Atrial High-Rate Episodes in Elderly Patients: The Anticoagulation Therapy Dilemma
Abstract
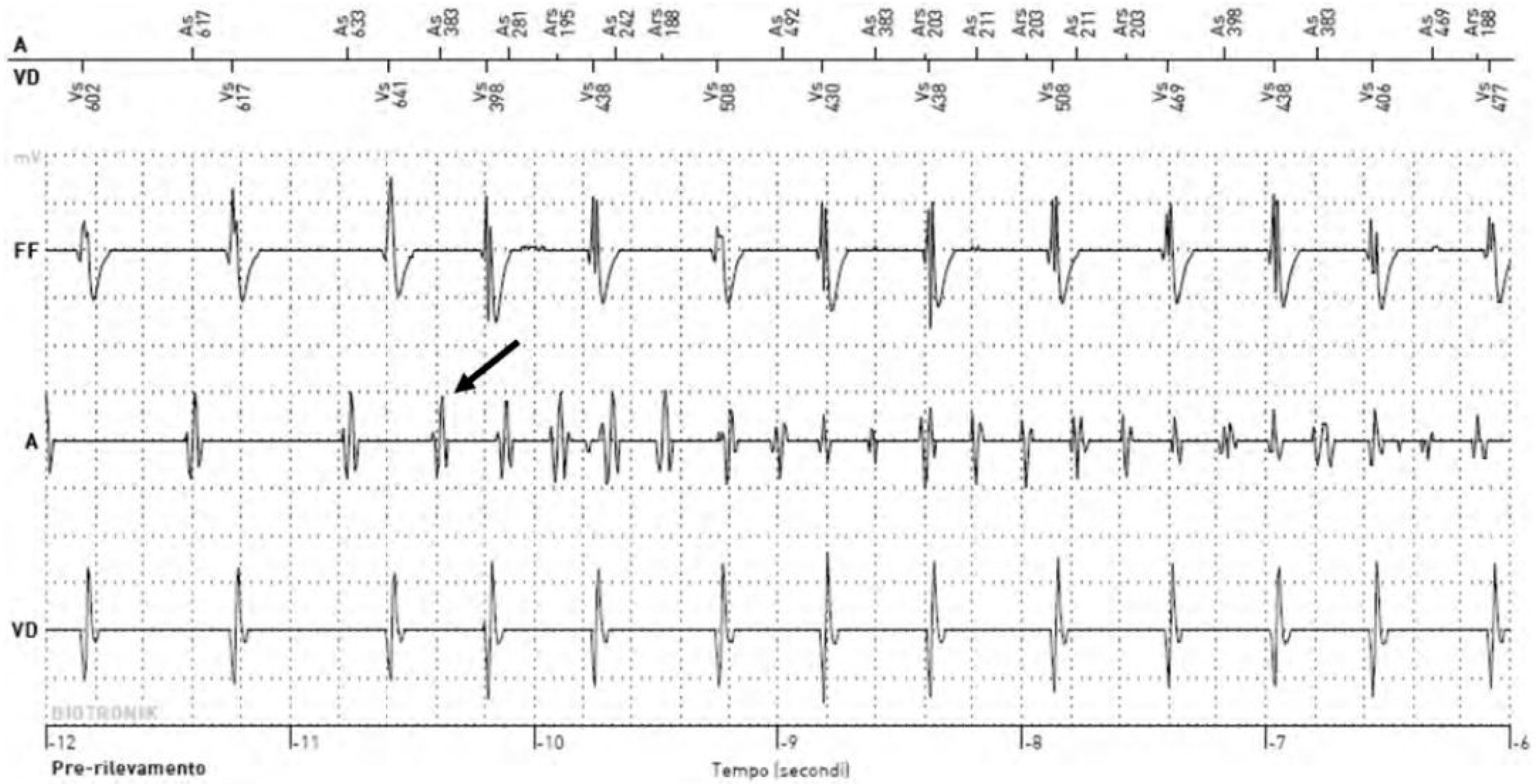
:1. Introduction
2. What Is the Appropriate Threshold for Detecting Atrial High-Rate Episodes?
3. Managing Subclinical Atrial Fibrillation in High-Risk Populations: Current Indications
4. Do We Need New Scores for a Better Prediction of Stroke and Systemic Embolism in Elderly Patients with Subclinical Atrial Fibrillation beyond CHA2DS2-VASc Score?
4.1. The Impact of Comorbidities Not Considered in the CHA2DS2-VASc Score on the Risk of Stroke
4.2. The Role of Frailty in Tailoring New Risk Scores for Elderly Individuals
5. Anticoagulation Therapy in the Elderly Patients with Atrial High-Rate Episodes Detected on Cardiac Implanted Electronic Devices: A Critical Issue
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Ruddox, V.; Sandven, I.; Munkhaugen, J.; Skattebu, J.; Edvardsen, T.; Otterstad, J.E. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2017, 24, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Wolf, P.A.; D’Agostino, R.B.; Silbershatz, H.; Kannel, W.B.; Levy, D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation 1998, 98, 946–952. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Champagne, J.; Sapp, J.; Essebag, V.; Novak, P.; Skanes, A.; Morillo, C.A.; Khaykin, Y.; Birnie, D. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): A prospective, multicenter study. JAMA Intern. Med. 2013, 173, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Migdady, I.; Russman, A.; Buletko, A.B. Atrial Fibrillation and Ischemic Stroke: A Clinical Review. Semin. Neurol. 2021, 41, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef] [PubMed]
- Glotzer, T.V.; Hellkamp, A.S.; Zimmerman, J.; Sweeney, M.O.; Yee, R.; Marinchak, R.; Cook, J.; Paraschos, A.; Love, J.; Radoslovich, G.; et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: Report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003, 107, 1614–1619. [Google Scholar] [CrossRef]
- Halcox, J.P.J.; Wareham, K.; Cardew, A.; Gilmore, M.; Barry, J.P.; Phillips, C.; Gravenor, M.B. Assessment of Remote Heart Rhythm Sampling Using the AliveCor Heart Monitor to Screen for Atrial Fibrillation: The REHEARSE-AF Study. Circulation 2017, 136, 1784–1794. [Google Scholar] [CrossRef]
- Proietti, M.; Romiti, G.F.; Vitolo, M.; Borgi, M.; Rocco, A.D.; Farcomeni, A.; Miyazawa, K.; Healey, J.S.; Lane, D.A.; Boriani, G.; et al. Epidemiology of subclinical atrial fibrillation in patients with cardiac implantable electronic devices: A systematic review and meta-regression. Eur. J. Intern. Med. 2022, 103, 84–94. [Google Scholar] [CrossRef]
- Healey, J.S.; Connolly, S.J.; Gold, M.R.; Israel, C.W.; Van Gelder, I.C.; Capucci, A.; Lau, C.P.; Fain, E.; Yang, S.; Bailleul, C.; et al. Subclinical atrial fibrillation and the risk of stroke. N. Engl. J. Med. 2012, 366, 120–129. [Google Scholar] [CrossRef]
- Sagris, D.; Georgiopoulos, G.; Pateras, K.; Perlepe, K.; Korompoki, E.; Milionis, H.; Tsiachris, D.; Chan, C.; Lip, G.Y.H.; Ntaios, G. Atrial High-Rate Episode Duration Thresholds and Thromboembolic Risk: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e022487. [Google Scholar] [CrossRef] [PubMed]
- Simu, G.; Rosu, R.; Cismaru, G.; Puiu, M.; Gusetu, G.; Minciuna, I.; Istratoaie, S.; Tomoaia, R.; Zdrenghea, D.; Pop, D. Atrial high-rate episodes: A comprehensive review. Cardiovasc. J. Afr. 2021, 32, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Singer, D.E.; Ziegler, P.D.; Koehler, J.L.; Sarkar, S.; Passman, R.S. Temporal Association Between Episodes of Atrial Fibrillation and Risk of Ischemic Stroke. JAMA Cardiol. 2021, 6, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.S.; Alings, M.; Ha, A.; Leong-Sit, P.; Birnie, D.H.; de Graaf, J.J.; Freericks, M.; Verma, A.; Wang, J.; Leong, D.; et al. Subclinical Atrial Fibrillation in Older Patients. Circulation 2017, 136, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, J.H.; Diederichsen, S.Z.; Hojberg, S.; Krieger, D.W.; Graff, C.; Kronborg, C.; Olesen, M.S.; Nielsen, J.B.; Holst, A.G.; Brandes, A.; et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): A randomised controlled trial. Lancet 2021, 398, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, S.A.; Atlas, S.J.; Ashburner, J.M.; Lipsanopoulos, A.T.T.; Borowsky, L.H.; Guan, W.; Khurshid, S.; Ellinor, P.T.; Chang, Y.; McManus, D.D.; et al. Screening for Atrial Fibrillation in Older Adults at Primary Care Visits: VITAL-AF Randomized Controlled Trial. Circulation 2022, 145, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, D.J.; Wachter, R.; Schmalstieg-Bahr, K.; Quinn, F.R.; Hummers, E.; Ivers, N.; Marsden, T.; Thornton, A.; Djuric, A.; Suerbaum, J.; et al. Screening for Atrial Fibrillation in the Older Population: A Randomized Clinical Trial. JAMA Cardiol. 2021, 6, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Svennberg, E.; Friberg, L.; Frykman, V.; Al-Khalili, F.; Engdahl, J.; Rosenqvist, M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): A multicentre, parallel group, unmasked, randomised controlled trial. Lancet 2021, 398, 1498–1506. [Google Scholar] [CrossRef]
- Soi, V.; Yee, J. Sodium Homeostasis in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2017, 24, 325–331. [Google Scholar] [CrossRef]
- Schweda, F. Salt feedback on the renin-angiotensin-aldosterone system. Pflugers Arch. 2015, 467, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Cheng, J.; Li, N.; Zhang, Z.; Nie, J.; Peng, Y.; Wang, Y.; Wang, D.W.; Zhou, N. Obesity and atrial fibrillation: A narrative review from arrhythmogenic mechanisms to clinical significance. Cardiovasc. Diabetol. 2023, 22, 192. [Google Scholar] [CrossRef]
- Ma, L.Z.; Sun, F.R.; Wang, Z.T.; Tan, L.; Hou, X.H.; Ou, Y.N.; Dong, Q.; Yu, J.T.; Tan, L. Metabolically healthy obesity and risk of stroke: A meta-analysis of prospective cohort studies. Ann. Transl. Med. 2021, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Horn, J.W.; Feng, T.; Morkedal, B.; Strand, L.B.; Horn, J.; Mukamal, K.; Janszky, I. Obesity and Risk for First Ischemic Stroke Depends on Metabolic Syndrome: The HUNT Study. Stroke 2021, 52, 3555–3561. [Google Scholar] [CrossRef]
- Babitt, J.L.; Lin, H.Y. Mechanisms of anemia in CKD. J. Am. Soc. Nephrol. 2012, 23, 1631–1634. [Google Scholar] [CrossRef]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Nattel, S. Implications of Inflammation and Fibrosis in Atrial Fibrillation Pathophysiology. Card. Electrophysiol. Clin. 2021, 13, 25–35. [Google Scholar] [CrossRef]
- Andrade, J.; Khairy, P.; Dobrev, D.; Nattel, S. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 2014, 114, 1453–1468. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, F.; Tajiri, K.; Yumino, D.; Ieda, M. Uremic Toxins and Atrial Fibrillation: Mechanisms and Therapeutic Implications. Toxins 2019, 11, 597. [Google Scholar] [CrossRef]
- Kelly, D.M.; Georgakis, M.K.; Franceschini, N.; Blacker, D.; Viswanathan, A.; Anderson, C.D. Interplay Between Chronic Kidney Disease, Hypertension, and Stroke: Insights From a Multivariable Mendelian Randomization Analysis. Neurology 2023, 101, e1960–e1969. [Google Scholar] [CrossRef]
- Chen, W.; Cai, X.; Yan, H.; Pan, Y. Causal Effect of Obstructive Sleep Apnea on Atrial Fibrillation: A Mendelian Randomization Study. J. Am. Heart Assoc. 2021, 10, e022560. [Google Scholar] [CrossRef] [PubMed]
- Simons, S.O.; Elliott, A.; Sastry, M.; Hendriks, J.M.; Arzt, M.; Rienstra, M.; Kalman, J.M.; Heidbuchel, H.; Nattel, S.; Wesseling, G.; et al. Chronic obstructive pulmonary disease and atrial fibrillation: An interdisciplinary perspective. Eur. Heart J. 2021, 42, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Jehan, S.; Farag, M.; Zizi, F.; Pandi-Perumal, S.R.; Chung, A.; Truong, A.; Jean-Louis, G.; Tello, D.; McFarlane, S.I. Obstructive sleep apnea and stroke. Sleep. Med. Disord. 2018, 2, 120–125. [Google Scholar]
- Linz, D.; Hohl, M.; Vollmar, J.; Ukena, C.; Mahfoud, F.; Bohm, M. Atrial fibrillation and gastroesophageal reflux disease: The cardiogastric interaction. Europace 2017, 19, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Damiris, K.; Suero-Abreu, G.; Xu, B.; Ahlawat, S. Reflux esophagitis is associated with higher risks of acute stroke and transient ischemic attacks in patients hospitalized with atrial fibrillation: A nationwide inpatient sample analysis. Medicine 2021, 100, e26502. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chen, W.; Ye, W. Stroke and the risk of gastrointestinal disorders: A Mendelian randomization study. Front. Neurol. 2023, 14, 1131250. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Zhang, X.; Yu, W.; Yin, G.; Chen, S.; Liu, H.; Wang, L.; Zhang, F. Association between gastroesophageal reflux disease and stroke: A bidirectional Mendelian randomization study. Front. Neurol. 2023, 14, 1295051. [Google Scholar] [CrossRef] [PubMed]
- Patten, M.; Pecha, S.; Aydin, A. Atrial Fibrillation in Hypertrophic Cardiomyopathy: Diagnosis and Considerations for Management. J. Atr. Fibrillation 2018, 10, 1556. [Google Scholar] [CrossRef] [PubMed]
- Vitolo, M.; Proietti, M.; Imberti, J.F.; Bonini, N.; Romiti, G.F.; Mei, D.A.; Malavasi, V.L.; Diemberger, I.; Fauchier, L.; Marin, F.; et al. Factors Associated with Progression of Atrial Fibrillation and Impact on All-Cause Mortality in a Cohort of European Patients. J. Clin. Med. 2023, 12, 768. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, L.; Zhang, L.; Han, Y.; Wang, P.; Yu, S. Left Atrial Enlargement and the Risk of Stroke: A Meta-Analysis of Prospective Cohort Studies. Front. Neurol. 2020, 11, 26. [Google Scholar] [CrossRef]
- van den Ham, H.A.; Klungel, O.H.; Singer, D.E.; Leufkens, H.G.; van Staa, T.P. Comparative Performance of ATRIA, CHADS2, and CHA2DS2-VASc Risk Scores Predicting Stroke in Patients With Atrial Fibrillation: Results From a National Primary Care Database. J. Am. Coll. Cardiol. 2015, 66, 1851–1859. [Google Scholar] [CrossRef]
- Rivera-Caravaca, J.M.; Roldan, V.; Esteve-Pastor, M.A.; Valdes, M.; Vicente, V.; Lip, G.Y.H.; Marin, F. Long-Term Stroke Risk Prediction in Patients With Atrial Fibrillation: Comparison of the ABC-Stroke and CHA(2)DS(2)-VASc Scores. J. Am. Heart Assoc. 2017, 6, e006490. [Google Scholar] [CrossRef]
- Xue, Q.L. The frailty syndrome: Definition and natural history. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef]
- Fried, L.P.; Xue, Q.L.; Cappola, A.R.; Ferrucci, L.; Chaves, P.; Varadhan, R.; Guralnik, J.M.; Leng, S.X.; Semba, R.D.; Walston, J.D.; et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2009, 64, 1049–1057. [Google Scholar] [CrossRef]
- Fried, L.P.; Cohen, A.A.; Xue, Q.L.; Walston, J.; Bandeen-Roche, K.; Varadhan, R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat. Aging 2021, 1, 36–46. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Theou, O.; Squires, E.; Mallery, K.; Lee, J.S.; Fay, S.; Goldstein, J.; Armstrong, J.J.; Rockwood, K. What do we know about frailty in the acute care setting? A scoping review. BMC Geriatr. 2018, 18, 139. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G. Prevalence of Frailty in Nursing Homes: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2015, 16, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Guo, Q.; Du, X.; Ma, C.S. Atrial fibrillation and frailty. J. Geriatr. Cardiol. 2020, 17, 105–109. [Google Scholar] [CrossRef]
- Proietti, M.; Romiti, G.F.; Raparelli, V.; Diemberger, I.; Boriani, G.; Dalla Vecchia, L.A.; Bellelli, G.; Marzetti, E.; Lip, G.Y.; Cesari, M. Frailty prevalence and impact on outcomes in patients with atrial fibrillation: A systematic review and meta-analysis of 1,187,000 patients. Ageing Res. Rev. 2022, 79, 101652. [Google Scholar] [CrossRef] [PubMed]
- Requena Calleja, M.A.; Arenas Miquelez, A.; Diez-Manglano, J.; Gullon, A.; Pose, A.; Formiga, F.; Mostaza, J.M.; Cepeda, J.M.; Suarez, C.; en nombre de los investigadores del estudio NONAVASC; et al. Sarcopenia, frailty, cognitive impairment and mortality in elderly patients with non-valvular atrial fibrillation. Rev. Clin. Esp. 2019, 219, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Perera, V.; Bajorek, B.V.; Matthews, S.; Hilmer, S.N. The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing 2009, 38, 156–162. [Google Scholar] [CrossRef]
- Liu, J.; Chai, K.; Zhu, W.; Du, M.; Meng, C.; Yang, L.; Cui, L.; Guo, D.; Sun, N.; Wang, H.; et al. Implication of different frailty criteria in older people with atrial fibrillation: A prospective cohort study. BMC Geriatr. 2023, 23, 604. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, M.; Holmes, D.N.; Piccini, J.P.; Ansell, J.E.; Fonarow, G.C.; Hylek, E.M.; Kowey, P.R.; Mahaffey, K.W.; Thomas, L.; Peterson, E.D.; et al. Association of frailty and cognitive impairment with benefits of oral anticoagulation in patients with atrial fibrillation. Am. Heart J. 2019, 211, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Romiti, G.F.; Vitolo, M.; Harrison, S.L.; Lane, D.A.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.S.; Dan, G.A.; et al. Epidemiology and impact of frailty in patients with atrial fibrillation in Europe. Age Ageing 2022, 51, afac192. [Google Scholar] [CrossRef]
- Wilkinson, C.; Wu, J.; Searle, S.D.; Todd, O.; Hall, M.; Kunadian, V.; Clegg, A.; Rockwood, K.; Gale, C.P. Clinical outcomes in patients with atrial fibrillation and frailty: Insights from the ENGAGE AF-TIMI 48 trial. BMC Med. 2020, 18, 401. [Google Scholar] [CrossRef] [PubMed]
- Blodgett, J.; Theou, O.; Kirkland, S.; Andreou, P.; Rockwood, K. Frailty in NHANES: Comparing the frailty index and phenotype. Arch. Gerontol. Geriatr. 2015, 60, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Sogaard, M.; Jensen, M.; Hojen, A.A.; Larsen, T.B.; Lip, G.Y.H.; Ording, A.G.; Nielsen, P.B. Net Clinical Benefit of Oral Anticoagulation Among Frail Patients With Atrial Fibrillation: Nationwide Cohort Study. Stroke 2024, 55, 413–422. [Google Scholar] [CrossRef]
- Abellan van Kan, G.; Rolland, Y.; Bergman, H.; Morley, J.E.; Kritchevsky, S.B.; Vellas, B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J. Nutr. Health Aging 2008, 12, 29–37. [Google Scholar] [CrossRef]
- Villani, E.R.; Tummolo, A.M.; Palmer, K.; Gravina, E.M.; Vetrano, D.L.; Bernabei, R.; Onder, G.; Acampora, N. Frailty and atrial fibrillation: A systematic review. Eur. J. Intern. Med. 2018, 56, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.S.; Lopes, R.D.; Granger, C.B.; Alings, M.; Rivard, L.; McIntyre, W.F.; Atar, D.; Birnie, D.H.; Boriani, G.; Camm, A.J.; et al. Apixaban for Stroke Prevention in Subclinical Atrial Fibrillation. N. Engl. J. Med. 2023, 390, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Toennis, T.; Goette, A.; Camm, A.J.; Diener, H.C.; Becher, N.; Bertaglia, E.; Blomstrom Lundqvist, C.; Borlich, M.; Brandes, A.; et al. Anticoagulation with Edoxaban in Patients with Atrial High-Rate Episodes. N. Engl. J. Med. 2023, 389, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Gerra, L.; Mei, D.A.; Bonini, N.; Vitolo, M.; Proietti, M.; Imberti, J.F. Detection of subclinical atrial fibrillation with cardiac implanted electronic devices: What decision making on anticoagulation after the NOAH and ARTESiA trials? Eur. J. Intern. Med. 2024, 123, 37–41. [Google Scholar] [CrossRef]
- McIntyre, W.F.; Benz, A.P.; Becher, N.; Healey, J.S.; Granger, C.B.; Rivard, L.; Camm, A.J.; Goette, A.; Zapf, A.; Alings, M.; et al. Direct Oral Anticoagulants for Stroke Prevention in Patients With Device-Detected Atrial Fibrillation: A Study-Level Meta-Analysis of the NOAH-AFNET 6 and ARTESiA Trials. Circulation 2024, 149, 981–988. [Google Scholar] [CrossRef]
- Aggarwal, R.; Ruff, C.T.; Virdone, S.; Perreault, S.; Kakkar, A.K.; Palazzolo, M.G.; Dorais, M.; Kayani, G.; Singer, D.E.; Secemsky, E.; et al. Development and Validation of the DOAC Score: A Novel Bleeding Risk Prediction Tool for Patients With Atrial Fibrillation on Direct-Acting Oral Anticoagulants. Circulation 2023, 148, 936–946. [Google Scholar] [CrossRef]
| Study and Year | Mean Age | Follow-Up | CHADSVASC Score (Mean) | Clinical Profile of Patients | Incidence of AF |
|---|---|---|---|---|---|
| RATE Registry, 2016 | 73.6 ± 11.8 for PPMs, 64.5 ± 12.6 for ICDs | 22.9 months (median) | 1.8 ± 1.0 for PPM 2.0 ± 0.8 for ICDs | All no permanent AF | 45/300 (48%) of PPM patients and 155/300 (52%) of ICD patients of the representative samples studied |
| Healey et al., 2013 | 71.7 ± 14.4 for no AHRE 74.3 ± 13.7 for AHRE detected | Single center retrospective | 2.02 ± 1.30 for no AHRE 2.23 ± 1.47 for AHRE detected | All | 55.3% (246/445) |
| ASSERT, 2012 | 76 ± 7 for no AHRE 77 ± 7 for AHRE detected | 2.5 years | 2.3 ± 1.0 for no AHRE 2.2 ± 1.1 for AHRE detected | History of hypertension, no history of AF, no OAC use | 34.7% (895/2580) |
| TRENDS, 2010 | 72.8 ± 9.9 for no AHRE 74.0 ± 9.1 for AHRE detected | 1.4 years (mean) | 4.1 ± 0.8 for no AHRE 4.2 ± 0.8 for AHRE detected | History of prior stroke, no history of AF, no OAC use, >1 stroke risk factor | 28% (45/163) |
| BEATS, 2006 | 12 months, prospective | All | 54% (137/254) | ||
| MOST, 2003 | Median 73 (68.81) for no AHRE Median 75 (68.79) for AHRE detected | 27 months (median) | NO | All | 50% (156/312) |
| Gillis et al., 2002 | 70 ± 12 | 718 ± 383 days | NA | All | 68% (157/231) |
| Trial and Year | Number of Patients | Follow-Up Duration | AF Burden Threshold | Hazard Ratio for TE Event | TE Event Rate (Below vs. Above AF Burden Threshold |
|---|---|---|---|---|---|
| RATE Registry, 2016 | 5379 (3141 with pacemakers and 2238 with ICDs) | 22.9 months (median) | Nonsustained atrial high-rate episodes with a duration from 3 atrial premature complexes to 15–20 s | 0.87 (p = 0.51) | For nonsustained atrial high-rate episodes: 0.55% (0.34–0.76%) per year for pacemakers and 0.81% (0.50–1.12%) per year for ICDs |
| SOS-AF, 2014 | 10,016 | 2 years (median) | 1 h | 2.11 (p = 0.008) | 0.39% per year overall |
| ASSERT, 2012 | 2580 | 2.5 years (mean) | 6 min | 2.5 (p = 0.007) | (0.69% vs. 1.69%) |
| Home Monitor CRT, 2012 | 560 | 370 days (median) | 3.8 h | 9.4 (p = 0.006) | 2.0% overall |
| TRENDS, 2009 | 2486 | 1.4 years (mean) | 5.5 h | 2.2 (p = 0.060) | 1.2% overall (1.1% vs. 2.4%) |
| Italian AT500 Registry, 2005 | 725 | 22 months (median) | 24 h | 3.1 (p = 0.044) | 1.2% annual rate |
| Ancillary MOST, 2003 | 312 | 27 months (median) | 5 min | 6.7 (p = 0.020) | 3.2% overall (1.3% vs. 5%) |
| Risk Factors | ATRIA | CHADS-VASC |
|---|---|---|
| Age 65–74 y | 3 | 1 |
| Age ≥ 75 y | 5 | 2 |
| Age ≥ 85 y | 6 | |
| Hypertension | 1 | |
| Female sex | 1 | 1 |
| Diabetes | 1 | 1 |
| Renal disease | 1 | |
| Current smoking | ||
| Congestive heart failure | 1 | 1 |
| Vascular disease | 1 | |
| Previous stroke or TIA | 2 (Age 75–84 y) 3 (Age > 85 y) 4 (Age 65–74 y) 8 (Age < 65 y) | 2 |
| Dementia | ||
| Previous bleeding | ||
| Proteinuria | 1 | |
| Low risk score | 0–5 | 0 |
| Intermediate risk score | 6 | 1 |
| High risk score | 7–15 | ≥2 |
| C-index | 0.66 | 0.63 |
| C-index | - | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimpini, L.; Biscetti, L.; Matacchione, G.; Giammarchi, C.; Barbieri, M.; Antonicelli, R. Atrial High-Rate Episodes in Elderly Patients: The Anticoagulation Therapy Dilemma. J. Clin. Med. 2024, 13, 3566. https://doi.org/10.3390/jcm13123566
Pimpini L, Biscetti L, Matacchione G, Giammarchi C, Barbieri M, Antonicelli R. Atrial High-Rate Episodes in Elderly Patients: The Anticoagulation Therapy Dilemma. Journal of Clinical Medicine. 2024; 13(12):3566. https://doi.org/10.3390/jcm13123566
Chicago/Turabian StylePimpini, Lorenzo, Leonardo Biscetti, Giulia Matacchione, Cinzia Giammarchi, Michelangela Barbieri, and Roberto Antonicelli. 2024. "Atrial High-Rate Episodes in Elderly Patients: The Anticoagulation Therapy Dilemma" Journal of Clinical Medicine 13, no. 12: 3566. https://doi.org/10.3390/jcm13123566
APA StylePimpini, L., Biscetti, L., Matacchione, G., Giammarchi, C., Barbieri, M., & Antonicelli, R. (2024). Atrial High-Rate Episodes in Elderly Patients: The Anticoagulation Therapy Dilemma. Journal of Clinical Medicine, 13(12), 3566. https://doi.org/10.3390/jcm13123566






