Chronic Kidney Disease Increases Mortality and Reduces the Chance of a Favorable Outcome in Stroke Patients Treated with Mechanical Thrombectomy—Single-Center Study
Abstract
:1. Introduction
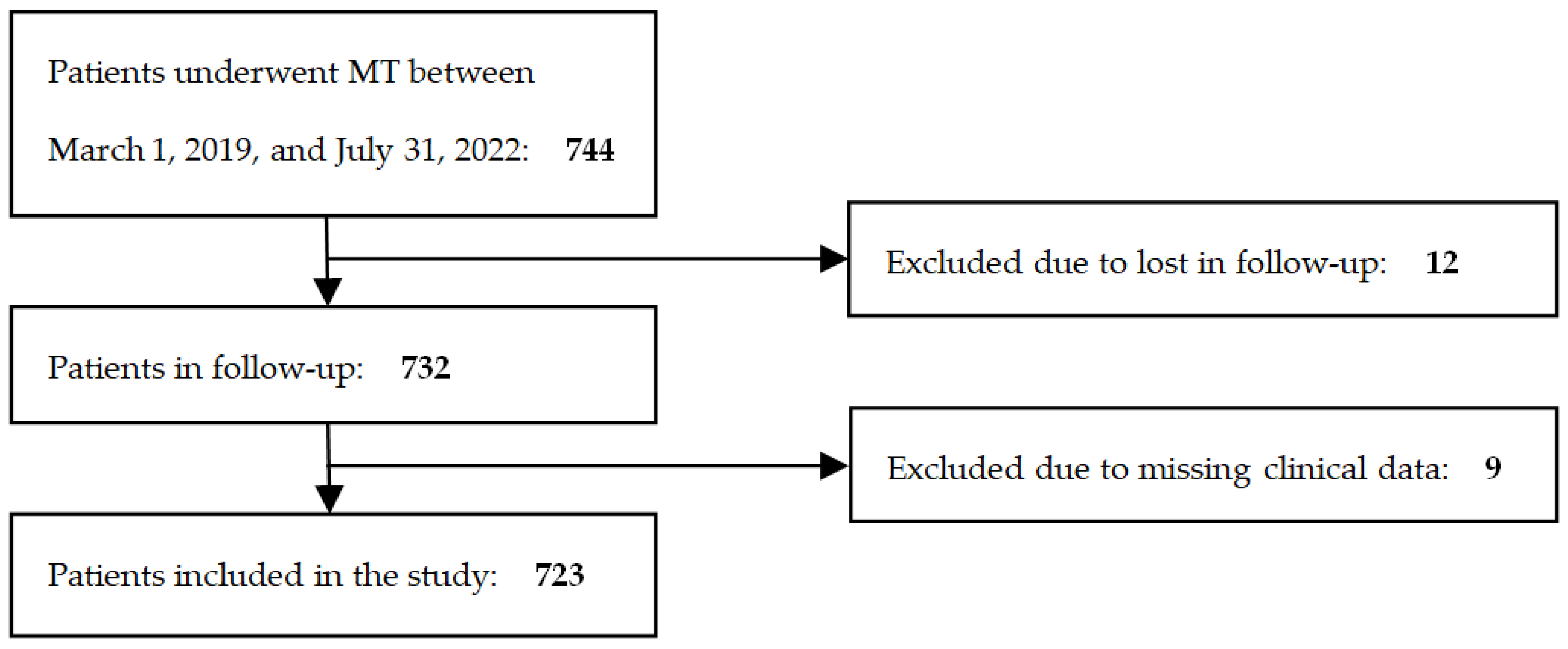
2. Materials and Methods
2.1. Patient Population
2.2. Clinical Characteristics of the Cohort
2.3. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Patient Groups
3.2. Arterial Hypertension and Medications Used
3.3. Predictors of Functional Independence
3.4. Predictors of Mortality
3.5. Predictors of Endovascular Revascularization in Patients with Chronic Kidney Disease
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A system. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Saver, J.L.; Chang, K.-H.; Liao, H.-W.; Chang, S.-C.; Ovbiagele, B. Low glomerular filtration rate and risk of stroke: Meta-analysis. BMJ 2010, 341, c4249. [Google Scholar] [CrossRef] [PubMed]
- Bobot, M.; Suissa, L.; Hak, J.F.; Burtey, S.; Guillet, B.; Hache, G. Kidney disease and stroke: Epidemiology and potential mechanisms of susceptibility. Nephrol. Dial. Transplant. 2023, 38, 1940–1951. [Google Scholar] [CrossRef]
- Arnold, J.; Sims, D.; Ferro, C.J. Modulation of stroke risk in chronic kidney disease. Clin. Kidney J. 2016, 9, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Webster, A.C.; Hong, M.; Turner, R.; Lindley, R.I.; Craig, J.C. Chronic kidney disease and the risk of stroke: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2015, 30, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Wyld, M.; Webster, A.C. Chronic Kidney Disease is a Risk Factor for Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 105730. [Google Scholar] [CrossRef] [PubMed]
- Krishna, P.R.; Naresh, S.; Krishna, G.S.R.; Lakshmi, A.Y.; Vengamma, B.; Kumar, V.S. Stroke in chronic kidney disease. Indian J. Nephrol. 2009, 19, 5–7. [Google Scholar] [PubMed]
- Jang, S.Y.; Sohn, M.K.; Lee, J.; Kim, D.Y.; Lee, S.G.; Shin, Y.I.; Oh, G.J.; Lee, Y.S.; Joo, M.C.; Han, E.Y.; et al. Chronic kidney disease and functional outcomes 6 months after ischemic stroke: A prospective multicenter study. Neuroepidemiology 2016, 46, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-M.; Kim, H.J.; Ahn, H.; Ahn, I.M.; Do, Y.; Choi, J.-Y.; Seo, W.-K.; Oh, K.; Cho, K.-H.; Yu, S. Chronic kidney disease and intravenous thrombolysis in acute stroke: A systematic review and meta-analysis. J. Neurol. Sci. 2015, 358, 345–350. [Google Scholar] [CrossRef]
- Malhotra, K.; Katsanos, A.H.; Goyal, N.; Tayal, A.; Gensicke, H.; Mitsias, P.D.; De Marchis, G.M.; Berge, E.; Alexandrov, A.W.; Alexandrov, A.V.; et al. Intravenous thrombolysis in patients with chronic kidney disease: A systematic review and meta-analysis. Neurology 2020, 95, e121–e130. [Google Scholar] [CrossRef]
- Laible, M.; Jenetzky, E.; Möhlenbruch, M.A.; Bendszus, M.; Ringleb, P.A.; Rizos, T. The Impact of Post-contrast Acute Kidney Injury on In-hospital Mortality after Endovascular Thrombectomy in Patients with Acute Ischemic Stroke. Front. Neurol. 2021, 12, 665614. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zheng, X.; Zhang, J.; Jiang, F.; Chen, N.; Xu, M.; Wu, Y.; Zou, J.; Cui, X.; Zhou, J. A Dynamic Nomogram to Identify Patients at High Risk of Poor Outcome in Stroke Patients with Chronic Kidney Disease. Clin. Interv. Aging 2022, 17, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Turc, G.; Bhogal, P.; Fischer, U.; Khatri, P.; Lobotesis, K.; Mazighi, M.; Schellinger, P.D.; Toni, D.; de Vries, J.; White, P.; et al. European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischemic Stroke. J. Neurointerv. Surg. 2023, 15, e8. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, L.J.; Diprose, W.K.; Wang, M.T.M.; Barber, P.A. Chronic Kidney Disease and Outcome Following Endovascular Thrombectomy for Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 104665. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Sulaiman, S.; Alqahtani, F.; Harris, A.H.; Hohmann, S.F.; Alkhouli, M. Association of Chronic Kidney Disease With In-Hospital Outcomes of Endovascular Stroke Interventions. Cardiovasc. Revasc. Med. 2022, 34, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Fandler-Höfler, S.; Odler, B.; Kneihsl, M.; Wünsch, G.; Haidegger, M.; Poltrum, B.; Beitzke, M.; Deutschmann, H.; Enzinger, C.; Rosenkranz, A.R.; et al. Acute and Chronic Kidney Dysfunction and Outcome After Stroke Thrombectomy. Transl. Stroke Res. 2021, 12, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Laible, M.; Möhlenbruch, M.A.; Pfaff, J.; Jenetzky, E.; Ringleb, P.A.; Bendszus, M.; Rizos, T. Influence of Renal Function on Treatment Results after Stroke Thrombectomy. Cerebrovasc. Dis. 2017, 44, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ma, M.; Gu, M.; Han, Y.; Wang, H.; Zi, W.; Yang, D.; Hao, Y.; Lv, Q.; Ye, R.; et al. Renal impairment on clinical outcomes following endovascular recanalization. Neurology 2020, 94, E464–E473. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Rocha, A.; Barbosa, F.; Barros, P.; Fonseca, L.; Ribeiro, M.; Afreixo, V.; Gregório, T. Acute kidney injury after endovascular therapy in acute stroke patients: Systematic review with meta-analysis. J. Neurointerv. Surg. 2023, 15, e468–e474. [Google Scholar] [CrossRef]
- Yao, Z.; Xu, H.; Cheng, Y.; Xu, Y. Relationship between estimated glomerular filtration rate and outcome of ischemic stroke patients after mechanical thrombectomy. CNS Neurosci. Ther. 2021, 27, 1281–1288. [Google Scholar] [CrossRef]
- Wang, R.; Xie, Z.; Li, B.; Zhang, P. Renal impairment and the prognosis of endovascular thrombectomy: A meta-analysis and systematic review. Ther. Adv. Neurol. Disord. 2022, 15, 17562864221083620. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, Y.D.; Nam, H.S.; Yoo, J.; Sohn, S.-I.; Hong, J.-H.; Kim, B.M.; Kim, D.J.; Bang, O.Y.; Seo, W.-K.; et al. Impact of Renal Function on Short-Term Outcome After Reperfusion Therapy in Patients With Ischemic Stroke. Stroke 2022, 53, 3622–3632. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Shen, H.; Tao, C.; Zhu, Y.; Xu, P.; Li, R.; Yang, P.; Zhang, Y.; Li, Z.; Zhang, Y.; et al. Effect of renal impairment on the efficacy and safety of intra-arterial treatment: A post-hoc analysis of DIRECT-MT study. Int. J. Stroke Off. J. Int. Stroke Soc. 2022, 17, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Diprose, W.K.; Sutherland, L.J.; Wang, M.T.M.; Alan Barber, P. Contrast-associated acute kidney injury in endovascular thrombectomy patients with and without baseline renal impairment. Stroke 2019, 50, 3527–3531. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; van Hal, R.; Stracke, P.; Hadisurya, J.; Nordmeyer, H.; Chapot, R. Incidence of Acute Kidney Injury After Computed Tomography Angiography ± Computed Tomography Perfusion Followed by Thrombectomy in Patients with Stroke Using a Postprocedural Hydration Protocol. J. Am. Heart Assoc. 2020, 9, e014418. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Yamamoto, N.; Kanematsu, Y.; Kuroda, K.; Yamaguchi, I.; Miyamoto, T.; Sogabe, S.; Shimada, K.; Takagi, Y.; Izumi, Y. High White Blood Cell Count Is a Risk Factor for Contrast-Induced Nephropathy following Mechanical Thrombectomy for Acute Ischemic Stroke. Cerebrovasc. Dis. Extra 2020, 10, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-W.; Chen, C.-H.; Lin, Y.-H.; Lee, C.-W.; Tsai, K.-C.; Tsai, L.-K.; Tang, S.-C.; Jeng, J.-S. Outcome of endovascular thrombectomy in patients with end-stage renal disease undergoing dialysis. J. Neurointerv. Surg. 2022, 15, e337–e342. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhou, F.; Shen, R.; Zhu, Y.; Arima, H.; Yang, J.; Zhou, J. Influence of renal function on stroke outcome after mechanical thrombectomy: A prospective cohort study. BMC Neurol. 2020, 20, 134. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Gu, M.; Lu, Y.; Xu, P.; Wang, J.; Lan, W.; Huang, Y.; Xu, G.; Zhu, S.; Wang, Q.; et al. Influence of renal impairment on clinical outcomes after endovascular recanalization in vertebrobasilar artery occlusions. J. Neurointerv. Surg. 2022, 14, 1077–1083. [Google Scholar] [CrossRef]
- Laible, M.; Jenetzky, E.; Möhlenbruch, M.A.; Neuberger, U.; Bendszus, M.; Ringleb, P.A.; Rizos, T. Renal Impairment Is Associated with Intracerebral Hemorrhage after Mechanical Thrombectomy in Vertebrobasilar Stroke. Cerebrovasc. Dis. 2019, 47, 48–56. [Google Scholar] [CrossRef]
- El Husseini, N.; Fonarow, G.C.; Smith, E.E.; Ju, C.; Schwamm, L.H.; Hernandez, A.F.; Schulte, P.J.; Xian, Y.; Goldstein, L.B. Renal Dysfunction Is Associated with Poststroke Discharge Disposition and In-Hospital Mortality: Findings from Get with the Guidelines-Stroke. Stroke 2017, 48, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Villwock, M.R.; Singla, A.; Padalino, D.J.; Deshaies, E.M. Acute ischaemic stroke outcomes following mechanical thrombectomy in the elderly versus their younger counterpart: A retrospective cohort study. BMJ Open 2014, 4, e004480. [Google Scholar] [CrossRef] [PubMed]
- Wessell, A.; Carvalho, H.; Le, E.; Cannarsa, G.; Kole, M.; Stokum, J.; Chryssikos, T.; Miller, T.; Chaturvedi, S.; Gandhi, D.; et al. A Critical Assessment of the Golden Hour and the Impact of Procedural Timing in Stroke Thrombectomy. Am. J. Neuroradiol. 2020, 41, 822–827. [Google Scholar] [CrossRef]
- Lasek-Bal, A.; Binek, Ł.; Żak, A.; Student, S.; Krzan, A.; Puz, P.; Bal, W.; Uchwat, U. Clinical and Non-Clinical Determinants of the Effect of Mechanical Thrombectomy and Post-Stroke Functional Status of Patients in Short and Long-Term Follow-Up. J. Clin. Med. 2021, 10, 5084. [Google Scholar] [CrossRef]
- Harlacher, E.; Wollenhaupt, J.; Baaten, C.C.F.M.J.; Noels, H. Impact of Uremic Toxins on Endothelial Dysfunction in Chronic Kidney Disease: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 531. [Google Scholar] [CrossRef]
- Podkowińska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef]
- Xu, T.-Y.; Zhang, Y.; Li, Y.; Zhu, D.-L.; Gao, P.-J. The association of serum inflammatory biomarkers with chronic kidney disease in hypertensive patients. Ren. Fail. 2014, 36, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Finck, T.; Sperl, P.; Hernandez-Petzsche, M.; Boeckh-Behrens, T.; Maegerlein, C.; Wunderlich, S.; Zimmer, C.; Kirschke, J.; Berndt, M. Inflammation in stroke: Initial CRP levels can predict poor outcomes in endovascularly treated stroke patients. Front. Neurol. 2023, 14, 1167549. [Google Scholar] [CrossRef]
- Huber, T.; Kleine, J.F.; Kaesmacher, J.; Bette, S.; Poppert, H.; Zimmer, C.; Boeckh-Behrens, T. Blood Leukocytes as Prognostic Parameter in Stroke Thrombectomy. Cerebrovasc. Dis. 2016, 42, 32–40. [Google Scholar] [CrossRef]
- Bian, J.; Guo, S.; Huang, T.; Li, X.; Zhao, S.; Chu, Z.; Li, Z. CRP as a potential predictor of outcome in acute ischemic stroke. Biomed. Rep. 2023, 18, 17. [Google Scholar] [CrossRef]
- Borończyk, M.; Kuźniak, M.; Borończyk, A.; Żak, A.; Binek, Ł.; Wagner-Kusz, A.; Lasek-Bal, A. Efficacy and safety of mechanical thrombectomy in the posterior cerebral circulation—A single center study. Sci. Rep. 2024, 14, 7700. [Google Scholar] [CrossRef] [PubMed]
- Spiotta, A.M.; Vargas, J.; Turner, R.; Chaudry, M.I.; Battenhouse, H.; Turk, A.S. The golden hour of stroke intervention: Effect of thrombectomy procedural time in acute ischemic stroke on outcome. J. Neurointerv. Surg. 2014, 6, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, M.; Saia, V.; Pracucci, G.; Fainardi, E.; Casetta, I.; Sallustio, F.; Nencini, P.; Bigliardi, G.; Saletti, A.; Ruggiero, M.; et al. Predictors for clinical and functional outcomes in stroke patients with first-pass complete recanalization after thrombectomy. Eur. J. Neurol. 2023, 30, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Boccardo, P.; Remuzzi, G.; Galbusera, M. Platelet dysfunction in renal failure. Semin. Thromb. Hemost. 2004, 30, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Pavord, S.; Myers, B. Bleeding and thrombotic complications of kidney disease. Blood Rev. 2011, 25, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.-H.; Lin, T.-C.; Shieh, Y.; Chang, T.-Y.; Hung, K.L.; Liu, C.-H.; Lee, T.H.; Chang, Y.J.; Der Lee, J.; Chang, C.H. Intracerebral hemorrhage after thrombolytic therapy in acute ischemic stroke patients with renal dysfunction. Eur. Neurol. 2013, 70, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Arnson, Y.; Hoshen, M.; Berliner-Sendrey, A.; Reges, O.; Balicer, R.; Leibowitz, M.; Tsadok, M.A.; Haim, M. Risk of Stroke, Bleeding, and Death in Patients with Nonvalvular Atrial Fibrillation and Chronic Kidney Disease. Cardiology 2020, 145, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Nogueira, R.G.; Zhu, Y.; Sun, J.; Han, H.; Yuan, G.; Wen, C.; Zhou, P.; Chen, W.; Zeng, G.; et al. Trial of Endovascular Treatment of Acute Basilar-Artery Occlusion. N. Engl. J. Med. 2022, 387, 1361–1372. [Google Scholar] [CrossRef] [PubMed]
- Jovin, T.G.; Li, C.; Wu, L.; Wu, C.; Chen, J.; Jiang, C.; Shi, Z.; Gao, Z.; Song, C.; Chen, W.; et al. Trial of Thrombectomy 6 to 24 Hours after Stroke Due to Basilar-Artery Occlusion. N. Engl. J. Med. 2022, 387, 1373–1384. [Google Scholar] [CrossRef]
- Pan, Y.; Jing, J.; Chen, W.; Wang, Y.; He, Y. Association between impaired renal function and stroke outcome in patients with versus without atrial fibrillation. Eur. J. Neurol. 2018, 25, 1041–1048. [Google Scholar] [CrossRef]
- Kaze, A.D.; Jaar, B.G.; Fonarow, G.C.; Echouffo-Tcheugui, J.B. Diabetic kidney disease and risk of incident stroke among adults with type 2 diabetes. BMC Med. 2022, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Mace-Brickman, T.; Eddeen, A.B.; Carrero, J.-J.; Mark, P.B.; Molnar, A.O.; Lam, N.N.; Zimmerman, D.; Harel, Z.; Sood, M.M. The Risk of Stroke and Stroke Type in Patients with Atrial Fibrillation and Chronic Kidney Disease. Can. J. Kidney Health Dis. 2019, 6, 2054358119892372. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.B.; Lip, G.Y.; Kamper, A.-L.; Hommel, K.; Køber, L.; Lane, D.A.; Lindhardsen, J.; Gislason, G.H.; Torp-Pedersen, C. Stroke and Bleeding in Atrial Fibrillation with Chronic Kidney Disease. N. Engl. J. Med. 2012, 367, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, M.; Hao, Z.; Tao, W. Association between reduced kidney function and clinical outcomes after ischaemic stroke with atrial fibrillation. Eur. J. Neurol. 2014, 21, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Seliger, S.L.; Gillen, D.L.; Longstreth, W.T.J.; Kestenbaum, B.; Stehman-Breen, C.O. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003, 64, 603–609. [Google Scholar] [CrossRef]
- Air, E.L.; Kissela, B.M. Diabetes, the metabolic syndrome, and ischemic stroke: Epidemiology and possible mechanisms. Diabetes Care 2007, 30, 3131–3140. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Wanner, C.; Zwiener, I.; Ofstad, A.P.; George, J.T.; Fitchett, D.; Zinman, B. Influence of Microvascular Disease on Cardiovascular Events in Type 2 Diabetes. J. Am. Coll. Cardiol. 2019, 73, 2780–2782. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Fioretto, P.; Dodson, P.M. Does microvascular disease predict macrovascular events in type 2 diabetes? Atherosclerosis 2011, 218, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Chen, Y.; Jing, J.; Zhao, X.; Wang, C.; Liu, L.; Wang, A.; Pan, Y.; Li, C.; Wang, Y. Association between polyvascular atherosclerosis and estimated glomerular filtration rate in patients with ischaemic stroke: Data analysis of the patients in the Chinese National stroke registry. Neurol. Res. 2015, 37, 415–420. [Google Scholar] [CrossRef]
- Yeh, S.-J.; Jeng, J.-S.; Tang, S.-C.; Liu, C.-H.; Hsu, S.-P.; Chen, C.-H.; Lien, L.-M.; Lin, H.-J.; Chen, C.-M.; Lin, R.-T.; et al. Low estimated glomerular filtration rate is associated with poor outcomes in patients who suffered a large artery atherosclerosis stroke. Atherosclerosis 2015, 239, 328–334. [Google Scholar] [CrossRef]
- Zhang, X.; Jing, J.; Zhao, X.; Liu, L.; Wang, C.; Pan, Y.; Meng, X.; Wang, Y.; Wang, Y. Statin Use during Hospitalization and Short-Term Mortality in Acute Ischaemic Stroke with Chronic Kidney Disease. Eur. Neurol. 2018, 79, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Ní Chróinín, D.; Asplund, K.; Åsberg, S.; Callaly, E.; Cuadrado-Godia, E.; Díez-Tejedor, E.; Di Napoli, M.; Engelter, S.T.; Furie, K.L.; Giannopoulos, S.; et al. Statin therapy and outcome after ischemic stroke: Systematic review and meta-analysis of observational studies and randomized trials. Stroke 2013, 44, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Landray, M.J.; Reith, C.; Emberson, J.; Wheeler, D.C.; Tomson, C.; Wanner, C.; Krane, V.; Cass, A.; Craig, J.; et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 2011, 377, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
| AMT (n = 648) | PMT (n = 75) | ||||||
|---|---|---|---|---|---|---|---|
| eGFR > 60 (nRI) (n = 519) | eGFR < 60 (CKD) (n = 129) | p-Value AMT + nRI vs. AMT + CKD | eGFR > 60 (nRI) n = 65 | eGFR < 60 (CKD) n = 10 | p-Value PMT + nRI vs. PMT + CKD | p-Value AMT + CKD vs. PMT + CKD | |
| Age (mean, median, range) | 65.65, 68, [19, 89] | 76.52, 77, [48, 92] | <0.001 1 | 61.54, 63, [23, 89] | 75.6, 77, [55, 91] | <0.001 1 | 0.763 1 |
| Female (n, %) | 221 (42.6%) | 77 (59.7%) | <0.001 2 | 18 (27.7%) | 5 (50%) | 0.291 3 | 0.790 3 |
| Hospitalization time (mean, median, range) | 12.06, 9, [1, 70] | 12.67, 9, [1, 60] | 0.823 1 | 10.38, 8, [0, 55] | 11.6, 11, [2, 20] | 0.1781 | 0.660 1 |
| Early ischemic changes in CT (n, %) | 211 (40.7%) | 64 (49.6%) | 0.166 2 | 19 (29.2%) | 4 (40%) | 0.750 3 | 0.800 3 |
| NIHSS1 (mean, median, range) | 13.25, 13 [1, 30] | 13.92, 14, [3, 29] | 0.279 1 0.033 2 | 11.4, 10, [1, 43] | 11.8, 11.5, [4, 22] | 0.691 1 | 0.273 1 |
| NIHSS2 (mean, median, range) | 12.31, 12, [0, 30] | 12.95, 13, [0, 29] | 0.404 1 0.740 2 | 11.52, 7, [0, 43] | 12.7, 13, [0, 26] | 0.503 1 | 0.935 1 |
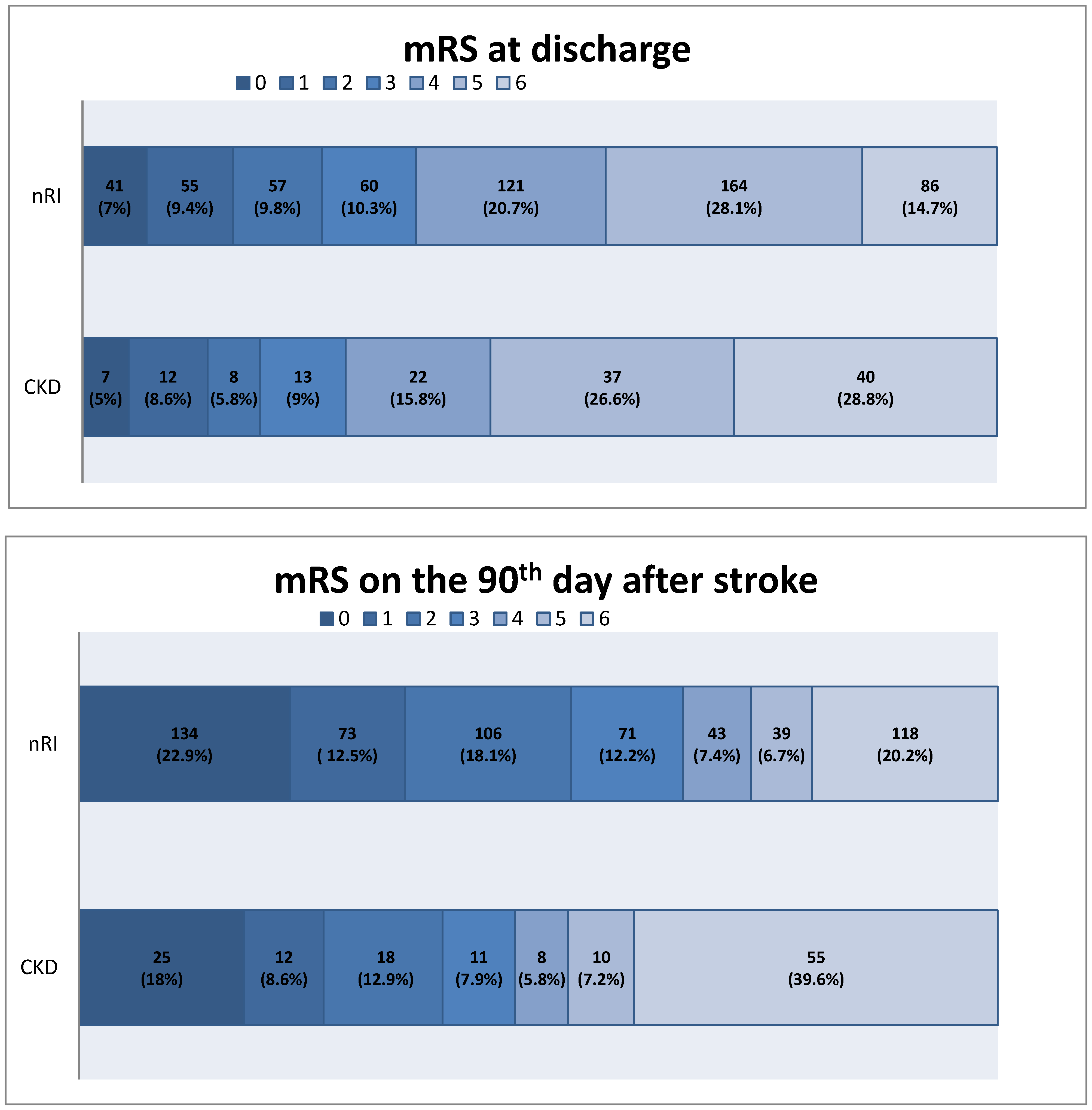
| mRS at discharge (mean, median, range) | 3.7, 4, [0, 6] | 4.19, 5, [0, 6] | 0.036 2 | 3.58, 4, [0, 6] | 3.9, 4.5, [0, 6] | 0.059 2 | 0.115 3 |
| mRS at discharge (0–2) (n, %) | 132 (25.4%) | 25 (19.4%) | 0.594 2 | 20 (30.8%) | 2 (20%) | 0.746 3 | 0.714 3 |
| mRS at 1 month (mean, median, range) | 3.02, 3, [0, 6] | 3.68, 4, [0, 6] | 0.001 2 | 3.12, 3, [0, 6] | 3.00, 3, [0, 6] | 0.674 2 | 0.595 2 |
| mRS at 1 month (0–2) (n, %) | 208 (40.1%) | 41 (31.8%) | 0.083 2 | 27 (41.5%) | 4 (40%) | 0.800 3 | 0.853 3 |
| mRS at 3 months (mean, median, range) | 2.67, 2, [0, 6] | 3.57, 4, [0, 6] | <0.001 2 | 2.93, 2, [0, 6] | 3.10, 3, [0, 6] | 0.560 2 | 0.786 2 |
| mRS at 3 months (0–2) (n, %) | 274 (52.8%) | 48 (37.2%) | 0.001 2 | 32 (49.2%) | 4 (40%) | 0.838 3 | 0.870 3 |
| AF (n, %) | 181 (34.9%) | 67 (51.9%) | <0.001 2 | 12 (18.5%) | 8 (80%) | <0.001 3 | 0.101 3 |
| AH (n, %) | 175 (33.7%) | 56 (43.4%) | 0.040 2 | 15 (23.1%) | 4 (40%) | 0.450 3 | 0.954 3 |
| DM (n, %) | 118 (22.7%) | 45 (34.9%) | 0.004 2 | 40 (61.5%) | 9 (90%) | 0.160 3 | 0.492 3 |
| CAS (n, %) | 352 (67.8%) | 110 (85.3%) | <0.001 2 | 15 (23.1%) | 6 (60%) | 0.041 3 | 0.986 3 |
| Smoking (n, %) | 142 (27.4%) | 12 (9.3%) | <0.001 2 | 19 (29.2%) | 1 (10%) | 0.221 3 | 0.300 3 |
| Dyslipidemia (n, %) | 172 (33.1%) | 57 (44.2%) | 0.019 2 | 25 (38.5%) | 2 (20%) | 0.436 3 | 0.247 3 |
| IVT (n, %) | 336 (64.7%) | 78 (60.5%) | 0.366 2 | 49 (75.4%) | 5 (50%) | 0.198 3 | 0.752 3 |
| mTICI 2b-3 (n, %) | 409 (78.8%) | 93 (72.1%) | 0.102 2 | 53 (81.5%) | 5 (50%) | 0.07 3 | 0.264 3 |
| ICH (n, %) | 97 (18.7%) | 28 (21.7%) | 0.437 2 | 9 (13.85%) | 5 (50%) | 0.022 3 | 0.100 3 |
| Hemicraniectomy (n, %) | 24 (4.6%) | 4 (3.1%) | 0.444 2 | 3 (4.6%) | 0 (0%) | 0.862 3 | 0.677 3 |
| Time from symptoms to MT (groin puncture) (mean, median, range) | 271.54, 270, [5, 870] | 261.44, 267, [60, 660] | 0.198 1 | 278.43, 267, [98, 780] | 315, 320, [150, 556] | 0.201 1 | 0.084 1 |
| MT time (mean, median, range) | 95.88, 90, [30, 270] | 98.05, 90, [30, 225] | 0.722 1 | 101.03, 100, [45, 190] | 92.2, 85, [57, 150] | 0.436 1 | 0.627 1 |
| CRP concentration (mean, median, range) | 20.02, 8.5, [3, 254] | 20.46, 9, [3, 254] | 0.359 1 | 16.54, 8, [4, 127] | 29.91, 21.5, [5, 72] | 0.045 1 | 0.009 1 |
| WBC (mean, median, range) | 11.05, 10, [4.63, 88] | 10.79, 10, [3, 22] | 0.943 1 | 10.77, 10.75, [5, 17] | 9.94, 10.41, [5.5, 15] | 0.454 1 | 0.613 1 |
| Thrombocytosis (n, %) | 11 (2.1%) | 4 (3.1%) | 0.292 2 | 2 (3.1%) | 0 (0%) | 0.623 3 | 0.720 3 |
| Thrombocytopenia (n, %) | 37 (7.2%) | 14 (10.85%) | 0.318 2 | 3 (4.6%) | 2 (20%) | 0.256 3 | 0.677 3 |
| Functional Independence at Discharge (mRS 0–2) | Functional Independence on 90th Day (mRS 0–2) | |
|---|---|---|
| Predictors | OR and CI95% | OR and CI95% |
| Age | 0.97 (0.95–0.98) | 0.97 (0.96–0.98) |
| Female | 1.07 (0.76–1.51) | 0.85 (0.62–1.17) |
| Time of the hospitalization | 0.97 (0.96–0.98) | 0.97 (0.95–0.98) |
| NIHSS1 | 0.85 (0.82–0.89) | 0.88 (0.85–0.91) |
| AF | 0.79 (0.55–1.13) | 0.81 (0.59–1.12) |
| AH | 0.66 (0.46–0.95) | 0.88 (0.62–1.24) |
| DM | 0.50 (0.32–0.78) | 0.77 (0.52–1.07) |
| CAS | 0.95 (0.67–1.36) | 0.76 (0.55–1.05) |
| Dyslipidemia | 0.94 (0.66–1.35) | 1.33 (0.74–1.43) |
| ICA | 0.71 (0.45–1.12) | 0.79 (0.53–1.17) |
| ACA | 1.19 (0.54–2.64) | 1.03 (0.43–2.42) |
| MCA | 1.19 (0.79–1.77) | 1.17 (0.81–1.70) |
| VA | 1.07 (0.41–2.76) | 1.18 (0.47–2.99) |
| BA | 0.86 (0.41–1.77) | 0.80 (0.39–1.61) |
| PCA | 2.41(1.04–5.60) | 1.89 (0.71–5.05) |
| WBC | 0.84 (0.80–0.89) | 0.91 (0.87–0.95) |
| CRP concentration | 0.98 (0.97–0.99) | 0.98 (0.98–0.99) |
| MT time | 0.98 (0.98–0.99) | 0.99 (0.98–0.99) |
| Time from symptoms to MT | 0.99 (0.98–0.99) | 0.99 (0.99–1.00) |
| mTICI 2b-3 | 4.59 (2.58–8.18) | 2.47 (1.70–3.60) |
| ICH | 0.41 (0.24–0.69) | 0.56 (0.37–0.86) |
| CKD | 0.69 (0.43–1.08) | 0.56 (0.38–0.81) |
| Creatinine level | 0.91 (0.57–1.47) | 0.69 (0.45–1.05) |
| Mortality at Discharge | Mortality at 90th Day | |
|---|---|---|
| Predictors | OR and CI95% | OR and CI95% |
| Age | 1.03 (1.01–1.04) | 1.03 (1.01–1.05) |
| Female | 1.14 (0.78–1.68) | 0.85 (0.62–1.17) |
| Time of the hospitalization | 0.98 (0.96–1.00) | 1.01 (0.99–1.02) |
| NIHSS1 | 1.09 (1.05–1.13) | 1.08 (1.04–1.12) |
| AF | 1.18 (0.79–1.74) | 0.81 (0.59–1.12) |
| AH | 1.12 (0.73–1.72) | 0.88 (0.62–1.24) |
| DM | 1.75 (1.16–2.64) | 1.63 (1.09–2.45) |
| CAS | 1.16 (0.78–1.73) | 0.76 (0.55–1.05) |
| Dyslipidemia | 1.29 (0.87–1.92) | 1.03 (0.74–1.43) |
| ICA | 0.97 (0.59–1.59) | 0.79 (0.53–1.17) |
| ACA | 1.93 (0.87–4.29) | 1.03 (0.43–2.42) |
| MCA | 0.86 (0.56–1.33) | 1.17 (0.81–1.70) |
| VA | 1.00 (0.33–3.01) | 1.89 (0.72–2.99) |
| BA | 2.30 (1.18–4.46) | 2.25 (1.21–4.19) |
| PCA | 1.15 (0.34–3.86) | 1.89 (0.71–5.05) |
| WBC | 1.09 (1.04–1.14) | 1.07 (1.02–1.12) |
| CRP concentration | 1.006 (1.001–1.012) | 1.003 (0.997–1.009) |
| MT time | 1.004 (1.000–1.009) | 1.006 (1.002–1.011) |
| Time from symptoms to MT | 1.002 (1.000–1.004) | 1.002 (1.000–1.003) |
| mTICI 2b-3 | 0.60 (0.39–0.92) | 0.48 (0.32–0.72) |
| ICH | 2.22 (1.44–3.42) | 2.03 (1.03–3.18) |
| CKD | 2.34 (1.52–3.61) | 2.59 (1.74–3.84) |
| Creatinine level | 2.16 (1.34–3.49) | 2.38 (1.38–3.81) |
| mTICI 2b-3 | |
|---|---|
| Predictors | OR and CI95% |
| Age | 0.98 (0.96–0.99) |
| AF | 0.60 (0.42–0.86) |
| CAS | 0.49 (0.34–0.69) |
| Time of MT | 0.985 (0.981–0.989) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borończyk, M.; Kuźniak, M.; Borończyk, A.; Barański, K.; Hawrot-Kawecka, A.; Lasek-Bal, A. Chronic Kidney Disease Increases Mortality and Reduces the Chance of a Favorable Outcome in Stroke Patients Treated with Mechanical Thrombectomy—Single-Center Study. J. Clin. Med. 2024, 13, 3469. https://doi.org/10.3390/jcm13123469
Borończyk M, Kuźniak M, Borończyk A, Barański K, Hawrot-Kawecka A, Lasek-Bal A. Chronic Kidney Disease Increases Mortality and Reduces the Chance of a Favorable Outcome in Stroke Patients Treated with Mechanical Thrombectomy—Single-Center Study. Journal of Clinical Medicine. 2024; 13(12):3469. https://doi.org/10.3390/jcm13123469
Chicago/Turabian StyleBorończyk, Michał, Mikołaj Kuźniak, Agnieszka Borończyk, Kamil Barański, Anna Hawrot-Kawecka, and Anetta Lasek-Bal. 2024. "Chronic Kidney Disease Increases Mortality and Reduces the Chance of a Favorable Outcome in Stroke Patients Treated with Mechanical Thrombectomy—Single-Center Study" Journal of Clinical Medicine 13, no. 12: 3469. https://doi.org/10.3390/jcm13123469
APA StyleBorończyk, M., Kuźniak, M., Borończyk, A., Barański, K., Hawrot-Kawecka, A., & Lasek-Bal, A. (2024). Chronic Kidney Disease Increases Mortality and Reduces the Chance of a Favorable Outcome in Stroke Patients Treated with Mechanical Thrombectomy—Single-Center Study. Journal of Clinical Medicine, 13(12), 3469. https://doi.org/10.3390/jcm13123469







