yun, T.J.; Kim, T.M.; Lee, S.H.; Park, C.K.; Kim, J.H.; Park, S.W.; et al. Paradoxical perfusion metrics of high-grade gliomas with an oligodendroglioma component: Quantitative analysis of dynamic susceptibility contrast perfusion MR imaging. Neuroradiology 2015, 57, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Friese, S.A.; Bitzer, M.; Freudenstein, D.; Voigt, K.; Küker, W. Classification of acquired lesions of the corpus callosum with MRI. Neuroradiology 2000, 42, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Ito, J.; Takahashi, N.; Ishikawa, K.; Furusawa, T.; Tokiguchi, S.; Sakai, K. MRI of high-grade astrocytic tumors: Early appearance and evolution. Neuroradiology 2002, 44, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Reulen, H.J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. ALA-Glioma Study Group. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2008, 62, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Fudaba, H.; Momii, Y.; Matsuta, H.; Onishi, K.; Kawasaki, Y.; Sugita, K.; Shimomura, T.; Fujiki, M. Perfusion Parameter Obtained on 3-Tesla Magnetic Resonance Imaging and the Ki-67 Labeling Index Predict the Overall Survival of Glioblastoma. World Neurosurg. 2021, 149, e469–e480. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.T.; Liu, M.X.; Chen, Z.Y. Differential Diagnostic Value of Texture Feature Analysis of Magnetic Resonance T2 Weighted Imaging between Glioblastoma and Primary Central Neural System Lymphoma. Chin. Med. Sci. J. 2019, 34, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, M.; Yamamoto, T.; Ishikawa, E.; Matsuda, M.; Takano, S.; Ishikawa, H.; Okumura, T.; Sakurai, H.; Matsumura, A.; Tsuboi, K. Proton beam therapy with concurrent chemotherapy for glioblastoma multiforme: Comparison of nimustine hydrochloride and temozolomide. J. Neurooncol. 2016, 130, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Isoardo, G.; Morra, I.; Chiarle, G.; Audrito, V.; Deaglio, S.; Melcarne, A.; Junemann, C.; Naddeo, M.; Cogoni, M.; Valentini, M.C.; et al. Different aquaporin-4 expression in glioblastoma multiforme patients with and without seizures. Mol. Med. 2012, 18, 1147–1151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stumpo, V.; Sebök, M.; van Niftrik, C.H.B.; Seystahl, K.; Hainc, N.; Kulcsar, Z.; Weller, M.; Regli, L.; Fierstra, J. Feasibility of glioblastoma tissue response mapping with physiologic BOLD imaging using precise oxygen and carbon dioxide challenge. MAGMA 2022, 35, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Iliadis, G.; Kotoula, V.; Chatzisotiriou, A.; Televantou, D.; Eleftheraki, A.G.; Lambaki, S.; Misailidou, D.; Selviaridis, P.; Fountzilas, G. Volumetric and MGMT parameters in glioblastoma patients: Survival analysis. BMC Cancer 2012, 12, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galldiks, N.; Langen, K.J.; Holy, R.; Pinkawa, M.; Stoffels, G.; Nolte, K.W.; Kaiser, H.J.; Filss, C.P.; Fink, G.R.; Coenen, H.H.; et al. Assessment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J. Nucl. Med. 2012, 53, 1048–1057, Erratum in J. Nucl. Med. 2013, 54, 1846. [Google Scholar] [CrossRef] [PubMed]
- Henker, C.; Kriesen, T.; Fürst, K.; Goody, D.; Glass, Ä.; Pützer, B.M.; Piek, J. Effect of 10 different polymorphisms on preoperative volumetric characteristics of glioblastoma multiforme. J. Neurooncol. 2016, 126, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Soltanian-Zadeh, H.; Jafari-Khouzani, K.; Scarpace, L.; Mikkelsen, T. Prediction of glioblastoma multiform response to bevacizumab treatment using multi-parametric MRI. PLoS ONE 2012, 7, e29945. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coburger, J.; Scheuerle, A.; Kapapa, T.; Engelke, J.; Thal, D.R.; Wirtz, C.R.; König, R. Sensitivity and specificity of linear array intraoperative ultrasound in glioblastoma surgery: A comparative study with high field intraoperative MRI and conventional sector array ultrasound. Neurosurg. Rev. 2015, 38, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Hakyemez, B.; Erdogan, C.; Ercan, I.; Ergin, N.; Uysal, S.; Atahan, S. High-grade and low-grade gliomas: Differentiation by using perfusion MR imaging. Clin. Radiol. 2005, 60, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lou, H.; Zou, T.; Wang, X.; Jiang, S.; Huang, Z.; Du, Y.; Jiang, C.; Ma, L.; Zhu, J.; et al. Applying protein-based amide proton transfer MR imaging to distinguish solitary brain metastases from glioblastoma. Eur. Radiol. 2017, 27, 4516–4524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laule, C.; Bjarnason, T.A.; Vavasour, I.M.; Traboulsee, A.L.; Wayne Moore, G.R.; Li, D.K.B.; MacKay, A.L. Characterization of brain tumours with spin-spin relaxation: Pilot case study reveals unique T 2 distribution profiles of glioblastoma, oligodendroglioma and meningioma. J. Neurol. 2017, 264, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Hirai, T.; Nakamura, H.; Murakami, R.; Kitajima, M.; Shigematsu, Y.; Nakashima, R.; Shiraishi, S.; Uetani, H.; Iwashita, K.; et al. Does adding FDG-PET to MRI improve the differentiation between primary cerebral lymphoma and glioblastoma? Observer performance study. Ann. Nucl. Med. 2011, 25, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.P.; Trantakis, C.; Rubach, M.; Schulz, T.; Dietrich, J.; Winkler, D.; Renner, C.; Schober, R.; Geiger, K.; Brosteanu, O.; et al. Intraoperative MRI to guide the resection of primary supratentorial glioblastoma multiforme--a quantitative radiological analysis. Neuroradiology 2005, 47, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.W.; Floeth, F.W.; Reifenberger, G.; Felsberg, J.; Aulich, A.; Kiwit, J.C.; Bock, W.J. MRI characteristics and histological changes in glioblastoma after gene therapy. Front. Radiat. Ther. Oncol. 1999, 33, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Oriuchi, N.; Tomiyoshi, K.; Inoue, T.; Ahmad, K.; Sarwar, M.; Tokunaga, M.; Suzuki, H.; Watanabe, N.; Hirano, T.; Horikoshi, S.; et al. Independent thallium-201 accumulation and fluorine-18-fluorodeoxyglucose metabolism in glioma. J. Nucl. Med. 1996, 37, 457–462. [Google Scholar] [PubMed]
- Anzai, Y.; Lufkin, R.; DeSalles, A.; Hamilton, D.R.; Farahani, K.; Black, K.L. Preliminary experience with MR-guided thermal ablation of brain tumors. Am. J. Neuroradiol. 1995, 16, 39–48, discussion 49–52. [Google Scholar] [PubMed] [PubMed Central]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakai, T.; Muraki, S.; Bagarinao, E.; Miki, Y.; Takehara, Y.; Matsuo, K.; Kato, C.; Sakahara, H.; Isoda, H. Application of independent component analysis to magnetic resonance imaging for enhancing the contrast of gray and white matter. Neuroimage 2004, 21, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Doknic, M.; Gasic, V.; Stojanovic, M.; Pavlovic, S.; Marinkovic, S.; Miljic, D.; Pekic, S.; Manojlovic-Gacic, E.; Damjanovic, D.; Soldatovic, I.; et al. Hypopituitarism in five PROP1 mutation siblings: Long-lasting natural course and the effects of growth hormone replacement introduction in middle adulthood. Pituitary 2020, 23, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Verburg, N.; Koopman, T.; Yaqub, M.M.; Hoekstra, O.S.; Lammertsma, A.A.; Barkhof, F.; Pouwels, P.J.W.; Reijneveld, J.C.; Heimans, J.J.; Rozemuller, A.J.M.; et al. Improved detection of diffuse glioma infiltration with imaging combinations: A diagnostic accuracy study. Neuro Oncol. 2020, 22, 412–422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishio, S.; Morioka, T.; Inamura, T.; Takeshita, I.; Fukui, M.; Sasaki, M.; Nakamura, K.; Wakisaka, S. Radiation-induced brain tumours: Potential late complications of radiation therapy for brain tumours. Acta Neurochir. 1998, 140, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Ballester, L.Y.; Huse, J.T.; Tang, G.; Fuller, G.N. Molecular classification of adult diffuse gliomas: Conflicting IDH1/IDH2, ATRX, and 1p/19q results. Hum. Pathol. 2017, 69, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lim, K.Y.; Park, J.W.; Kang, J.; Won, J.K.; Lee, K.; Shim, Y.; Park, C.K.; Kim, S.K.; Choi, S.H.; et al. Sporadic and Lynch syndrome-associated mismatch repair-deficient brain tumors. Lab. Investig. 2022, 102, 160–171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prasanna, P.; Mitra, J.; Beig, N.; Nayate, A.; Patel, J.; Ghose, S.; Thawani, R.; Partovi, S.; Madabhushi, A.; Tiwari, P. Mass Effect Deformation Heterogeneity (MEDH) on Gadolinium-contrast T1-weighted MRI is associated with decreased survival in patients with right cerebral hemisphere Glioblastoma: A feasibility study. Sci. Rep. 2019, 9, 1145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, C.X.; Wu, J.F.; Zhao, W.; Cai, Z.W.; Cai, R.Z.; Chen, C.M. Primary spinal glioblastoma multiforme: A case report and review of the literature. Medicine 2017, 96, e6634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petzold, J.; Severus, E.; Meyer, S.; Bauer, M.; Daubner, D.; Krex, D.; Juratli, T.A. Glioblastoma multiforme presenting as postpartum depression: A case report. J. Med. Case Rep. 2018, 12, 374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, C.; Kong, X.; Yin, H.; Wang, Y.; He, H.; Zhang, H.; Gao, J.; Li, Y.; Ma, W. Glioblastoma multiforme in conus medullaris with intracranial metastasis after postoperative adjuvant therapy. Medicine 2017, 96, e6500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faguer, R.; Tanguy, J.Y.; Rousseau, A.; Clavreul, A.; Menei, P. Early presentation of primary glioblastoma. Neurochirurgie 2014, 60, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Karthigeyan, M.; Ahuja, C.K.; Chatterjee, D.; Salunke, P. Radiologic Dilemma in an Extra-Axial Petroclival Lesion: Low Grade versus High Grade. World Neurosurg. 2017, 100, 713.e1–713.e3. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Schmidt, R.H.; Salzman, K.L.; Chin, S.S.; Couldwell, W.T. Glioblastoma multiforme of the pineal region. J. Neurooncol. 2006, 79, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kajitani, T.; Kanamori, M.; Saito, R.; Watanabe, Y.; Suzuki, H.; Watanabe, M.; Kure, S.; Tominaga, T. Three case reports of radiation-induced glioblastoma after complete remission of acute lymphoblastic leukemia. Brain Tumor Pathol. 2018, 35, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Roemer, S.F.; Scheithauer, B.W.; Varnavas, G.G.; Lucchinetti, C.F. Tumefactive demyelination and glioblastoma: A rare collision lesion. Clin. Neuropathol. 2011, 30, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Kiang, K.M.; Chan, A.A.; Leung, G.K. Secondary gliosarcoma: The clinicopathological features and the development of a patient-derived xenograft model of gliosarcoma. BMC Cancer 2021, 21, 265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boikov, A.S.; Schweitzer, A.D.; Young, R.J.; Lavi, E.; Tsiouris, A.J.; Gupta, A. Glioblastoma-arteriovenous fistula complex: Imaging characteristics and treatment considerations. Clin. Imaging. 2014, 38, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tsuda, M.; Kanno, H.; Murata, J.; Mahabir, R.; Ishida, Y.; Kimura, T.; Tanino, M.; Nishihara, H.; Nagashima, K.; et al. Differential diagnosis of small cell glioblastoma and anaplastic oligodendroglioma: A case report of an elderly man. Brain Tumor Pathol. 2014, 31, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.C.; Giverso, C.; Faggiano, E.; Boffano, C.; Acerbi, F.; Ciarletta, P. Towards the Personalized Treatment of Glioblastoma: Integrating Patient-Specific Clinical Data in a Continuous Mechanical Model. PLoS ONE 2015, 10, e0132887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nestler, U.; Schmidinger, A.; Schulz, C.; Huegens-Penzel, M.; Gamerdinger, U.A.; Koehler, A.; Kuchelmeister, K.W. Glioblastoma simultaneously present with meningioma--report of three cases. Zentralbl. Neurochir. 2007, 68, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Lobbous, M.; Nabors, L.B.; Markert, J.M.; Kim, J. Undesired impact of iron supplement on MRI assessment of post-treatment glioblastoma. CNS Oncol. 2022, 11, CNS90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gu, S.; Chakraborty, G.; Champley, K.; Alessio, A.M.; Claridge, J.; Rockne, R.; Muzi, M.; Krohn, K.A.; Spence, A.M.; Alvord, E.C., Jr.; et al. Applying a patient-specific bio-mathematical model of glioma growth to develop virtual [18F]-FMISO-PET images. Math. Med. Biol. 2012, 29, 31–48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soleman, J.; Roth, J.; Ram, Z.; Yalon, M.; Constantini, S. Malignant transformation of a conservatively managed incidental childhood cerebral mass lesion: Controversy regarding management paradigm. Childs Nerv. Syst. 2017, 33, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Lrhezzioui, J.; Emery, E.; Chapon, F. Glioblastome frontal à cellules géantes: Tumeur radio-induite ? A propos d’un cas, avec revue de la littérature [Frontal giant cell glioblastoma: Radio-induced tumor? Case report and literature review]. Neurochirurgie 2007, 53, 486–490. (In French) [Google Scholar] [CrossRef] [PubMed]
- Cohen-Gadol, A.A.; DiLuna, M.L.; Bannykh, S.I.; Piepmeier, J.M.; Spencer, D.D. Non-enhancing de novo glioblastoma: Report of two cases. Neurosurg. Rev. 2004, 27, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.W.; Ng, D.C.; Wong, W.Y.; Ong, S.C.; Yu, S.W.; See, S.J. Promising role of [18F] fluorocholine PET/CT vs [18F] fluorodeoxyglucose PET/CT in primary brain tumors-early experience. Clin. Neurol. Neurosurg. 2011, 113, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Chen, C.Y.; Chung, H.W.; Juan, C.J.; Hsueh, C.J.; Gao, H.W. Discrepant MR spectroscopic and perfusion imaging results in a case of malignant transformation of cerebral glioma. Am. J. Neuroradiol. 2002, 23, 1775–1778. [Google Scholar] [PubMed] [PubMed Central]
- Franco, C.M.; Malheiros, S.M.; Nogueira, R.G.; Batista, M.A.; Santos, A.J.; Abdala, N.; Stávale, J.N.; Ferraz, F.A.; Gabbai, A.A. Gliomas múltiplos: Casos ilustrativos de quatro formas de apresentação [Multiple gliomas. Illustrative cases of 4 different presentations]. Arq. Neuropsiquiatr. 2000, 58, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, T.; Li, B.; Luo, B.; Li, Z. Clinicopathologic characteristics of unusual rhabdoid glioblastoma. Zhonghua Bing Li Xue Za Zhi 2015, 44, 772–777. (In Chinese) [Google Scholar] [PubMed]
- Dilber, B.; Havalı, C.; Eroglu, N.; Aydın, K.; Şahin, S.; Cansu, A. Cerebral neoplasm in L-2-hydroxyglutaric aciduria: Two different presentations. Childs Nerv. Syst. 2020, 36, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Ota, Y.; Nagai, M.; Kusaka, G.; Tanaka, Y.; Naritaka, H. Ultrasonography Monitoring with Superb Microvascular Imaging Technique in Brain Tumor Surgery. World Neurosurg. 2017, 97, 749.e11–749.e20. [Google Scholar] [CrossRef] [PubMed]
- Roetzer, T.; Leskovar, K.; Peter, N.; Furtner, J.; Muck, M.; Augustin, M.; Lichtenegger, A.; Nowosielski, M.; Hainfellner, J.A.; Baumann, B.; et al. Evaluating cellularity and structural connectivity on whole brain slides using a custom-made digital pathology pipeline. J. Neurosci. Methods 2019, 311, 215–221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, H.S.; Milbach, N.; Hurrell, S.L.; Cochran, E.; Connelly, J.; Bovi, J.A.; Schultz, C.J.; Mueller, W.M.; Rand, S.D.; Schmainda, K.M.; et al. Progressing Bevacizumab-Induced Diffusion Restriction Is Associated with Coagulative Necrosis Surrounded by Viable Tumor and Decreased Overall Survival in Patients with Recurrent Glioblastoma. Am. J. Neuroradiol. 2016, 37, 2201–2208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schiff, D.; Rosenblum, M.K. Herpes simplex encephalitis (HSE) and the immunocompromised: A clinical and autopsy study of HSE in the settings of cancer and human immunodeficiency virus-type 1 infection. Hum. Pathol. 1998, 29, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Cloughesy, T.F.; Lai, A.; Nghiemphu, P.L.; Liau, L.M.; Pope, W.B. High order diffusion tensor imaging in human glioblastoma. Acad. Radiol. 2011, 18, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Jallo, G.I.; Friedlander, D.R.; Kelly, P.J.; Wisoff, J.H.; Grumet, M.; Zagzag, D. Tenascin-C expression in the cyst wall and fluid of human brain tumors correlates with angiogenesis. Neurosurgery 1997, 41, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Maslehaty, H.; Cordovi, S.; Hefti, M. Symptomatic spinal metastases of intracranial glioblastoma: Clinical characteristics and pathomechanism relating to GFAP expression. J. Neurooncol. 2011, 101, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, K.A.; Henry, B.M.; Kumar Ramakrishnan, P.; Roy, J.; Vikse, J.; Loukas, M.; Tubbs, R.S.; Walocha, J.A. Development of the Anatomical Quality Assurance (AQUA) checklist: Guidelines for reporting original anatomical studies. Clin. Anat. 2017, 30, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, A. Guidelines for reporting original anatomical studies-Quality and ethics. Clin. Anat. 2017, 30, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019, 22, 407–411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zarnett, O.J.; Sahgal, A.; Gosio, J.; Perry, J.; Berger, M.S.; Chang, S.; Das, S. Treatment of elderly patients with glioblastoma: A systematic evidence-based analysis. JAMA Neurol. 2015, 72, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Corr, F.; Grimm, D.; Saß, B.; Pojskić, M.; Bartsch, J.W.; Carl, B.; Nimsky, C.; Bopp, M.H.A. Radiogenomic Predictors of Recurrence in Glioblastoma-A Systematic Review. J. Pers. Med. 2022, 12, 402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Author | Study Type and Number of Subjects. | Incidence and Anatomical Location of GB | Relevant Statistical Data | Geographic Location | Laterality | Sex of Patients with GBM | Relevant Clinical Considerations |
|---|---|---|---|---|---|---|---|
| Hashiguchi 2022 [10] | Retrospective study, 51 patients. | 51/51 (100%) Frontal: 15 Temporal: 16 Parietal: 8 Occipital: 3 Cerebellum: 2 Others (including midline lesion or multiple lesions): 7 | Not specified | Japan | Laterality not reported. | 25 female; 26 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Drabycz 2009 [11] | Retrospective study, 72 patients | 72/72 (100%) Frontal: 19 Temporal: 32 Parietal: 17 Occipital: 2 Declassified: 2 | p-value: 0.85 in relation to the grouping of the right and left sides or predominant lobe; p-value: 0.88 in relation to the comparison of the occupation of the left and right sides. | Canada | R. hemisphere: 34 L. hemisphere: 38 | 24 female; 48 male. | There was no association between the anatomical location or radial distribution of GBM and the MGMT promoter methylation status. |
| Ko 2016 [12] | Retrospective study, 126 patients | 104/126 (82.5%) Frontal: 38 Parietal: 25 Temporal: 27 Occipital: 3 Basal ganglia: 2 Corpus callosum: 3 Thalamus: 5 Brainstem: 1. | p-value: 0.02 in relation to the location of the tumor. | Taiwan | Laterality not reported | 46 female; 58 male. | There were statistically significant differences in tumor location between GBM and primary brain lymphoma. |
| Abecassis 2020 [13] | Retrospective study, 100 patients | 31/100 (31%) Frontal: 17 Parietal: 8 Temporal: 24 Occipital: 0 Brainstem: 1 Cerebellum: 0 These data correspond to the locations of tumors from patients with gliomas of different grades, not specifically GBM. | p-value: < 0.001 in relation to tumor location of patients with glioma, not specifically GBM. | USA | R. hemisphere: 20 L. hemisphere: 30; for patients with gliomas of different grades. | Not specified | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Ferreira 2004 [14] | Retrospective study, 67 patients | 67/67 100% Frontal: 32 (2 bilateral) Temporal: 12 Parietal: 11 Occipital: 4 (1 bilateral) Frontal and temporal: 1 Temporo-parietal: 1 Parieto-occipital: 1 Parieto-occipital + Corpus callosum: 1 Corpus callosum: 3 Brainstem: 1 | Not specified | Brazil | Laterality not reported | 29 female; 38 male. | Regarding their location, they were more frequent in the frontal and temporal lobes. In children, the most frequent location was infratentorial. |
| Stark 2010 [15] | Retrospective study, 7 patients | 7/7 (100%) Cerebellum: 4 Brainstem: 2 Inferior to the floor of the fourth ventricle: 1 | Not specified | Germany | L. hemisphere: 1; lateralities of the remaining cases were not reported. | 1 female; 1 male. Gender was not reported for the remaining cases, so it was considered as NS. | The incidence of infratentorial glioblastomas (iGB) in adults is in the range of 1.2% of all GB patients. The most common clinical features of iGB are rapid deterioration of ataxia and dysmetria. Diagnosing iGB can be challenging due to nonspecific symptoms and radiological characteristics, leading to misdiagnosis. The pathological findings of iGB are comparable to supratentorial glioblastomas. |
| Liu 2018 [16] | Retrospective study, 167 patients | 107/167 (64.07%) Basal ganglia and thalamus: 4 Cortex: 68 Subventricular zone: 64 Corpus callosum: 17 several patients presented with more than one location. | p-value: <0.001 in relation to location in the basal ganglia and thalamus and cortex; p-value: 0.076 in relation to location in the subventricular zone; p-value: 1.000 in relation to location in the corpus callosum. | China | Laterality not reported | 34 female; 73 male. | GBMs located in infratentorial regions or multiple lesions are rare. There are significant differences in the anatomical location between GBM and primary central nervous system lymphoma. |
| Chen 2022 [17] | Retrospective study, 15 patients | 15/15 (100%) Frontal: 5 Temporal: 3 Occipital: 1 Temporo-occipital: 1 Parieto-occipital: 1 Corpus callosum: 1 Cerebellum: 1 Brainstem: 1 Spinal (C1–C7): 1 | Not specified | China | R. hemisphere: 4 L. hemisphere: 9 | 6 female; 9 male. | Compression of the affected segments of the spinal cord results in dysfunction, often presenting as lower limb movement disorders, pain, and abnormal urination. As spinal metastatic dissemination usually occurs simultaneously or sequentially with the progression of intracranial GBM, patients are often asymptomatic or of late onset. Progression of intracranial GBM leads to motor disorders and sensory abnormalities, which may additionally mask symptoms of spinal metastatic spread. |
| Jaskólski 2013 [18] | Retrospective study, 89 patients | 26/89 (29.2%) Frontal: 11 Temporal: 7 Parietal: 6 Occipital: 2 | Not specified | Poland | Laterality not reported | 12 female; 14 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Kim 2019 [19] | Retrospective study, 83 patients | 83/83 100% Frontal or temporal: 29 Others: 54 | p-value: 0.19 in relation to the tumor location. | South Korea | Laterality not reported | 37 female; 46 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Hatakeyama 2021 [20] | Retrospective study, 75 patients | 55/75 (73.3%) Cerebral hemisphere: 49 Supratentorial central structures: 5 Vermis: 1 | Not specified | Japan | Laterality not reported | 22 female; 33 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Yamashita 2019 [21] | Retrospective study, 112 patients | 112/112 (100%) Frontal: 36 Parietal: 25 Temporal: 21 Occipital: 3 Insula: 7 Basal ganglia or thalamus: 16 Brainstem or cerebellum: 4 | Not specified | Japan | Laterality not reported | 56 female; 56 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Kuroiwa 1995 [22] | Retrospective study, 9 patients | 9/9 (100%) Cerebellar vermis: 3 Cerebellar vermis and brainstem: 2 Cerebellar hemisphere: 1 Vermis and cerebellar hemisphere: 1 Cerebellar hemisphere and brainstem: 1 Brainstem: 1. | Not specified | USA | Laterality not reported | 1 female; 8 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Awad 2017 [23] | Retrospective study, 330 patients | 330/330 (100%) Frontal: 132 Temporal:139 Parietal: 99 Occipital: 50 Periventricular: 11 Hippocampal: 51 Brainstem: 4 Basal ganglia/deep nuclei: 32 Cerebellum: 3 | Univariate p-value: 0.32 in relation to frontal location; 0.918 in relation to temporal location; 0.336 in relation to parietal location; 0.121 in relation to occipital location; 0.006 in relation to periventricular location; 0.304 in relation to hippocampal location; 0.114 in relation to brainstem location; 0.002 in relation to deep nuclei/basal ganglia location; 0.852 in relation to cerebellar location. Multivariate p-value: 0.816 in relation to occipital location; 0.518 in relation to periventricular location; 0.28 in relation to brainstem location; 0.045 in relation to deep nuclei/basal ganglia location. | USA | R. hemisphere: 168 L. hemisphere: 139 Bilateral: 23 | 130 female; 200 male. | It was suggested that specific tumor locations may play a significant role in better understanding the aggressive nature of GBM and how it impacts patient survival. |
| Quan 2023 [24] | Retrospective study, 110 patients | 110/110 (100%) Frontal: 42 Others: 68 | p-value: 0.204 in relation to the location, or not, of the tumor in the frontal lobe. | China | Laterality not reported | 49 female; 61 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Onuma 2013 [25] | Retrospective study, 33 patients | 33/33 (100%) Frontal: 17 Others: 16 | p-value: 0.157 in relation to the location, or not, of the tumor in the frontal lobe. | Japan | Laterality not reported | Not specified | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Cui 2021 [26] | Retrospective study, 77 patients | 77/77 (100%) Frontal: 44 Frontal/temporal insula lobe: 22 Parietal/parieto-occipital lobe: 11 All 77 had the corpus callosum affected. | p-value: 0.121 in relation to location in the frontal lobe; p-value: 0.571 in relation to location in the frontal/temporal insula lobe; p-value: 0.273 in relation to location in the parietal/parieto-occipital lobe. | China | Unilateral: 41 Blateral: 36; for unilateral cases, the hemisphere was not specified. | 44 female; 43 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Chen 2023 [27] | Retrospective study, 42 patients | 20/42 (47.6%) Frontal: 12 Parietal: 3 Temporal: 2 Basal ganglia: 1 Cerebellar hemisphere: 2 The corpus callosum was invaded by 3 frontal lesions. | Not specified | China | Laterality not reported | 8 female; 12 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Wach 2020 [28] | Retrospective study, 198 patients | 198/198 (100%) R. hemisphere: 111 L. hemisphere: 87 | p-value: 0.398 in relation to the location in the right hemisphere. | Germany | R. hemisphere: 111 L. hemisphere: 87 | 80 female; 118 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Steidl 2023 [29] | Retrospective study, 65 patients | 65/65 (100%) Temporal: 27 Frontal: 16 Parietal: 15 Occipital: 12 Thalamus: 2 Insula: 1 Basal ganglia: 1 | Not specified | Germany | R. hemisphere: 36 L. hemisphere: 28 Bilateral: 1 | 17 female; 48 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Park 2017 [30] | Retrospective study, 108 patients | 108/108 (100%) Frontal: 45 Others: 63 | p-value: 0.955 in relation to the location of GBM in other areas. | South Korea | Laterality not reported | 54 female; 54 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Ideguchi 2015 [31] | Retrospective study, 5 patients | 5/5 (100%) Frontal: 1 Temporal: 1 Parietal: 1 Occipital: 1 Basal ganglia: 1 | Not specified | Japan | R. hemisphere: 3 L. hemisphere: 2 | 4 female; 1 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Wang 2018 [32] | Retrospective study, 34 patients | 19/34 (55.9%) Frontal: 7 (1 bilateral) Temporal: 1 Parietal: 2 Frontal, occipital, temporal: 1 Fronto-temporal: 1 Corpus callosum: 1 Brainstem: 1 Basal ganglia: 1 Not specified: 4 | Not specified | China | R. hemisphere: 6 L. hemisphere: 6 Bilateral: 1. Not specified: 6. | 4 female; 15 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Muller 2019 [33] | Retrospective study, 275 patients | 275/275 (100%) R. hemisphere: 141 L. hemisphere: 134 | Not specified | USA | R. hemisphere: 141 L. hemisphere: 134 | 105 female; 170 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Thomas 2016 [34] | Retrospective study, 21 patients | 21/21 (100%) Corpus callosum both hemispheres (butterfly): 5 Insula: 4 Thalamus/basal ganglia: 1 Cingulate: 2 Splenium: 2 Temporal: 1 Motor area: 3 Speech area (broca): 3 | Not specified | USA | Laterality not reported | Not specified | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Koike 2022 [35] | Retrospective study, 22 patients | 11/22 (50%) Infratentorial: 11 Supratentorial: 0 | p-value: 0.279 | Japan | Laterality not reported | 5 female; 6 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Xing 2018 [36] | Retrospective study, 75 patients | 75/75 (100%) Frontal: 32 Parietal: 12 Temporal: 13 Occipital: 5 Insula: 1 Others: 12 | p-value: 0.002 | China | Laterality not reported | 34 female; 41 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Smedley 2018 [37] | Retrospective study, 304 patients | 304/304 (100%) Frontal: 114 Temporal: 91 Parietal: 76 Occipital: 18 Thalamus: 8 Corpus callosum: 4 Cerebellum: 1 Pineal gland: 1 Midbrain: 1 In 7 cases it was bilateral and in 11 cases it involved 2 locations. | Not specified | USA | R. hemisphere: 162 L. hemisphere: 149 | 116 female; 188 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Miquelini 2016 [38] | Retrospective study, 84 patients | 42/84 (50%) Supratentorial: 41 Infratentorial: 1 Three cases presented involvement of the corpus callosum | p-value: 0.007 in relation to infratentorial location; p-value: 0.048 in relation to the cases involving the corpus callosum. | Argentina | Laterality not reported | 19 female; 23 male. | The article did not establish a relationship between the region of the glioblastoma and clinical |
| Han 2018 [39] | Retrospective study, 92 patients | 92/92 (100%) R. hemisphere: 39 L. hemisphere: 39 Midline: 14. | p-value: 0.72 in relation to presence in the left hemisphere. | China | R. hemisphere: 39 L. hemisphere: 39 Midline: 14. | Not specified | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Mathew 2018 [40] | Retrospective study, 47 patients | 47/47 (100%) Ipsilateral cerebral lobes: 15 Lateral ventricle: 41 | Not specified | India | Laterality not reported | 14 female; 33 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Jiang 2017 [41] | Retrospective study, 10 patients | 10/10 (100%) Single lobe: 7 Parieto-occipital: 1 Third ventricle: 2 | Not specified | China | Laterality not reported | Not specified | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Li 2018 [42] | Retrospective study, 406 patients | 406/406 (100%) Frontal: 182 Temporal: 224 | p-value: 0.879 in relation to the hemispheres. | China | R. hemisphere: 179 L. hemisphere: 227 | 195 female; 211 male. Female: 77 frontal lobe and 118 temporal lobe; male: 105 frontal lobe and 106 temporal lobe. | The results demonstrated that tumor location was an important factor, and glioblastomas in the frontal lobe and temporal lobe had different clinical properties. |
| Utsuki 2005 [43] | Retrospective study, 37 patients | 37/37 (100%) Frontal: 16 Temporal: 8 Parietal: 3 Occipital: 5 Parietoocipital: 4 Frontoparietal: 1 | Not specified | Japan | Laterality not reported | 18 female; 19 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Fan 2017 [44] | Retrospective study, 133 patients | 133/133 (100%) R. hemisphere: 56 L. hemisphere: 77 | Not specified | China | R. hemisphere: 56 L. hemisphere: 77 | 51 female; 82 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Hart 2016 [45] | Retrospective study, 5 patients | 5/5 (100%) Parietal: 2 Occipital: 1 Paracentral: 1 Postcentral and supramarginal gyri: 1 | Not specified | UK | R. hemisphere: 5 | Not specified | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Wang 2014 [46] | Retrospective study, 153 patients | 153/153 (100%) R. hemisphere: 73 L. hemisphere: 80 | Not specified | China | R. hemisphere: 73 L. hemisphere: 80 | 56 female; 97 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Smets 2013 [47] | Retrospective study, 24 patients | 24/24 (100%) Frontal: 4 Fronto-parietal: 1 Occipital: 2 Parietal: 5 Parieto-occipital: 4 Temporal: 6 Temporo-parietal: 2 | Not specified | Belgium | R. hemisphere: 15 L. hemisphere: 9 | 11 female; 13 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Eoli 2007 [48] | Retrospective study, 86 patients | 86/86 (100%) Frontal: 36 Temporal: 35 Others: 15 | p-value: 0.005 in relation to the frontal lobe and temporal lobe. | Italy | Laterality not reported | 21 female; 65 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| sugimoto 2015 [49] | Retrospective study, 4 patients | 4/4 (100%) Temporal: 1 Frontal: 3 Case 3 presented a satellite lesion in the right frontal lobe via the corpus callosum. | Not specified | japan | R. hemisphere: 0 L. hemisphere: 3 Bilateral: 1 | 4 female | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| seidel 2011 [50] | Retrospective study, 122 patients | 122/122 (100%) Frontal: 70 Temporal: 62 Central: 62 Parieto-occipital: 38 Basal ganglia: 10 Others: 2 | Not specified | Germany | Laterality not reported | Not specified | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Cho 2018 [51] | Retrospective study, 60 patients | 60/60 (100%) Frontal: 33 Parietal: 21 Temporal: 34 Occipital: 4 Insula: 8 Deep gray matter: 14 Corpus callosum: 8 Midbrain: 5 Infratentorial: 2 several patients presented with more than one location. | Not specified | South Korea | Laterality not reported | 25 female; 35 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Olar 2012 [52] | Retrospective study, 9 patients | 4/9 (44.44%) Temporal: 1 Parietal: 1 Fronto-temporo-parietal: 1 Basal ganglia and insula: 1 | Not specified | USA | R. hemisphere: 1 L. hemisphere: 3 | 1 female; 3 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| woo 2019 [53] | Retrospective study, 147 patients | 147/147 (100%) Temporal: 47 Frontal: 46 Parietal: 34 Occipital: 5 Insula: 3 Corpus callosum: 3 Cerebellum: 6 Intraventricular: 3 | Not specified | China | Laterality not reported | Not specified | Although total resection was an independent factor for survival, it could not be achieved in the majority of cases. |
| Ali 2014 [54] | Retrospective study, 9 patients | 9/9 (100%) Frontal: 3 Parietal: 1 Occipital: 1 Temporal:1 Fronto-temporal: 2 Fronto-temporo-parietal: 1 | Not specified | USA | R. hemisphere: 7 L. hemisphere: 2 | 5 female; 4 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Mohan 2019 [55] | Retrospective study, 65 patients | 48/65 (73.85%) Frontal: 11 Temporal: 12 Parietal: 10 Occipital: 3 Two lobes involved: 13 | Not specified | USA | Laterality not reported | 18 female; 30 men. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Mangla 2010 [56] | Retrospective study, 36 patients | 36/36 (100%) Fronto-temporal: 22 Others: 14 | Not specified | USA | Laterality not reported | 12 female; 24 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Adeberg 2014 [57] | Retrospective study, 100 patients | 100/100 (100%) R. hemisphere: 40 L. hemisphere: 47 Bilateral: 13 | Not specified | Germany | R. hemisphere: 40 L.hemisphere: 47 Bilateral: 13 | 41 female; 59 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| wright 2016 [58] | Retrospective study, 10 patients | 8/10 (80%) Frontal: 3 Anterior corpus callosum: 1 Corpus callosum: 1 Thalamus: 1 Fronto-parietal: 1 Fronto-temporal: 1 | Not specified | USA | R. hemisphere: 4 L. hemisphere: 2 Bilateral: 2 | 5 female; 5 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| shibahara 2019 [59] | Retrospective study, 87 patients | 87/87 (100%) Frontal: 28. Temporal: 30. Parietal: 15. Infratentorial: 2. Others: 12. | Not specified | Japan | Laterality not reported | 26 female; 61 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| tykocinski 2012 [60] | Retrospective study, 132 patients | 132/132 (100%) Frontal, parietal, occipital: 81 Temporal, insula: 50 Posterior fossa: 1 | Not specified | USA | Laterality not reported | 61 female; 71 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Kanas 2017 [61] | Retrospective study, 86 patients | 86/86 (100%) Frontal: 25 Temporal: 38 Parietal: 17 Occipital: 6 | Not specified | France | R. hemisphere: 47 L. hemisphere: 39 | 27 female; 59 men. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| reimer 2017 [62] | Retrospective study, 35 patients | 35/35 (100%) Frontal and temporal: 33 Parietal: 2. | Not specified | Germany | Laterality not reported | 9 female; 26 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Jiguet-Jiglaire 2022 [63] | Retrospective study, 38 patients | 38/38 (100%) Frontal: 17. Temporal: 15. Corpus callosum: 12. Basal ganglia: 11. several patients presented with more than one location. | Not specified | France | Laterality not reported | 27 female; 11 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Senders 2020 [64] | Retrospective study, 562 patients | 562/562 (100%) Frontal: 235. Temporal: 250. Parietal: 175. Occipital: 73. Corpus callosum:: 59. several patients presented with more than one location. | Not specified | USA | R. hemisphere: 302 L. hemisphere: 281; the number of bilateral patients was not specified | Not specified | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Zhang 2021 [65] | Retrospective study, 60 patients | 60/60 (100%) Frontal: 29. Temporal: 13. Parietal: 7. Occipital: 5. Insula, thalamus, others: 6. | Not specified | China | R. hemisphere: 33 L. hemisphere: 27 | 26 female; 34 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Liu 2023 [66] | Retrospective study, 60 patients | 76/118 (64.41%) R. hemisphere: 35 L. hemisphere: 25 Bilateral: 6 Only 66 were mentioned in terms of laterality, but there were 76 patients with GBM | Not specified | China | R. hemisphere: 35 L. hemisphere: 25 Bilateral: 6;. only 66 were mentioned in terms of laterality, but there were 76 patients with GBM. | Not specified | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| cohen 2000 [67] | Retrospective study, 7 patients | 1/7 (14.29%) Occipital | Not specified | Israel | L. hemisphere: 1 | 1 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| nishio 1997 [68] | Retrospective study, 20 patients | 4/20 (20%) Thalamus: 4 | Not specified | Japan | Laterality not reported | Not specified | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| wang 2015 [69] | Retrospective study, 400 patients | 200/400 (50%) Frontal: 109 Temporal: 92 Others: 76 | Not specified | China | R. hemisphere: 87 L. hemisphere: 113 | 71 female; 129 male. | In the GBM cohort, the brain region (Cluster 3) associated with advanced age at tumor diagnosis was mainly located in the bilateral temporal lobe, particularly at the posterior region of the subventricular zone (SVZ). Meanwhile, the brain region associated with younger age at tumor diagnosis was preferentially located in the left inferior frontal region. |
| simonet-redondo 2012 [70] | Retrospective study, 6 patients | 5/6 (83.33%) Frontal: 2 Temporal: 3 | Not specified | Spain | R. hemisphere: 37 L. hemisphere: 2 | 2 female; 3 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| sunwoo 2015 [71] | Retrospective study, 72 patients | 20/72 (27.78%) Frontal: 11 Temporal: 7 Parietal: 7 Occipital: 2 Multiple patients presented with more than one location | Not specified | South Korea | Laterality not reported | 9 female; 11 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| friese 2000 [72] | Retrospective study, 59 patients | 1/59 (1.69%) Corpus callosum: 1 | Not specified | Germany | Midline: 1 | 1 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| okamoto 2002 [73] | Retrospective study, 5 patients | 2/5 (40%) Parietal: 1 Corpus callosum to right hemisphere: 1 | Not specified | Japan | R. hemisphere: 1 L. hemisphere: 1 | 1 female; 1 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Stummer 2008 [74] | Prospective study, 243 patients. | 221/243 (90.9%) R. hemisphere: 156 L. hemisphere: 87 | p-value 0.4734 in relation to the difference between hemispheres. | Germany | R. hemisphere: 156 L. hemisphere: 87 These numbers are from the total studied patients, but they were not specifically reported for glioblastoma. | 90 female; 153 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Fudaba 2021 [75] | Prospective study, 35 patients | 35/35 (100%) R. hemisphere: 18 L. hemisphere and bilateral: 17 | p-value: 0.739 in relation to the location in the hemisphere of the tumor. p-value: 0.023 in relation to patients who with total or subtotal resection of the tumor showing significantly better progression-free survival. | Japan | R. hemisphere: 18. L. hemisphere and bilateral: 17; the difference between left and bilateral locations was not specified. | 14 female; 21 male. | The extension and tumor location on the hemispheric side were not identified as significant predictors of overall survival. However, patients with total or subtotal resection showed significantly better progression-free survival. |
| Wang 2019 [76] | Prospective study, 109 patients | 81/109 (74,3%) Frontal: 13 Parietal: 8 Temporal: 32 Occipital: 3 Insula: 3 Corpus callosum: 6 Basal ganglia: 9 Thalamus: 4 Hippocampus: 3 | Not specified | China | Laterality not reported | 39 female; 42 male. | Differences in predilection sites were found between GBM and primary central neural system lymphoma. |
| Mizumoto 2016 [77] | Prospective study, 46 patients | 46/46 (100%) Frontal: 23 Temporal: 16 Parietal: 3 Occipital: 4 | Not specified | Japan | Laterality not reported | 22 female; 24 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Isoardo 2012 [78] | Prospective study, 19 patients | 19/19 (100%) Frontal: 9 Temporal: 5 Parietal: 5 | Not specified | Italy | Laterality not reported | 6 female; 13 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Stumpo 2021 [79] | Prospective study, 7 patients | 7/7 (100%) Frontal: 4 Temporal: 2 Parietal: 1 | Not specified | Switzerland | R. hemisphere: 2 L. hemisphere: 4 Midline: 1 | 1 female; 6 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Iliadis 2011 [80] | Prospective study, 65 patients | 65/65 (100%) Parietal: 20 Temporal: 20 Frontal: 17 Occipital: 3 | Not specified | Greece | R. hemisphere: 29 L. hemisphere: 33 Bilateral: 3 | 28 female; 37 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Galldiks 2012 [81] | Prospective study, 25 patients | 25/25 (100%) Frontal: 9 Temporal: 7 Parietal: 8 Occipital: 1 | Not specified | Germany | Laterality not reported | 10 female; 15 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Henker 2015 [82] | Prospective study, 20 patients | 20/20 (100%) Frontal: 6 Temporal: 10 Parietal: 2 Others: 2 | Not specified | Germany | R. hemisphere: 11 L. hemisphere: 9 | 11 female; 9 male. | PTE volume potentially represents infiltration into the tumor area instead of a simple accumulation of water as a side effect of the tumor. (XXXX) may have a benefit in the survival of patients with GBM. |
| Najafi 2012 [83] | Prospective study, 12 patients | 12/12 (100%) Frontal: 4 Temporal: 5 Parietal: 1 Occipital: 1 Multiple: 1 | Not specified | Iran | R. hemisphere: 5 L. hemisphere: 6 Multiple: 1 | 3 female; 9 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Coburger 2015 [84] | Prospective study, 20 patients | 20/20 (100%) Frontal: 10 Temporal: 5 Parietal: 3 Occipital: 2 | Not specified | Germany | Laterality not reported | Not specified | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Hakyemez 2004 [85] | Prospective study, 33 patients | 18/33 (55%) Frontal: 4 Temporal: 4 Parietal: 5 Occipital: 3Thalamus: 2 | Not specified | Turkey | Laterality not reported | 3 female; 15 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Yu 2017 [86] | Prospective study, 88 patients | 43/88 (48%) Cerebral parenchyma: 35 Brainstem: 2 Cerebellar hemisphere: 6 | Not specified | China | Laterality not reported | 26 female; 62 male. | There was no difference between sexes for GBM and SBM; however, patients with SBM were older than those with GBM. |
| Laule 2017 [87] | Prospective study, 3 patients | 1/3 (33.33%) Frontal: 1 | Not specified | Canada | L. hemisphere: 1 | 1 female | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Makino 2011 [88] | Prospective study, 21 patients | 7/21 (33.33%) Temporal: 3 Frontal: 1 Basal ganglia: 1 Corpus callosum: 1 Thalamus: 1 | Not specified | Japan | Laterality not reported | 8 female; 13 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| schneider 2005 [89] | Prospective study, 31 patients | 31/31 (100%) Parietal: 5 Temporal: 6 Frontal: 7 Fronto-parietal: 7 Fronto-temporal: 2 Parieto-temporal: 2 Parieto-occipital:1 Occipito-temporal: 1 | Not specified | Germany | R. hemisphere: 14 L. hemisphere: 17 | 12 female; 19 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| weber 1999 [90] | Prospective study, 10 patients | 10/10 (100%) Frontal: 3 Occipital: 1 Parietal:1 Fronto-parietal: 1 Parieto-occipital: 1 Temporo-parieto-occipital: 2 Fronto-temporal: 1 | Not specified | Germany | R. hemisphere: 5 L. hemisphere: 5 | 3 female; 7 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Oriuchi 1996 [91] | Prospective study, 20 patients | 5/20 (25%) Parietal: 3 Thalamus: 2 | Not specified | Japan | R. hemisphere: 2 L. hemisphere: 3 | 5 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Anzai 1995 [92] | Prospective study, 12 patients | 1/12 (8.33%) Frontal: 1 | Not specified | USA | L. hemisphere: 1 | 1 female | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Todo 2022 [93] | Prospective study, 19 patients | 19/19 (100%) Frontal: 11 Temporal: 4 Parietal: 3 Corpus callosum: 1 | Not specified | Japan | R. hemisphere: 11 L. hemisphere: 8 Midline: 1 | 4 female; 15 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Nakai 2004 [94] | Prospective study, 10 patients | 2/10 (20%) Fronto-temporal:1 Temporal: 1 | Not specified | Japan | L. hemisphere: 2 | 1 female; 1 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Doknic 2020 [95] | Prospective study, 5 patients | 1/5 (20%) Temporal: 1 | Not specified | Serbia | L. hemisphere: 1 according to the image of the CT presented. | 1 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Verburg 2020 [96] | Prospective study, 20 patients | 12/20 (60%) Frontal: 3 Parietal: 6 Occipital: 2 Temporal: 1 | Not specified | Netherlands | R. hemisphere: 7 L. hemisphere: 5 | 5 female; 7 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Nishio 1998 [97] | Prospective study, 11 patients | 1/11 (9.09%) Cerebellum and brainstem: 1 | Not specified | Japan | Laterality not reported | 1 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| ballester 2017 [98] | Prospective study, 6 patients | 3/6 (50%) Frontal: 1 Temporal: 2 | Not specified | USA | R. hemisphere: 3 | 3 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| kim 2022 [99] | Prospective study, 13 patients | 8/13 (61.54%) Temporal: 3 Parietal: 1 Occipital: 1 Frontal: 1 Cerebellum: 1 Thalamus, basal ganglia, and midbrain: 1 | Not specified | South Korea | R. hemisphere: 5 L. hemisphere: 2 | 5 female; 3 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Prasanna 2019 [100] | Prospective study, 138 patients | 138/138 (100%) R. hemisphere: 60 L. hemisphere: 78 | Not specified | USA | R. hemisphere: 60 L. hemisphere: 78 | 52 female; 86 male. | MEDH in AAL regions due to the mass effect was associated with survival for right-hemispheric tumors. |
| Shen 2017 [101] | Case report, 1 patient | 1/1 (100%) Cervical spinal cord (C4–C7): 1 | Not specified | China | Laterality not reported | 1 female | Primary spinal GBM is a clinically rare entity that progresses rapidly with a dismal prognosis and short survival time. |
| Petzold 2018 [102] | Case report, 1 patient | 1/1 (100%) Frontal: 1 | Not specified | Germany | L. hemisphere: 1. | 1 female | A supposed but not significant association was found between tumors located in the frontal lobe and mood-related symptoms. |
| Yan 2017 [103] | Case report, 1 patient | 1/1 (100%) Conus medullaris: 1 | Not specified | China | Laterality not reported | 1 male | Spinal GBM located in the conus medullaris is rare. |
| Faguer 2014 [104] | Case report, 4 patients | 4/4 (100%) Temporal: 1 Parietal: 2 Frontal and parietal:1 | Not specified | France | R. hemisphere: 3 L. hemisphere: 1 | 4 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Karthigeyan 2017 [105] | Case report, 1 patient | 1/1 (100%) Petroclival: 1 | Not specified | India | L. hemisphere: 1 | 1 female | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Amini 2006 [106] | Case report, 3 patients | 3/3 (100%) Pineal: 3 Posterior ventricular region: 1 one patient had 2 affected areas. | Not specified | USA | Laterality not reported | 1 female; 2 male | GBM of the pineal region is extremely rare and is associated with a bad prognosis. Most patients present signs and symptoms of hydrocephalus and Parinaud syndrome, requiring placement of a ventriculoperitoneal shunt or endoscopic biopsy and third ventriculostomy. |
| Kajitani 2018 [107] | Case report, 3 patients | 3/3 (100%) Cerebellum and Pons: 1 Fronto-temporo-parietal and insula: 1 Fronto-parietal: 1 | Not specified | Japan | R. hemisphere: 3 L. hemisphere: 0 | 2 female; 1 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Roemer 2011 [108] | Case report, 1 patient | 1/1 (100%) R. hemisphere | Not specified | USA | R. hemisphere: 1 | Female | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Kiang 2021 [109] | Case report, 1 patient | 1/1 (100%) Basal ganglia and frontal: 1 | Not specified | China | L. hemisphere: 1 | Female | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Boikov 2013 [110] | Case report, 1 patient | 1/1 (100%) Thalamus: 1 | Not specified | USA | R. hemisphere: 1 | Female | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| takahashi 2013 [111] | Case report, 1 patient | 1/1 (100%) Frontal: 1 | Not specified | Japan | L. hemisphere:1 | Male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Colombo 2015 [112] | Case report, 1 patient | 1/1 (100%) Parietal: 1 | Not specified | italy | R. hemisphere: 1 | Not specified | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Nestler 2007 [113] | Case report, 3 patients | 3/3 (100%) Cerebellar:1 Parasagittal: 1 Frontal: 1 | Not specified | Germany | R. hemisphere: 0 L. hemisphere: 3 | 1 female; 2 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Park 2022 [114] | Case report, 1 patient | 1/1 (100%) Right posterior temporal and occipital: 1 | Not specified | USA | R. hemisphere: 1 | 1 female | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Gu 2011 [115] | Case report, 1 patient | 1/1 (100%) Fronto-temporal: 1 | Not specified | USA | R. hemisphere: 1 | 1 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| soleman 2017 [116] | Case report, 1 patient | 1/1 (100%) Frontal: 1 | Not specified | Israel | R. hemisphere: 1 | 1 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Lrhezzioui 2007 [117] | Case report, 1 patient | 1/1 (100%) Frontal: 1 | Not specified | France | R. hemisphere:1 | 1 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| cohen-gadol 2004 [118] | Case report, 2 patients | 2/2 (100%) Precentral gyrus: 1 Temporal: 1 | Not specified | USA | R. hemisphere: 2 | 2 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Lam 2011 [119] | Case report, 2 patients | 1/2 (50%) Thalamus and corpus callosum: 1 | Not specified | Singapore | L. hemisphere: 1 | 1 female | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| wu 2002 [120] | Case report, 1 patient | 1/1 (100%) Temporal: 1 | Not specified | China | L. hemisphere: 1 | 1 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| franco 2000 [121] | Case report, 4 patient | 3/4 (75%) Frontal: 1 Right temporo-thalamic and left frontal horn: 1 Frontal and ear of the right lateral ventricle: 1 | Not specified | Brazil | R. hemisphere: 2 L. hemisphere: 0 Bilateral: 1 | 2 female; 1 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Li 2015 [122] | Case report, 2 patients | 2/2 (100%) Temporal: 1 Cerebellum: 1 | Not specified | China | R. hemisphere: 1 L. hemisphere: 1 | 2 female | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Dilber 2020 [123] | Case report, 2 patients | 1/2 (50%) Parieto-temporo-occipital and corpus callosum: 1 | Not specified | Turkey | L. hemisphere: 1 | 1 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| ishikawa 2017 [124] | Case report, 15 patients | 5/15 (33.33%) Frontal: 1 Temporal: 1 Occipital: 2 Parietal: 1 | Not specified | Japan | R. hemisphere: 2 L. hemisphere: 3 | 3 female; 2 male. | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Roetzer 2018 [125] | Cadaveric study, 3 patients | 3/3 (100%) Temporo-cccipital: 1 Temporal: 1 Pons: 1 | Not specified | Austria | R. hemisphere: 2 L. hemisphere: 0 Midline: 1 | 2 female; 1 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Nguyen 2016 [126] | Cadaveric study, 6 patients | 6/6 (100%) Corpus callosum: 2 Corona radiate: 2 Centrum semiovale: 2 | Not specified | USA | Laterality not reported | 1 female; 5 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Schiff 1998 [127] | Cadaveric study, 3 patients | 1/3 (33.33%) Temporo-parietal and basal ganglia: 1 | Not specified | USA | L. hemisphere | 1 female | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Ellingson 2011 [128] | Transversal study, 25 patients | 25/25 (100%) Frontal: 7 Parietal: 10 Temporal: 5 Occipital: 2 | Not specified | USA | Laterality not reported | 11 female; 14 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Jallo 1997 [129] | Transversal study, 26 patients | 5/26 (19.23%) Frontal: 3 Parietal: 1 Temporal: 1 | Not specified | USA | Laterality not reported | 1 female; 4 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Maslehaty 2011 [130] | Case report and literature review (1 report/19 articles reviewed) | 20/20 (100%) Frontal: 5 Temporal: 5 Parietal: 2 temporo-parietal: 7 fronto-temporal: 2 | Not specified | Switzerland | L. hemisphere: 1 (case); literature review not reported | 7 female; 13 male | The article did not establish a relationship between the region of the glioblastoma and clinical implications. |
| Author y N | Right Hemisphere | Left Hemisphere | Bilateral |
|---|---|---|---|
| Stummer 221 patients | 156 | 87 | - |
| Drabycz 72 patients | 34 | 38 | - |
| Fudaba 35 patients | 18 | 17 | - |
| Abecassis 31 patients | 20 | 30 (patients with gliomas) | - |
| Stark 7 patients | - | 1 | - |
| Petzold 1 patients | - | 1 | - |
| Chen 15 patients | 4 | 9 | - |
| Stumpo 7 patients | 2 | 4 | 1 |
| Awad 330 patients | 168 | 139 | 23 |
| Cui 77 patients | - | - | 36 |
| Wach 198 patients | 111 | 87 | - |
| Steidl 65 patients | 36 | 28 | 1 |
| Ideguchi 5 patients | 3 | 2 | - |
| Wang 19 patients | 6 | 6 | 1 |
| Faguer 4 patients | 3 | 1 | - |
| Muller 275 patients | 141 | 134 | - |
| Iliadis 65 patients | 29 | 33 | 3 |
| Karthigeyan 1 patient | - | 1 | - |
| Smedley 304 patients | 162 | 149 | - |
| Han 92 patients | 39 | 39 | 14. |
| Henker 20 patients | 11 | 9 | - |
| Roetzer 3 patients | 2 | - | - |
| Fan 133 patients | 56 | 77 | - |
| Najafi 12 patients | 5 | 6 | - |
| Wang 153 patients | 73 | 80 | - |
| Smets 24 patients | 15 | 9 | - |
| Kajitani 3 patients | 3 | - | - |
| Roemer 1 patient | 1 | - | |
| Kiang 1 patient | - | 1 | - |
| Boikov 1 patient | 1 | - | - |
| Sugimoto 4 patients | - | 4 | - |
| Laule 1 patient | - | 1 | - |
| Olar 4 patients | 1 | 3 | - |
| Takahashi 1 patient | - | 1 | - |
| Ali 9 patients | 7 | 2 | - |
| Colombo 1 patient | 1 | - | - |
| Nestler 3 patients | - | 3 | - |
| Mohan 48 patients | - | - | - |
| Schiff 1 patient | - | 1 | - |
| Maslehaty 19 patients | - | 1 | - |
| Park 1 patient | 1 | - | - |
| Adaberg 100 patients | 40 | 47 | 13 |
| Gu 1 patient | 1 | - | - |
| Wright 8 patients | 4 | 2 | 2 |
| Schneider 31 patients | 14 | 17 | - |
| Soleman 1 patient | 1 | - | - |
| Irhezzioui 1 patient | 1 | - | - |
| Cohen-gadol 2 patients | 2 | - | - |
| Iam 1 patient | - | 1 | - |
| Weber 10 patients | 5 | 5 | - |
| Wu 1 patient | - | 1 | - |
| Franco 3 patients | 2 | - | 1 |
| Oriuchi 5 patients | 2 | 3 | - |
| Kanas 86 patients | 47 | 39 | - |
| Anzai 1 patient | - | 1 | - |
| Li 2 patients | 1 | 1 | - |
| Senders 562 patients | 302 | 281 | - |
| Todo 19 patients | 11 | 8 | 1 |
| Nakai 2 patients | - | 2 | - |
| Zhang 60 patients | 33 | 27 | - |
| Liu 76 patients | 25 | 35 | 6 |
| Doknic 1 patient | - | 1 | - |
| Dilber 1 patient | - | 1 | - |
| Cohen 1 patient | - | 1 | - |
| Verburg 20 patients | 7 | 5 | - |
| Wang 200 patients | 87 | 113 | - |
| Simonet Redondo 5 patients | 3 | 2 | - |
| Friese 1 patient | - | - | 1 (callosum body) |
| Okamoto 2 patients | 1 | 1 | 1 (callosum body) |
| Nishio 1 patient | - | - | 1 (cerebellum) |
| Ballester 3 patients | 3 | - | - |
| Ishikawa 5 patients | 2 | 3 | - |
| Kim 8 patients | 5 | 2 | 1 (posterior fossa) |
| Prasanna 138 patients | 60 | 78 | - |
| Hemisphere | Right Hemisphere | Left Hemisphere |
|---|---|---|
| Median | 33.36 | 34.70 |
| Standard deviation | 58.00 | 65.07 |
| Author and Number of Patients | Frontal Lobe | Parietal Lobe | Temporal Lobe | Occipital Lobe | Insula | Diencephalom | Brain Stem | Cerebellum | Other Structures |
|---|---|---|---|---|---|---|---|---|---|
| Hashiguchi 51 patients | 15 | 8 | 16 | 3 | - | - | - | 2 | 7 |
| Drabycz 72 patients | 19 | 17 | 32 | 2 | - | - | - | - | 2 |
| Chung Ko 104 patients | 38 | 25 | 27 | 3 | - | 5 | 1 | - | 5 |
| Abecassis 31 patients | 17 | 8 | 24 | - | - | - | 1 | - | - |
| Wang 81 patients | 13 | 8 | 32 | 3 | 3 | 7 | - | - | 15 |
| Shen 1 patients | - | - | - | - | - | - | - | - | 1 |
| Mizumoto 46 patients | 23 | 3 | 16 | 4 | - | - | - | - | - |
| Isoardo 19 patients | 9 | 5 | 5 | - | - | - | - | - | - |
| Ferreira 67 patients | 33 | 14 | 14 | 6 | - | - | 1 | - | 4 |
| Stark 7 patients | - | - | - | - | - | - | 2 | 4 | 1 |
| Liu 107 patients | - | - | - | - | - | - | - | - | 153 |
| Petzold 1 patient | 1 | - | - | - | - | - | - | - | - |
| Chen 15 patients | 5 | 1 | 4 | 3 | - | - | 1 | 1 | 2 |
| Stumpo 7 patients | 4 | 1 | 2 | - | - | - | - | - | - |
| Jaskólski 26 patients | 11 | 6 | 7 | 2 | - | - | - | - | - |
| Kim 83 patients | - | - | 29 | - | - | - | - | - | 54 |
| Hatakeyama 55 patients | - | - | - | - | - | - | - | 1 | 54 |
| Yamashita 112 patients | 36 | 25 | 21 | 3 | 7 | - | - | - | 20 |
| Kuroiwa 9 patients | - | - | - | - | - | - | 4 | 9 | - |
| Awad 330 patients | 132 | 99 | 139 | 50 | - | - | 4 | 3 | 200 |
| Quan 110 patients | 42 | - | - | - | - | - | - | - | 68 |
| Onuma 33 patients | 17 | - | - | - | - | - | - | - | 16 |
| Cui 77 patients | 44 | 11 | - | 11 | 22 | - | - | - | 77 |
| Steidl 65 patients | 16 | 15 | 27 | 12 | 1 | 2 | - | - | 1 |
| Park 108 patients | 45 | - | - | - | - | - | - | - | 63 |
| Yan 1 patient | - | - | - | - | - | - | - | - | 1 |
| Ideguchi 5 patients | 1 | 1 | 1 | 1 | - | - | - | - | 1 |
| Wang 19 patients | 9 | 2 | 3 | 1 | - | - | 1 | - | 6 |
| Faguer 4 patients | 1 | 3 | 1 | - | - | - | - | - | - |
| Thomas 21 patients | 6 | - | 1 | - | 4 | - | - | - | 10 |
| Iliadis 65 patients | 17 | 20 | 20 | 3 | - | - | - | - | 8 |
| Galldiks 25 patients | 9 | 8 | 7 | 1 | - | - | - | - | - |
| Koike 11 patients | - | - | - | - | - | - | - | - | 11 |
| Xing 75 patients | 32 | 12 | 13 | 5 | 1 | - | - | - | 12 |
| Karthigeyan 1 patient | - | - | - | - | - | - | - | - | 1 |
| Smedley 304 patients | 114 | 76 | 91 | 18 | - | 10 | - | 1 | - |
| Michelini 42 patients | - | - | - | - | - | - | - | - | 42 |
| Henker 20 patients | 6 | 2 | 10 | - | - | - | - | - | 2 |
| Mathew 47 patients | - | - | - | - | - | - | - | - | 56 |
| Jiang 10 patients | - | 1 | - | 1 | - | - | - | - | 9 |
| Roetzer 3 patients | - | - | 2 | 1 | - | - | 1 | - | - |
| Yu li 406 patients | 182 | - | 224 | - | - | - | - | - | - |
| Utsuki 37 patients 5 patients | 17 | 8 | 8 | 9 | - | - | - | - | - |
| Hart 5 patients | 1 | 3 | - | 1 | - | - | - | - | - |
| Najafi 12 patients | 4 | 1 | 5 | 1 | - | - | - | - | 1 |
| Smets 24 patients | 5 | 12 | 8 | 6 | - | - | - | - | - |
| Amini 3 patients | - | - | - | - | - | 3 | - | - | 1 |
| Kajitani 3 patients | 2 | 2 | 1 | - | 1 | - | 1 | 1 | - |
| Coburger 20 patients | 10 | 3 | 5 | 2 | - | - | - | - | - |
| Kiang 1 patient | 1 | - | - | - | - | - | - | - | 1 |
| Boikov 1 patient | - | - | - | - | - | 1 | - | - | - |
| Hakyemez 18 patients | 4 | 5 | 4 | 3 | - | 2 | - | - | - |
| Eoli 86 patients | 36 | - | 35 | - | - | - | - | - | 15 |
| Yu 43 patients | - | - | - | - | - | - | 2 | 6 | 35 |
| Sugimoto 4 patients | 3 | - | 1 | - | - | - | - | 1 | 1 |
| Laule 1 patient | 1 | - | - | - | - | - | - | - | - |
| Makino 7 patients | 1 | - | 3 | - | - | 1 | - | - | 2 |
| Seidel 122 patients | 70 | 38 | 62 | 38 | 74 | ||||
| Cho 60 patients | 33 | 21 | 34 | 4 | 8 | 5 | - | - | 24 |
| Olar 4 patients | 1 | 2 | 2 | 1 | 1 | ||||
| Takahashi 1 patient | 1 | - | - | - | - | - | - | - | - |
| Woo 147 patients | 46 | 34 | 47 | 5 | 3 | - | - | 6 | 6 |
| Nguyen 6 patients | - | - | - | - | - | - | - | - | 6 |
| Ali 9 patients | 6 | 2 | 4 | 1 | - | - | - | - | - |
| Colombo 1 patient | - | 1 | - | - | - | - | - | - | - |
| Nestler 3 patients | 1 | - | - | - | - | - | - | 1 | 1 |
| Mohan 48 patients | 11 | 10 | 12 | 3 | - | - | - | - | - |
| Mangla 36 patients | 22 | - | 22 | - | - | - | - | - | 14 |
| Schiff 1 patient | - | 1 | 1 | - | - | - | - | - | 1 |
| Maslehaty 19 patients | 7 | 9 | 14 | - | - | - | - | - | - |
| Park 1 patient | - | - | 1 | 1 | - | - | - | - | - |
| Ellingson 25 patients | 7 | 10 | 5 | 2 | - | - | - | - | - |
| Gu 1 patient | 1 | - | 1 | - | - | - | - | - | - |
| Wright 8 patients | 5 | 1 | 1 | - | - | 1 | - | - | 2 |
| Schneider 31 patients | 16 | 15 | 11 | 2 | - | - | - | - | - |
| Soleman 1 patient | 1 | - | - | - | - | - | - | - | - |
| Irhezzioui 1 patient | 1 | - | - | - | - | - | - | - | - |
| Cohen-gadol 2 patients | 1 | - | 1 | - | - | - | - | - | - |
| Iam 1 patient | - | - | - | - | - | 1 | - | - | 1 |
| Weber 10 patients | 5 | 4 | 3 | 3 | - | - | - | - | - |
| Wu 1 patient | - | - | 1 | - | - | - | - | - | - |
| Franco 3 patients | 3 | - | 1 | - | - | 1 | - | - | 1 |
| Oriuchi 5 patients | - | 3 | - | - | - | 2 | - | - | - |
| Shibahara 87 patients | 28 | 15 | 30 | - | - | - | - | - | 14 |
| Tykocinski 132 patients | 81 | 81 | 50 | 81 | 50 | - | - | - | 1 |
| Kanas 86 patients | 25 | 17 | 38 | 6 | - | - | - | - | - |
| Anzai 1 patient | 1 | - | - | - | - | - | - | - | - |
| Reimer 35 patients | 33 | 2 | 33 | - | - | - | - | - | - |
| Li 2 patients | - | - | 1 | - | - | - | - | 1 | - |
| Jiguet-Jiglaire 38 patients | 17 | - | 15 | - | - | - | - | - | 23 |
| Senders 562 patients | 235 | 175 | 250 | 73 | - | - | - | - | 59 |
| Todo 19 patients | 11 | 3 | 4 | - | - | - | - | - | 1 |
| Nakai 2 patients | 1 | - | 2 | - | - | - | - | - | - |
| Zhang 60 patients | 29 | 7 | 13 | 5 | - | - | - | - | 6 |
| Doknic 1 patient | - | - | 1 | - | - | - | - | - | - |
| Jallo 5 patients | 3 | 1 | 1 | - | - | - | - | - | - |
| Dilber 1 patient | - | 1 | 1 | 1 | - | - | - | - | 2 |
| Cohen 1 patient | - | - | - | 1 | - | - | - | - | |
| Nishio 4 patients | - | - | - | - | - | 4 | - | - | - |
| Verburg 12 patients | 3 | 6 | 1 | 2 | - | - | - | - | - |
| Wang 200 patients | 109 | - | 92 | - | - | - | - | - | 76 |
| Simonet Redondo 5 patients | 2 | - | 3 | - | - | - | - | - | - |
| Sunwoo 20 patients | 11 | 7 | 7 | 2 | - | - | - | - | - |
| Friese 1 patient | - | - | - | - | - | - | - | - | 1 |
| Okamoto 2 patients | - | 1 | - | - | - | - | - | - | 1 |
| Nishio 1 patient | - | - | - | - | - | - | 1 | 1 | - |
| Ballester 3 patients | 1 | - | 2 | - | - | - | - | - | - |
| Ishikawa 5 patients | 1 | 1 | 1 | 2 | - | - | - | - | - |
| Kim 8 patients | 1 | 1 | 3 | 1 | - | 1 | 1 | - | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela-Fuenzalida, J.J.; Moyano-Valarezo, L.; Silva-Bravo, V.; Milos-Brandenberg, D.; Orellana-Donoso, M.; Nova-Baeza, P.; Suazo-Santibáñez, A.; Rodríguez-Luengo, M.; Oyanedel-Amaro, G.; Sanchis-Gimeno, J.; et al. Association between the Anatomical Location of Glioblastoma and Its Evaluation with Clinical Considerations: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 3460. https://doi.org/10.3390/jcm13123460
Valenzuela-Fuenzalida JJ, Moyano-Valarezo L, Silva-Bravo V, Milos-Brandenberg D, Orellana-Donoso M, Nova-Baeza P, Suazo-Santibáñez A, Rodríguez-Luengo M, Oyanedel-Amaro G, Sanchis-Gimeno J, et al. Association between the Anatomical Location of Glioblastoma and Its Evaluation with Clinical Considerations: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(12):3460. https://doi.org/10.3390/jcm13123460
Chicago/Turabian StyleValenzuela-Fuenzalida, Juan Jose, Laura Moyano-Valarezo, Vicente Silva-Bravo, Daniel Milos-Brandenberg, Mathias Orellana-Donoso, Pablo Nova-Baeza, Alejandra Suazo-Santibáñez, Macarena Rodríguez-Luengo, Gustavo Oyanedel-Amaro, Juan Sanchis-Gimeno, and et al. 2024. "Association between the Anatomical Location of Glioblastoma and Its Evaluation with Clinical Considerations: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 12: 3460. https://doi.org/10.3390/jcm13123460





