Benefits of Quercetin on Glycated Hemoglobin, Blood Pressure, PiKo-6 Readings, Night-Time Sleep, Anxiety, and Quality of Life in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phytochemical Supplement Specifications
2.2. Study Conduct: Ethical Approval and Research Compliance
2.3. Study Design
2.4. Clinical Safety and Tolerability
2.5. Sample Size Estimation
2.6. Statistical Methods
3. Results
4. Discussion
Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herman, W.H. The Global Burden of Diabetes: An Overview. In Diabetes Mellitus in Developing Countries and Underserved Communities; Dagogo-Jack, S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–5. ISBN 978-3-319-41559-8. [Google Scholar]
- IDF. 2021 International Diabetes Federation Diabetes Atlas, 10th ed.; IDF: Brussels, Belgium, 2021. [Google Scholar]
- Sinclair, A.; Saeedi, P.; Kaundal, A.; Karuranga, S.; Malanda, B.; Williams, R. Diabetes and Global Ageing among 65–99-Year-Old Adults: Findings from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2020, 162, 108078. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative Stress and Vascular Disease. Arteroscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef]
- Ali, A.; Kumar, M.; Srivastava, N.; Khan, M.M.; Khan, M.A. Free Radicals and Diabetes Mellitus. Int. J. Pharm. Sci. Med. 2023, 8, 1–19. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Cheng, D. Prevalence, Predisposition and Prevention of Type II Diabetes. Nutr. Metab. 2005, 2, 29. [Google Scholar] [CrossRef]
- Iwasaki, K.; Abarca, C.; Aguayo-Mazzucato, C. Regulation of Cellular Senescence in Type 2 Diabetes Mellitus: From Mechanisms to Clinical Applications. Diabetes Metab. J. 2023, 47, 441–453. [Google Scholar] [CrossRef]
- Murakami, T.; Inagaki, N.; Kondoh, H. Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells. Front. Endocrinol. 2022, 13, 869414. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Standards of Medical Care in Diabetes—2014|Diabetes Care|American Diabetes Association. Available online: https://diabetesjournals.org/care/article/37/Supplement_1/S14/37696/Standards-of-Medical-Care-in-Diabetes-2014 (accessed on 23 April 2024).
- Marton, L.T.; Pescinini-e-Salzedas, L.M.; Camargo, M.E.C.; Barbalho, S.M.; Haber, J.F.D.S.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V.; Cincotto dos Santos Bueno, P. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front. Endocrinol. 2021, 12, 669448. [Google Scholar] [CrossRef]
- Zahedi, M.; Ghiasvand, R.; Feizi, A.; Asgari, G.; Darvish, L. Does Quercetin Improve Cardiovascular Risk Factors and Inflammatory Biomarkers in Women with Type 2 Diabetes: A Double-Blind Randomized Controlled Clinical Trial. Int. J. Prev. Med. 2013, 4, 777–785. [Google Scholar]
- Hasani-Ranjbar, S.; Zahedi, H.S.; Abdollahi, M.; Larijani, B. Trends in Publication of Evidence-Based Traditional Iranian Medicine in Endocrinology and Metabolic Disorders. J. Diabetes Metab. Disord. 2013, 12, 49. [Google Scholar] [CrossRef]
- Scroggie, D.A.; Albright, A.; Harris, M.D. The Effect of Glucosamine-Chondroitin Supplementation on Glycosylated Hemoglobin Levels in Patients with Type 2 Diabetes Mellitus: A Placebo-Controlled, Double-Blinded, Randomized Clinical Trial. Arch. Intern. Med. 2003, 163, 1587–1590. [Google Scholar] [CrossRef]
- Scheen, A.J. Pharmacotherapy of “treatment Resistant” Type 2 Diabetes. Expert Opin. Pharmacother. 2017, 18, 503–515. [Google Scholar] [CrossRef]
- Ghasemi-Dehnoo, M.; Amini-Khoei, H.; Lorigooini, Z.; Rafieian-Kopaei, M. Oxidative Stress and Antioxidants in Diabetes Mellitus. Asian Pac. J. Trop. Med. 2020, 13, 431. [Google Scholar] [CrossRef]
- Karunakaran, U.; Park, K.-G. A Systematic Review of Oxidative Stress and Safety of Antioxidants in Diabetes: Focus on Islets and Their Defense. Diabetes Metab. J. 2013, 37, 106–112. [Google Scholar] [CrossRef]
- Utami, A.R.; Maksum, I.P.; Deawati, Y. Berberine and Its Study as an Antidiabetic Compound. Biology 2023, 12, 973. [Google Scholar] [CrossRef]
- Stolf, A.M.; Cardoso, C.C.; Acco, A. Effects of Silymarin on Diabetes Mellitus Complications: A Review. Phytother. Res. 2017, 31, 366–374. [Google Scholar] [CrossRef]
- Rchid, H.; Chevassus, H.; Nmila, R.; Guiral, C.; Petit, P.; Chokaïri, M.; Sauvaire, Y. Nigella Sativa Seed Extracts Enhance Glucose-Induced Insulin Release from Rat-Isolated Langerhans Islets. Fundam. Clin. Pharmacol. 2004, 18, 525–529. [Google Scholar] [CrossRef]
- Chandra, S.; Mondal, D.; Agrawal, K.C. HIV-1 Protease Inhibitor Induced Oxidative Stress Suppresses Glucose Stimulated Insulin Release: Protection with Thymoquinone. Exp. Biol. Med. 2009, 234, 442–453. [Google Scholar] [CrossRef]
- Safamansouri, H.; Nikan, M.; Amin, G.; Sarkhail, P.; Gohari, A.R.; Kurepaz-Mahmoodabadi, M.; Saeidnia, S. α-Amylase Inhibitory Activity of Some Traditionally Used Medicinal Species of Labiatae. J. Diabetes Metab. Disord. 2014, 13, 114. [Google Scholar] [CrossRef]
- Ferenczyova, K.; Kalocayova, B.; Bartekova, M. Potential Implications of Quercetin and Its Derivatives in Cardioprotection. Int. J. Mol. Sci. 2020, 21, 1585. [Google Scholar] [CrossRef]
- Zoico, E.; Nori, N.; Darra, E.; Tebon, M.; Rizzatti, V.; Policastro, G.; De Caro, A.; Rossi, A.P.; Fantin, F.; Zamboni, M. Senolytic Effects of Quercetin in an in Vitro Model of Pre-Adipocytes and Adipocytes Induced Senescence. Sci. Rep. 2021, 11, 23237. [Google Scholar] [CrossRef]
- Islam, M.T.; Tuday, E.; Allen, S.; Kim, J.; Trott, D.W.; Holland, W.L.; Donato, A.J.; Lesniewski, L.A. Senolytic Drugs, Dasatinib and Quercetin, Attenuate Adipose Tissue Inflammation, and Ameliorate Metabolic Function in Old Age. Aging Cell 2023, 22, e13767. [Google Scholar] [CrossRef]
- Hollman, P.C.; van Trijp, J.M.; Buysman, M.N.; van der Gaag, M.S.; Mengelers, M.J.; de Vries, J.H.; Katan, M.B. Relative Bioavailability of the Antioxidant Flavonoid Quercetin from Various Foods in Man. FEBS Lett. 1997, 418, 152–156. [Google Scholar] [CrossRef]
- Margină, D.; Olaru, O.T.; Ilie, M.; Grădinaru, D.; Guțu, C.; Voicu, S.; Dinischiotu, A.; Spandidos, D.A.; Tsatsakis, A.M. Assessment of the Potential Health Benefits of Certain Total Extracts from Vitis Vinifera, Aesculus Hyppocastanum and Curcuma Longa. Exp. Ther. Med. 2015, 10, 1681–1688. [Google Scholar] [CrossRef]
- Wilsher, N.E.; Arroo, R.R.; Matsoukas, M.; Tsatsakis, A.M.; Spandidos, D.A.; Androutsopoulos, V.P. Cytochrome P450 CYP1 Metabolism of Hydroxylated Flavones and Flavonols: Selective Bioactivation of Luteolin in Breast Cancer Cells. Food Chem. Toxicol. 2017, 110, 383–394. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin Reduces Blood Pressure in Hypertensive Subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef]
- Erdman, J.W.; Balentine, D.; Arab, L.; Beecher, G.; Dwyer, J.T.; Folts, J.; Harnly, J.; Hollman, P.; Keen, C.L.; Mazza, G.; et al. Flavonoids and Heart Health: Proceedings of the ILSI North America Flavonoids Workshop, May 31-June 1, 2005, Washington, DC. J. Nutr. 2007, 137, 718S–737S. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and Apoptosis-Inducing Effects of Quercetin in Vitro and in Vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef]
- Notas, G.; Nifli, A.-P.; Kampa, M.; Pelekanou, V.; Alexaki, V.-I.; Theodoropoulos, P.; Vercauteren, J.; Castanas, E. Quercetin Accumulates in Nuclear Structures and Triggers Specific Gene Expression in Epithelial Cells. J. Nutr. Biochem. 2012, 23, 656–666. [Google Scholar] [CrossRef]
- Liu, J.; Yu, H.; Ning, X. Effect of Quercetin on Chronic Enhancement of Spatial Learning and Memory of Mice. Sci. China Ser. C 2006, 49, 583–590. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Somboonporn, W.; Thukham-Mee, W.; Sungkamnee, S. Memory-Enhancing Effect of 8-Week Consumption of the Quercetin-Enriched Culinary Herbs-Derived Functional Ingredients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Foods 2022, 11, 2678. [Google Scholar] [CrossRef]
- de Barros, D.P.C.; Santos, R.; Reed, P.; Fonseca, L.P.; Oliva, A. Design of Quercetin-Loaded Natural Oil-Based Nanostructured Lipid Carriers for the Treatment of Bacterial Skin Infections. Molecules 2022, 27, 8818. [Google Scholar] [CrossRef]
- Jin, F.; Nieman, D.C.; Shanely, R.A.; Knab, A.M.; Austin, M.D.; Sha, W. The Variable Plasma Quercetin Response to 12-Week Quercetin Supplementation in Humans. Eur. J. Clin. Nutr. 2010, 64, 692–697. [Google Scholar] [CrossRef]
- Lu, N.T.; Crespi, C.M.; Liu, N.M.; Vu, J.Q.; Ahmadieh, Y.; Wu, S.; Lin, S.; McClune, A.; Durazo, F.; Saab, S.; et al. A Phase I Dose Escalation Study Demonstrates Quercetin Safety and Explores Potential for Bioflavonoid Antivirals in Patients with Chronic Hepatitis C. Phytother. Res. 2016, 30, 160–168. [Google Scholar] [CrossRef]
- Umathe, S.; Dixit, P.; Vaghasiya, M.J.M.; Jain, N. Influence of Quercetin on Diabetes-Induced Alteration in CYP3A Activity and Bioavailability of Pioglitazone in Rats. Am. J. Infect. Dis. 2009, 5, 125–132. [Google Scholar] [CrossRef]
- Kim, K.-A.; Park, P.-W.; Kim, H.-K.; Ha, J.-M.; Park, J.-Y. Effect of Quercetin on the Pharmacokinetics of Rosiglitazone, a CYP2C8 Substrate, in Healthy Subjects. J. Clin. Pharmacol. 2005, 45, 941–946. [Google Scholar] [CrossRef]
- Mantadaki, A.E.; Linardakis, M.; Vafeiadi, M.; Anastasiou, F.; Tsatsakis, A.; Symvoulakis, E.K. The Impact of Three-Month Quercetin Intake on Quality of Life and Anxiety in Patients With Type II Diabetes Mellitus: An Early Data Analysis From a Randomized Controlled Trial. Cureus 2024, 16, e58219. [Google Scholar] [CrossRef]
- Jeong, S.-M.; Kang, M.-J.; Choi, H.-N.; Kim, J.-H.; Kim, J.-I. Quercetin Ameliorates Hyperglycemia and Dyslipidemia and Improves Antioxidant Status in Type 2 Diabetic Db/Db Mice. Nutr. Res. Pract. 2012, 6, 201–207. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, BMI.S38440. [Google Scholar] [CrossRef]
- Abdul Murad, N.A.; Abdullah, N.; Kamaruddin, M.A.; Abd Jalal, N.; Ismail, N.; Yusof, N.A.M.; Mustafa, N.; Jamal, R. Discordance between Fasting Plasma Glucose (FPG) and HbA1c in Diagnosing Diabetes and Pre-Diabetes in The Malaysian Cohort. J. ASEAN Fed. Endocr. Soc. 2021, 36, 127–132. [Google Scholar] [CrossRef]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in Idiopathic Pulmonary Fibrosis: Results from a First-in-Human, Open-Label, Pilot Study. eBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef]
- Peyrot, M.; Rubin, R.R. Levels and Risks of Depression and Anxiety Symptomatology among Diabetic Adults. Diabetes Care 1997, 20, 585–590. [Google Scholar] [CrossRef]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Sotoudeh, G. Beneficial Effects of Nano-Curcumin Supplement on Depression and Anxiety in Diabetic Patients with Peripheral Neuropathy: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Phytother. Res. 2020, 34, 896–903. [Google Scholar] [CrossRef]
- Mast, R.; Rauh, S.P.; Groeneveld, L.; Koopman, A.D.; Beulens, J.W.J.; Jansen, A.P.D.; Bremmer, M.; van der Heijden, A.A.W.A.; Elders, P.J.; Dekker, J.M.; et al. The Use of Antidepressants, Anxiolytics, and Hypnotics in People with Type 2 Diabetes and Patterns Associated with Use: The Hoorn Diabetes Care System Cohort. Biomed Res. Int. 2017, 2017, 5134602. [Google Scholar] [CrossRef]
- Edwards, J.G. Adverse Effects of Antianxiety Drugs. Drugs 1981, 22, 495–514. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Vieira, K.F. Nutritional and Herbal Supplements for Anxiety and Anxiety-Related Disorders: Systematic Review. Nutr. J. 2010, 9, 42. [Google Scholar] [CrossRef]
- Srivastava, A.; Kumar, P. Chapter 4—Nutraceuticals in Anxiety and Stress. In Nutraceuticals, 2nd ed.; Gupta, R.C., Lall, R., Srivastava, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 63–72. ISBN 978-0-12-821038-3. [Google Scholar]
- Gupta, C. Chapter 7—Nutraceuticals for Prevention and Management of Anxiety. In Nutraceutical Fruits and Foods for Neurodegenerative Disorders; Keservani, R.K., Kesharwani, R.K., Emerald, M., Sharma, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 123–149. ISBN 978-0-443-18951-7. [Google Scholar]
- Vida, R.G.; Fittler, A.; Somogyi-Végh, A.; Poór, M. Dietary Quercetin Supplements: Assessment of Online Product Informations and Quantitation of Quercetin in the Products by High-Performance Liquid Chromatography. Phytother. Res. 2019, 33, 1912–1920. [Google Scholar] [CrossRef]
- ISRCTN13131584: Study on the Benefit of Quercetin Intake in Diabetic Patients Treated with Antidiabetic Tablets. Available online: https://www.isrctn.com/ISRCTN13131584 (accessed on 15 January 2024).
- WMA 2013 World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects|Research, Methods, Statistics|JAMA|JAMA Network. Available online: https://jamanetwork.com/journals/jama/fullarticle/1760318 (accessed on 18 January 2024).
- ICH Harmonised Guideline Integrated Addendum to ICH E6(R1): Guideline for Good Clinical Practice ICH E6(R2) ICH Consensus Guideline. Available online: https://ichgcp.net/home (accessed on 25 April 2024).
- World Health Organization. Handbook for Good Clinical Research Practice (GCP): Guidance for Implementation; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- European Medicines Agency Guideline for Good Clinical Practice E6 (R2). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-good-clinical-practice-e6r2-4-step-2b_en.pdf (accessed on 22 March 2024).
- European Parliament and the Council of the European Union Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on Clinical Trials on Medicinal Products for Human Use, and Repealing Directive 2001/20/EC. Available online: https://health.ec.europa.eu/document/download/f724d198-9ec8-4cad-9ce7-b6d2ac1ec44e_en (accessed on 22 March 2024).
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual Framework and Item Selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Brazier, J.E.; Harper, R.; Jones, N.M.; O’Cathain, A.; Thomas, K.J.; Usherwood, T.; Westlake, L. Validating the SF-36 Health Survey Questionnaire: New Outcome Measure for Primary Care. BMJ 1992, 305, 160–164. [Google Scholar] [CrossRef]
- Pappa, E.; Kontodimopoulos, N.; Niakas, D. Validating and Norming of the Greek SF-36 Health Survey. Qual. Life Res. 2005, 14, 1433–1438. [Google Scholar] [CrossRef]
- Sinoff, G.; Ore, L.; Zlotogorsky, D.; Tamir, A. Short Anxiety Screening Test—A Brief Instrument for Detecting Anxiety in the Elderly. Int. J. Geriatr. Psychiatry 1999, 14, 1062–1071. [Google Scholar] [CrossRef]
- Grammatikopoulos, I.A.; Sinoff, G.; Alegakis, A.; Kounalakis, D.; Antonopoulou, M.; Lionis, C. The Short Anxiety Screening Test in Greek: Translation and Validation. Ann. Gen. Psychiatry 2010, 9, 1. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Thomas, J.V.; Smina, T.P.; Khanna, A.; Kunnumakkara, A.B.; Maliakel, B.; Mohanan, R.; Krishnakumar, I.M. Influence of a Low-Dose Supplementation of Curcumagalactomannoside Complex (CurQfen) in Knee Osteoarthritis: A Randomized, Open-Labeled, Active-Controlled Clinical Trial. Phytother. Res. 2021, 35, 1443–1455. [Google Scholar] [CrossRef]
- Dehghani, F.; Sezavar Seyedi Jandaghi, S.H.; Janani, L.; Sarebanhassanabadi, M.; Emamat, H.; Vafa, M. Effects of Quercetin Supplementation on Inflammatory Factors and Quality of Life in Post-myocardial Infarction Patients: A Double Blind, Placebo-controlled, Randomized Clinical Trial. Phytother. Res. 2021, 35, 2085–2098. [Google Scholar] [CrossRef]
- Duijker, G.; Bertsias, A.; Symvoulakis, E.K.; Moschandreas, J.; Malliaraki, N.; Derdas, S.P.; Tsikalas, G.K.; Katerinopoulos, H.E.; Pirintsos, S.A.; Sourvinos, G.; et al. Reporting Effectiveness of an Extract of Three Traditional Cretan Herbs on Upper Respiratory Tract Infection: Results from a Double-Blind Randomized Controlled Trial. J. Ethnopharmacol. 2015, 163, 157–166. [Google Scholar] [CrossRef]
- Salek, R.; Dehghani, M.; Mohajeri, S.A.; Talaei, A.; Fanipakdel, A.; Javadinia, S.A. Amelioration of Anxiety, Depression, and Chemotherapy Related Toxicity after Crocin Administration during Chemotherapy of Breast Cancer: A Double Blind, Randomized Clinical Trial. Phytother. Res. 2021, 35, 5143–5153. [Google Scholar] [CrossRef]
- Mazloom, Z.; Abdollahzadeh, S.M.; Dabbaghmanesh, M.H.; Rezaianzadeh, A. The Effect of Quercetin Supplementation on Oxidative Stress, Glycemic Control, Lipid Profile and Insulin Resistance in Type 2 Diabetes: A Randomized Clinical Trial. J. Health Sci. Surveill. Syst. 2014, 2, 8–14. [Google Scholar]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Stehle, P.; et al. No Effects of Quercetin from Onion Skin Extract on Serum Leptin and Adiponectin Concentrations in Overweight-to-Obese Patients with (Pre-)Hypertension: A Randomized Double-Blinded, Placebo-Controlled Crossover Trial. Eur. J. Nutr. 2017, 56, 2265–2275. [Google Scholar] [CrossRef]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A Review on the Role of Antioxidants in the Management of Diabetes and Its Complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef]
- AL-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef]
- Liu, F.; Sirisena, S.; Ng, K. Efficacy of Flavonoids on Biomarkers of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2023, 63, 4916–4941. [Google Scholar] [CrossRef]
- Xie, W.; Su, F.; Wang, G.; Peng, Z.; Xu, Y.; Zhang, Y.; Xu, N.; Hou, K.; Hu, Z.; Chen, Y.; et al. Glucose-Lowering Effect of Berberine on Type 2 Diabetes: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 1015045. [Google Scholar] [CrossRef]
- Elfaituri, M.K.; Alzubi, A.S.; Khaled, T.; Faraj, H.A.A.; BenGhatnsh, A.; Msherghi, A. Abstract 13683: Deciphering the Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis. Circulation 2023, 148, A13683. [Google Scholar] [CrossRef]
- Marunaka, Y.; Marunaka, R.; Sun, H.; Yamamoto, T.; Kanamura, N.; Inui, T.; Taruno, A. Actions of Quercetin, a Polyphenol, on Blood Pressure. Molecules 2017, 22, 209. [Google Scholar] [CrossRef]
- Barreiro, E.; de la Puente, B.; Minguella, J.; Corominas, J.M.; Serrano, S.; Hussain, S.N.A.; Gea, J. Oxidative Stress and Respiratory Muscle Dysfunction in Severe Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2005, 171, 1116–1124. [Google Scholar] [CrossRef]
- Patel, S.; Marchetti, N.; Ganjian, H.; Kelsen, S.G.; Criner, G.J.; Sajjan, U. Oral Treatment with Quercetin Reduces Markers of Inflammation in COPD Patients. In Proceedings of the C40. Clinical Trials and Novel Interventions in Copd, Washington, DC, USA, 19–24 May 2023; American Thoracic Society: New York, NY, USA, 2023; p. A5001. [Google Scholar]
- Araújo, N.P.D.S.; de Matos, N.A.; Oliveira, M.; de Souza, A.B.F.; Castro, T.D.F.; Machado-Júnior, P.A.; de Souza, D.M.S.; Talvani, A.; Cangussú, S.D.; de Menezes, R.C.A.; et al. Quercetin Improves Pulmonary Function and Prevents Emphysema Caused by Exposure to Cigarette Smoke in Male Mice. Antioxidants 2022, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Maturu, P.; Wei-Liang, Y.; Androutsopoulos, V.P.; Jiang, W.; Wang, L.; Tsatsakis, A.M.; Couroucli, X.I. Quercetin Attenuates the Hyperoxic Lung Injury in Neonatal Mice: Implications for Bronchopulmonary Dysplasia (BPD). Food Chem. Toxicol. 2018, 114, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Carvalhas-Almeida, C.; Cavadas, C.; Álvaro, A.R. The Impact of Insomnia on Frailty and the Hallmarks of Aging. Aging Clin. Exp. Res. 2023, 35, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2018, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hong, K.-B.; Jo, K.; Suh, H.J. Quercetin-3-O-Glucuronide in the Ethanol Extract of Lotus Leaf (Nelumbo Nucifera) Enhances Sleep Quantity and Quality in a Rodent Model via a GABAergic Mechanism. Molecules 2021, 26, 3023. [Google Scholar] [CrossRef] [PubMed]
- Kambe, D.; Kotani, M.; Yoshimoto, M.; Kaku, S.; Chaki, S.; Honda, K. Effects of Quercetin on the Sleep–Wake Cycle in Rats: Involvement of Gamma-Aminobutyric Acid Receptor Type A in Regulation of Rapid Eye Movement Sleep. Brain Res. 2010, 1330, 83–88. [Google Scholar] [CrossRef]
- Rondanelli, M.; Riva, A.; Petrangolini, G.; Gasparri, C.; Perna, S. Two-Month Period of 500 Mg Lecithin-Based Delivery Form of Quercetin Daily Dietary Supplementation Counterbalances Chronic Fatigue Symptoms: A Double-Blind Placebo Controlled Clinical Trial. Biomed. Pharmacother. 2023, 167, 115453. [Google Scholar] [CrossRef] [PubMed]
- Bigelman, K.A.; Chapman, D.P.; Freese, E.C.; Trilk, J.L.; Cureton, K.J. Effects of 6 Weeks of Quercetin Supplementation on Energy, Fatigue, and Sleep in ROTC Cadets. Mil. Med. 2011, 176, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J.; Ali, S.; Metse, A.P.; Kalns, J.; Drummond, P.D. Effects of a Bacopa Monnieri Extract (Bacognize®) on Stress, Fatigue, Quality of Life and Sleep in Adults with Self-Reported Poor Sleep: A Randomised, Double-Blind, Placebo-Controlled Study. J. Funct. Foods 2021, 85, 104671. [Google Scholar] [CrossRef]
- Firoozabadi, A.; Kolouri, S.; Zarshenas, M.M.; Salehi, A.; Mosavat, S.H.; Dastgheib, S.A. Efficacy of a Freeze-Dried Aqueous Extract of Nepeta Menthoides Boiss. & Buhse in the Treatment of Anxiety in Patients with Depression: A Double-Blind, Randomized, Controlled Trial. J. Herb. Med. 2017, 10, 17–23. [Google Scholar] [CrossRef]
- Spencer, J.P.E. The Interactions of Flavonoids within Neuronal Signalling Pathways. Genes Nutr. 2007, 2, 257–273. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Vissiennon, C.; Nieber, K.; Kelber, O.; Butterweck, V. Route of Administration Determines the Anxiolytic Activity of the Flavonols Kaempferol, Quercetin and Myricetin—Are They Prodrugs? J. Nutr. Biochem. 2012, 23, 733–740. [Google Scholar] [CrossRef]
- Kim, D.H.; Jung, E.A.; Sohng, I.S.; Han, J.A.; Kim, T.H.; Han, M.J. Intestinal Bacterial Metabolism of Flavonoids and Its Relation to Some Biological Activities. Arch. Pharm. Res. 1998, 21, 17–23. [Google Scholar] [CrossRef]
- Bhutada, P.; Mundhada, Y.; Bansod, K.; Ubgade, A.; Quazi, M.; Umathe, S.; Mundhada, D. Reversal by Quercetin of Corticotrophin Releasing Factor Induced Anxiety- and Depression-like Effect in Mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Parashar, A.; Udayabanu, M. Quercetin Prevents Chronic Unpredictable Stress Induced Behavioral Dysfunction in Mice by Alleviating Hippocampal Oxidative and Inflammatory Stress. Physiol. Behav. 2017, 171, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kosari-Nasab, M.; Shokouhi, G.; Ghorbanihaghjo, A.; Mesgari-Abbasi, M.; Salari, A.-A. Quercetin Mitigates Anxiety-like Behavior and Normalizes Hypothalamus-Pituitary-Adrenal Axis Function in a Mouse Model of Mild Traumatic Brain Injury. Behav. Pharmacol. 2019, 30, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.-H. Protective Effects of Quercetin on Anxiety-Like Symptoms and Neuroinflammation Induced by Lipopolysaccharide in Rats. Evid. Based Complement. Altern. Med. 2020, 2020, 4892415. [Google Scholar] [CrossRef]
- Netuveli, G.; Wiggins, R.D.; Hildon, Z.; Montgomery, S.M.; Blane, D. Quality of Life at Older Ages: Evidence from the English Longitudinal Study of Aging (Wave 1). J. Epidemiol. Community Health 2006, 60, 357–363. [Google Scholar] [CrossRef]
- Noto, S. Perspectives on Aging and Quality of Life. Healthcare 2023, 11, 2131. [Google Scholar] [CrossRef]
- Perna, G.; Iannone, G.; Alciati, A.; Caldirola, D. Are Anxiety Disorders Associated with Accelerated Aging? A Focus on Neuroprogression. Neural Plast. 2016, 2016, 8457612. [Google Scholar] [CrossRef] [PubMed]
- Yawar, R.; Khan, S.; Rafiq, M.; Fawad, N.; Shams, S.; Navid, S.; Khan, M.A.; Taufiq, N.; Touqir, A.; Imran, M.; et al. Aging Is Inevitable: Understanding Aging Anxiety Related to Physical Symptomology and Quality of Life with the Mediating Role of Self-Esteem in Adults. Int. J. Hum. Rights Healthc. 2022, 17, 170–185. [Google Scholar] [CrossRef]
- Jing, X.; Chen, J.; Dong, Y.; Han, D.; Zhao, H.; Wang, X.; Gao, F.; Li, C.; Cui, Z.; Liu, Y.; et al. Related Factors of Quality of Life of Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis. Health Qual. Life Outcomes 2018, 16, 189. [Google Scholar] [CrossRef] [PubMed]
- Saleh, F.; Ara, F.; Mumu, S.J.; Hafez, M.A. Assessment of Health-Related Quality of Life of Bangladeshi Patients with Type 2 Diabetes Using the EQ-5D: A Cross-Sectional Study. BMC Res. Notes 2015, 8, 497. [Google Scholar] [CrossRef] [PubMed]
- Khonche, A.; Fallah Huseini, H.; Abdi, H.; Mohtashami, R.; Nabati, F.; Kianbakht, S. Efficacy of Mentha Pulegium Extract in the Treatment of Functional Dyspepsia: A Randomized Double-Blind Placebo-Controlled Clinical Trial. J. Ethnopharmacol. 2017, 206, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Knab, A.M.; Shanely, R.A.; Henson, D.A.; Jin, F.; Heinz, S.A.; Austin, M.D.; Nieman, D.C. Influence of Quercetin Supplementation on Disease Risk Factors in Community-Dwelling Adults. J. Am. Diet. Assoc. 2011, 111, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liao, D.; Dong, Y.; Pu, R. Clinical Effectiveness of Quercetin Supplementation in the Management of Weight Loss: A Pooled Analysis of Randomized Controlled Trials. Diabetes Metab. Syndr. Obes. 2019, 12, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Shatylo, V.; Antoniuk-Shcheglova, I.; Naskalova, S.; Bondarenko, O.; Havalko, A.; Krasnienkov, D.; Zabuga, O.; Kukharskyy, V.; Guryanov, V.; Vaiserman, A. Cardio-Metabolic Benefits of Quercetin in Elderly Patients with Metabolic Syndrome. PharmaNutrition 2021, 15, 100250. [Google Scholar] [CrossRef]
- Pfeuffer, M.; Auinger, A.; Bley, U.; Kraus-Stojanowic, I.; Laue, C.; Winkler, P.; Rüfer, C.E.; Frank, J.; Bösch-Saadatmandi, C.; Rimbach, G.; et al. Effect of Quercetin on Traits of the Metabolic Syndrome, Endothelial Function and Inflammation in Men with Different APOE Isoforms. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 403–409. [Google Scholar] [CrossRef]
- Sahebkar, A. Effects of Quercetin Supplementation on Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2017, 57, 666–676. [Google Scholar] [CrossRef]
- Ren, K.; Jiang, T.; Zhao, G.-J. Quercetin Induces the Selective Uptake of HDL-Cholesterol via Promoting SR-BI Expression and the Activation of the PPARγ/LXRα Pathway. Food Funct. 2018, 9, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Franczyk, B.; Rysz, J.; Ławiński, J.; Rysz-Górzyńska, M.; Gluba-Brzózka, A. Is a High HDL-Cholesterol Level Always Beneficial? Biomedicines 2021, 9, 1083. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liao, D.; Dong, Y.; Pu, R. Effect of Quercetin Supplementation on Plasma Lipid Profiles, Blood Pressure, and Glucose Levels: A Systematic Review and Meta-Analysis. Nutr. Rev. 2020, 78, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.I. Platelet Function and Ageing. Mamm. Genome 2016, 27, 358–366. [Google Scholar] [CrossRef]
- Balduini, C.L.; Noris, P. Platelet Count and Aging. Haematologica 2014, 99, 953–955. [Google Scholar] [CrossRef]
- Piva, E.; Brugnara, C.; Spolaore, F.; Plebani, M. Clinical Utility of Reticulocyte Parameters. Clin. Lab. Med. 2015, 35, 133–163. [Google Scholar] [CrossRef]
- Jain, D.P.; Jain, D.R.; Shah, D.C.; Jindal, D.M.; Jain, D.A.K.; Dixit, D.R. A Comparative Study of Reticulocyte Count in Healthy Young Adult and Elderly Age Group Subjects: Study of Reticulocyte Count in Healthy Young Adult and Elderly. Natl. J. Integr. Res. Med. 2013, 4, 118–122. [Google Scholar]
- Pérez-Ruixo, J.J.; Krzyzanski, W.; Hing, J. Pharmacodynamic Analysis of Recombinant Human Erythropoietin Effect on Reticulocyte Production Rate and Age Distribution in Healthy Subjects. Clin. Pharmacokinet. 2008, 47, 399–415. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A Flavonol with Multifaceted Therapeutic Applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef]
- Brouwer, A.; van Raalte, D.H.; Rutters, F.; Elders, P.J.M.; Snoek, F.J.; Beekman, A.T.F.; Bremmer, M.A. Sleep and HbA1c in Patients with Type 2 Diabetes: Which Sleep Characteristics Matter Most? Diabetes Care 2019, 43, 235–243. [Google Scholar] [CrossRef]
- Lee, S.W.H.; Ng, K.Y.; Chin, W.K. The Impact of Sleep Amount and Sleep Quality on Glycemic Control in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2017, 31, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Maan, H.B.; Meo, S.A.; Al Rouq, F.; Meo, I.M.U.; Gacuan, M.E.; Alkhalifah, J.M. Effect of Glycated Hemoglobin (HbA1c) and Duration of Disease on Lung Functions in Type 2 Diabetic Patients. Int. J. Environ. Res. Public Health 2021, 18, 6970. [Google Scholar] [CrossRef] [PubMed]
- Koloverou, E.; Tentolouris, N.; Bakoula, C.; Darviri, C.; Chrousos, G. Implementation of a Stress Management Program in Outpatients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Hormones 2014, 13, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Ding, J.; Su, H.; Du, Y.; Pan, T.; Zhong, X. Association of Long-Term HbA1c Variability with Anxiety and Depression in Patients with Type 2 Diabetes: A Cross-Sectional Retrospective Study. Psychol. Res. Behav. Manag. 2023, 16, 5053–5068. [Google Scholar] [CrossRef] [PubMed]
- Camara, A.; Baldé, N.M.; Enoru, S.; Bangoura, J.S.; Sobngwi, E.; Bonnet, F. Prevalence of Anxiety and Depression among Diabetic African Patients in Guinea: Association with HbA1c Levels. Diabetes Metab. 2015, 41, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qin, C.; Guo, X.; Cao, F.; Tang, C. Association of Hemoglobin A1c with the Incidence of Hypertension: A Large Prospective Study. Front. Endocrinol. 2023, 13, 1098012. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, Y.; Jin, S.; Zhao, D.; Guo, J.; Chen, L.; Huang, Y. Association of Glycemic Control with Hypertension in Patients with Diabetes: A Population-Based Longitudinal Study. BMC Cardiovasc. Disord. 2023, 23, 501. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Stampfer, M.J. Insulin Resistance and Hypertension. Circulation 2005, 112, 1678–1680. [Google Scholar] [CrossRef] [PubMed]
- Lionis, C.; Symvoulakis, E.K.; Vardavas, C.I. Implementing Family Practice Research in Countries with Limited Resources: A Stepwise Model Experienced in Crete, Greece. Fam. Pract. 2010, 27, 48–54. [Google Scholar] [CrossRef]
- Lionis, C.; Symvoulakis, E.K.; Markaki, A.; Petelos, E.; Papadakis, S.; Sifaki-Pistolla, D.; Papadakakis, M.; Souliotis, K.; Tziraki, C. Integrated People-Centred Primary Health Care in Greece: Unravelling Ariadne’s Thread. Prim. Health Care Res. Dev. 2019, 20, e113. [Google Scholar] [CrossRef]
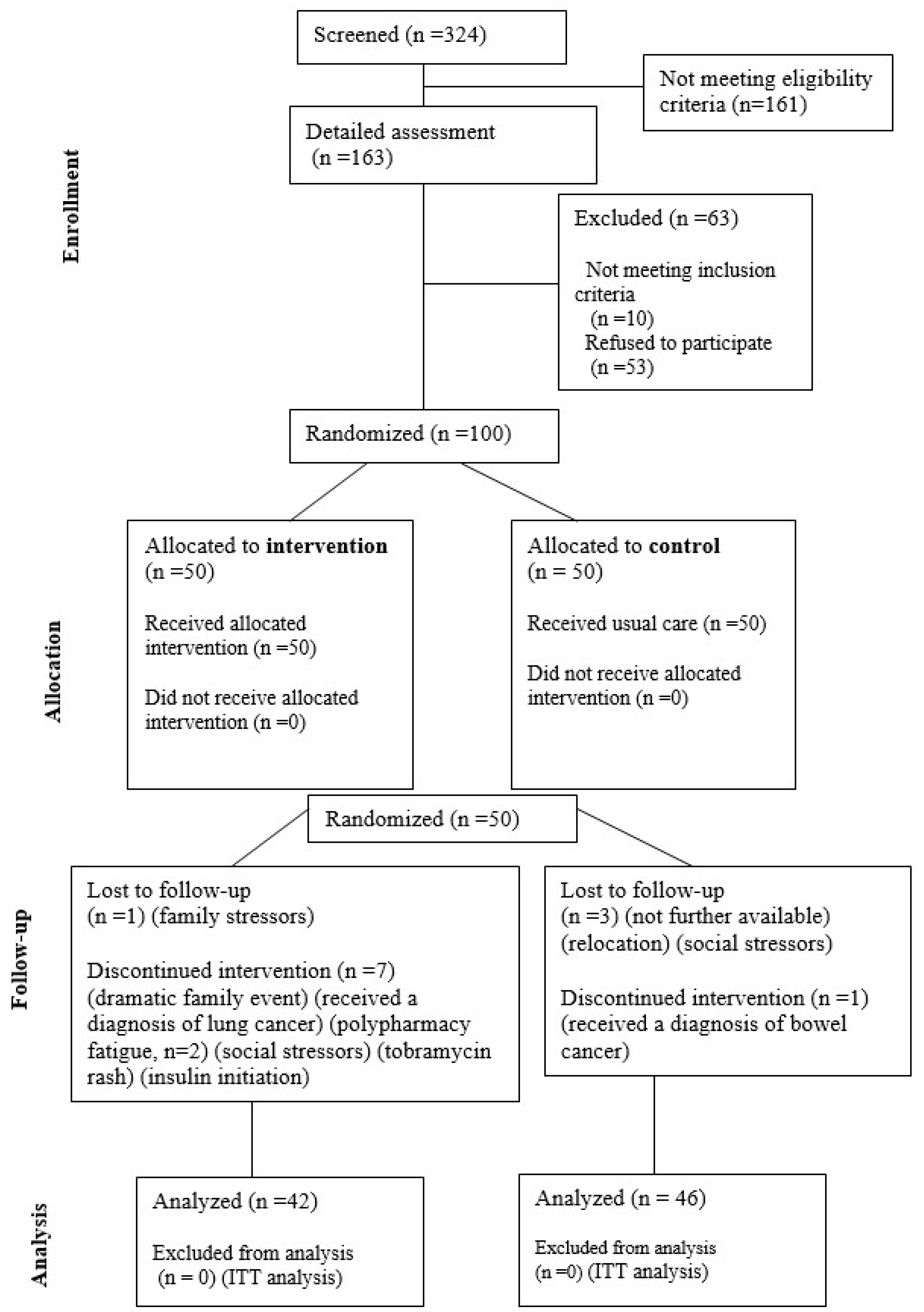

| Groups | |||
|---|---|---|---|
| Intervention (n = 42) | Control (n = 46) | ||
| n (%) | |||
| Gender | male | 24 (57.1) | 24 (52.2) |
| female | 18 (42.9) | 22 (47.8) | |
| Age, years | mean ± stand. dev. | 66.9 ± 7.7 | 68.7 ± 6.6 |
| Nationality | Greek | 38 (90.5) * | 46 (100.0) |
| Current smoker | yes | 14 (33.3) | 16 (34.8) |
| Current drinker | yes | 29 (69.0) | 30 (65.2) |
| Multimorbidity | 3+ chronic conditions | 33 (78.6) | 31 (67.4) |
| Polypharmacy | 4+ medications | 29 (69.0) | 32 (69.6) |
| Vaccination | influenza | 35 (83.3) | 40 (87.0) |
| pneumococcal | 30 (71.4) | 32 (69.6) | |
| shingles (herpes zoster) | 14 (33.3) | 20 (43.5) | |
| COVID-19 | 41 (97.6) | 45 (100.0) | |
| Years diagnosed with T2DM | mean ± stand. dev. | 11.1 ± 6.9 | 10.2 ± 6.6 |
| Groups | Cohen’s d Effect Size | ||||
|---|---|---|---|---|---|
| Intervention | Control | p-Value | |||
| Mean ± Stand. Dev. | |||||
| Lymphocytes (LYMPH) (%) | beginning | 31.79 ± 6.20 | 31.65 ± 6.87 | ||
| 8 months | 31.22 ± 8.75 | 30.98 ± 7.85 | |||
| Δ-change | −0.57 | −0.67 | 0.941 | 0.02 | |
| Δ%-change | −1.8 | −2.1 | |||
| Monocytes (MONO) (%) | beginning | 5.92 ± 1.98 | 6.35 ± 2.07 | ||
| 8 months | 6.65 ± 2.47 | 6.40 ± 1.71 | |||
| Δ-change | 0.72 | 0.05 | 0.186 | 0.29 | |
| Δ%-change | 12.2 | 0.8 | |||
| Neutrophils (NEUT) (%) | beginning | 58.83 ± 7.22 | 58.84 ± 7.18 | ||
| 8 months | 58.84 ± 9.71 | 59.00 ± 8.52 | |||
| Δ-change | 0.02 | 0.15 | 0.931 | 0.02 | |
| Δ%-change | 0.0 | 0.3 | |||
| Hemoglobin (HGB) (g/dL) | beginning | 15.69 ± 4.49 | 13.75 ± 1.44 | ||
| 8 months | 14.42 ± 1.38 | 13.75 ± 1.43 | |||
| Δ-change | −1.26 | 0.00 | 0.092 | 0.37 | |
| Δ%-change | −8.1 | 0.0 | |||
| Platelet count (PLT) × 103/mm3 | beginning | 227.0 ± 53.1 | 243.0 ± 54.1 | ||
| 8 months | 236.9 ± 53.9 | 240.9 ± 65.0 | |||
| Δ-change | 9.8 | −2.1 | 0.171 | 0.30 | |
| Δ%-change | 4.3 | −0.9 | |||
| White blood cells (WBCs) × 103/μL | beginning | 7.87 ± 2.12 | 7.48 ± 2.03 | ||
| 8 months | 7.85 ± 2.41 | 7.47 ± 1.94 | |||
| Δ-change | −0.01 | −0.01 | 0.987 | 0.00 | |
| Δ%-change | −0.2 | −0.1 | |||
| Reticulocytes (RTCs) % | beginning | 1.36 ± 0.50 | 1.57 ± 2.03 | ||
| 8 months | 1.45 ± 0.51 | 1.33 ± 0.42 | |||
| Δ-change | 0.13 | −0.24 | 0.277 | 0.24 | |
| Δ%-change | 9.3 | −15.2 | |||
| Groups | Cohen’s d Effect Size | ||||
|---|---|---|---|---|---|
| Intervention | Control | p-Value | |||
| Mean ± Stand. Dev. | |||||
| Serum urea (mg/dL) | beginning | 34.95 ± 9.62 | 37.37 ± 11.80 | ||
| 8 months | 35.69 ± 7.90 | 36.66 ± 10.81 | |||
| Δ-change | 0.74 | −0.72 | 0.478 | 0.15 | |
| Δ%-change | 2.1 | −1.9 | |||
| Blood creatinine (mg/dL) | beginning | 0.80 ± 0.22 | 0.83 ± 0.25 | ||
| 8 months | 0.82 ± 0.25 | 0.87 ± 0.27 | |||
| Δ-change | 0.02 | 0.04 | 0.461 | 0.16 | |
| Δ%-change | 1.9 | 4.4 | |||
| Total cholesterol (mg/dL) | beginning | 153.8 ± 35.4 | 162.6 ± 37.4 | ||
| 8 months | 159.1 ± 36.1 | 162.4 ± 44.1 | |||
| Δ-change | 5.3 | −0.2 | 0.417 | 0.18 | |
| Δ%-change | 3.5 | −0.1 | |||
| Triglycerides (mg/dL) | beginning | 146.9 ± 85.3 | 136.7 ± 59.0 | ||
| 8 months | 154.4 ± 80.5 | 146.3 ± 62.5 | |||
| Δ-change | 7.5 | 9.6 | 0.825 | 0.05 | |
| Δ%-change | 5.1 | 7.0 | |||
| Low-density lipoprotein (LDL) (mg/dL) | beginning | 79.8 ± 33.7 | 86.0 ± 32.6 | ||
| 8 months | 85.4 ± 34.4 | 87.3 ± 33.8 | |||
| Δ-change | 5.6 | 1.3 | 0.509 | 0.14 | |
| Δ%-change | 7.1 | 1.5 | |||
| C-reactive protein (CRP) (mg/dL) | beginning | 0.97 ± 2.80 | 0.76 ± 1.67 | ||
| 8 months | 0.78 ± 1.58 | 0.62 ± 1.11 | |||
| Δ-change | −0.19 | −0.14 | 0.892 | 0.03 | |
| Δ%-change | −19.7 | −18.2 | |||
| Glycated hemoglobin (HbA1c) (%) | beginning | 7.06 ± 1.11 | 6.76 ± 0.82 | ||
| 8 months | 6.78 ± 0.89 | 6.76 ± 0.92 | |||
| Δ-change | −0.28 | 0.01 | 0.011 | 0.56 | |
| Δ%-change | −4.0 | 0.1 | |||
| 25-hydroxy vitamin D (ng/mL) | beginning | 33.8 ± 15.8 | 30.4 ± 16.3 | ||
| 8 months | 28.6 ± 11.6 | 29.0 ± 9.7 | |||
| Δ-change | −5.2 | −1.4 | 0.248 | 0.25 | |
| Δ%-change | −15.4 | −4.5 | |||
| DHEA sulfate (DHEAS) (μg/mL) | beginning | 0.91 ± 0.57 | 0.87 ± 0.64 | ||
| 8 months | 0.81 ± 0.48 | 0.94 ± 0.89 | |||
| Δ-change | −0.10 | 0.07 | 0.078 | 0.39 | |
| Δ%-change | −11.2 | 7.5 | |||
| Cholesterol ratio | beginning | 3.93 ± 1.96 | 3.35 ± 0.92 | ||
| 8 months | 3.85 ± 1.44 | 4.09 ± 2.10 | |||
| Δ-change | −0.08 | 0.74 | 0.061 | 0.41 | |
| Δ%-change | −1.9 | 22.0 | |||
| Groups | Cohen’s d Effect Size | ||||
|---|---|---|---|---|---|
| Intervention | Control | p-Value | |||
| Mean ± Stand. Dev. | |||||
| Night-time sleep (hours) | beginning | 6.36 ± 1.84 | 6.50 ± 1.58 | ||
| 8 months | 7.10 ± 1.38 | 6.03 ± 1.72 | |||
| Δ-change | 0.74 | −0.47 | <0.001 | 0.91 | |
| Δ%-change | 11.6 | −7.3 | |||
| Moderate-intensity activities in the last 7 days (days) | beginning | 1.69 ± 2.04 | 1.26 ± 1.98 | ||
| 8 months | 2.25 ± 2.11 | 2.05 ± 1.99 | |||
| Δ-change | 0.56 | 0.79 | 0.586 | 0.12 | |
| Δ%-change | 33.1 | 62.9 | |||
| Body mass index (kg/m2) | beginning | 31.36 ± 4.97 | 30.60 ± 5.49 | ||
| 8 months | 30.89 ± 5.00 | 30.30 ± 5.22 | |||
| Δ-change | −0.47 | −0.30 | 0.463 | 0.16 | |
| Δ%-change | −1.5 | −1.0 | |||
| Waist circumference (cm) | beginning | 108.64 ± 12.01 | 108.39 ± 10.82 | ||
| 8 months | 107.76 ± 13.82 | 107.94 ± 10.54 | |||
| Δ-change | −0.88 | −0.46 | 0.704 | 0.08 | |
| Δ%-change | −0.8 | −0.4 | |||
| Forced expiratory volume in the first second, FEV1 (L) | beginning | 2.18 ± 0.72 | 2.08 ± 0.61 | ||
| 8 months | 2.30 ± 0.69 | 2.04 ± 0.61 | |||
| Δ-change | 0.12 | −0.03 | 0.002 | 0.70 | |
| Δ%-change | 5.6 | −1.5 | |||
| Forced expiratory volume in the sixth second, FEV6 (L) | beginning | 2.64 ± 0.84 | 2.52 ± 0.73 | ||
| 8 months | 2.91 ± 0.89 | 2.62 ± 0.82 | |||
| Δ-change | 0.27 | 0.10 | 0.055 | 0.42 | |
| Δ%-change | 10.1 | 4.1 | |||
| Systolic blood pressure (mmHg) | beginning | 130.83 ± 14.08 | 129.85 ± 16.84 | ||
| 8 months | 124.29 ± 13.66 | 129.61 ± 16.58 | |||
| Δ-change | −6.55 | −0.24 | 0.029 | 0.47 | |
| Δ%-change | −5.0 | −0.2 | |||
| Diastolic blood pressure (mmHg) | beginning | 75.40 ± 8.97 | 75.76 ± 10.41 | ||
| 8 months | 77.14 ± 8.91 | 76.87 ± 8.84 | |||
| Δ-change | 1.74 | 1.11 | 0.741 | 0.07 | |
| Δ%-change | 2.3 | 1.5 | |||
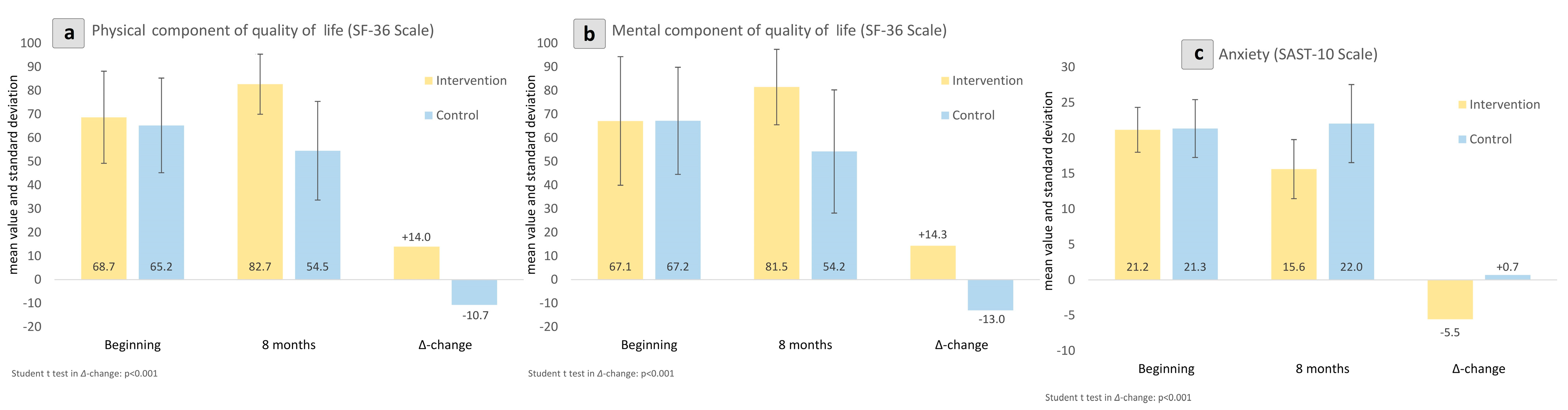
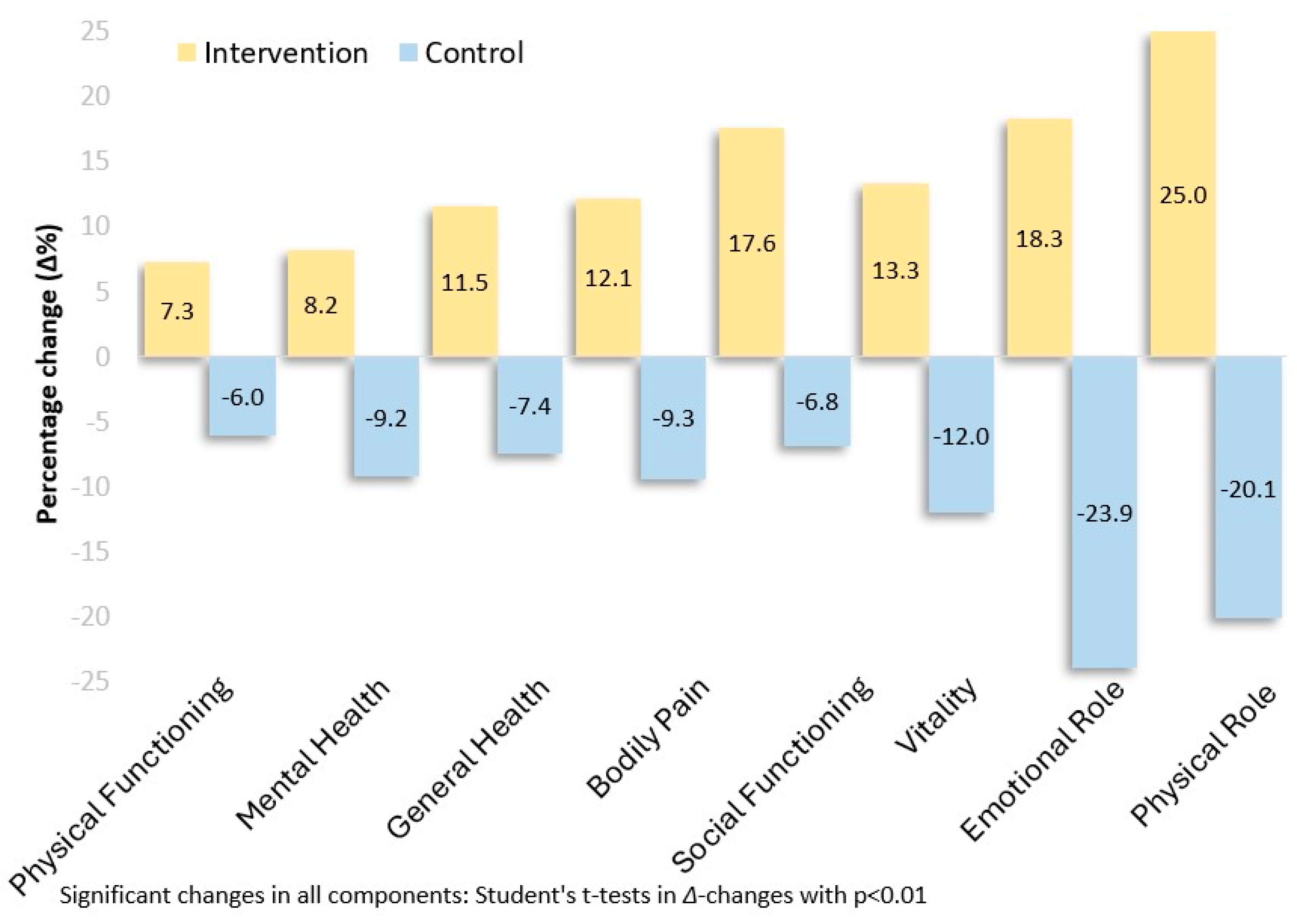
| Physical component of quality of life (SF-36 Scale) a | beginning | 68.69 ± 19.48 | 65.24 ± 20.02 | ||
| 8 months | 82.68 ± 12.72 | 54.54 ± 20.88 | |||
| Δ-change | 13.99 | −10.71 | <0.001 | 1.58 | |
| Δ%-change | 20.4 | −16.4 | |||
| Mental component of quality of life (SF-36 Scale) a | beginning | 67.13 ± 27.20 | 67.19 ± 22.66 | ||
| 8 months | 81.47 ± 15.97 | 54.22 ± 26.05 | |||
| Δ-change | 14.33 | −12.97 | <0.001 | 1.46 | |
| Δ%-change | 21.4 | −19.3 | |||
| Anxiety (SAST-10 Scale) b | beginning | 21.17 ± 3.16 | 21.35 ± 4.08 | ||
| 8 months | 15.62 ± 4.16 | 22.04 ± 5.51 | |||
| Δ-change | −5.55 | 0.70 | <0.001 | 1.40 | |
| Δ%-change | −26.2 | 3.3 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantadaki, A.E.; Linardakis, M.; Tsakiri, M.; Baliou, S.; Fragkiadaki, P.; Vakonaki, E.; Tzatzarakis, M.N.; Tsatsakis, A.; Symvoulakis, E.K. Benefits of Quercetin on Glycated Hemoglobin, Blood Pressure, PiKo-6 Readings, Night-Time Sleep, Anxiety, and Quality of Life in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. J. Clin. Med. 2024, 13, 3504. https://doi.org/10.3390/jcm13123504
Mantadaki AE, Linardakis M, Tsakiri M, Baliou S, Fragkiadaki P, Vakonaki E, Tzatzarakis MN, Tsatsakis A, Symvoulakis EK. Benefits of Quercetin on Glycated Hemoglobin, Blood Pressure, PiKo-6 Readings, Night-Time Sleep, Anxiety, and Quality of Life in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Journal of Clinical Medicine. 2024; 13(12):3504. https://doi.org/10.3390/jcm13123504
Chicago/Turabian StyleMantadaki, Aikaterini E., Manolis Linardakis, Maria Tsakiri, Stella Baliou, Persefoni Fragkiadaki, Elena Vakonaki, Manolis N. Tzatzarakis, Aristidis Tsatsakis, and Emmanouil K. Symvoulakis. 2024. "Benefits of Quercetin on Glycated Hemoglobin, Blood Pressure, PiKo-6 Readings, Night-Time Sleep, Anxiety, and Quality of Life in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial" Journal of Clinical Medicine 13, no. 12: 3504. https://doi.org/10.3390/jcm13123504
APA StyleMantadaki, A. E., Linardakis, M., Tsakiri, M., Baliou, S., Fragkiadaki, P., Vakonaki, E., Tzatzarakis, M. N., Tsatsakis, A., & Symvoulakis, E. K. (2024). Benefits of Quercetin on Glycated Hemoglobin, Blood Pressure, PiKo-6 Readings, Night-Time Sleep, Anxiety, and Quality of Life in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Journal of Clinical Medicine, 13(12), 3504. https://doi.org/10.3390/jcm13123504







