Abstract
Background/Objectives: In oral and maxillofacial surgery, the reconstruction of defects often involves the transfer of skin tissue into the oral cavity utilizing microvascular grafts. This study investigates postoperative changes in microbial colonization following intraoral microvascular transplantation, as well as potential influencing factors. Methods: In 37 patients undergoing intraoral reconstructions, pre- and postoperative swabs were taken from the donor and recipient regions to quantify the seven selected marker bacteria using TaqMan PCRs. Patient-specific factors and clinical data were also recorded. Results: The infection-associated Acinetobacter baumannii tended to decrease postoperatively, while the infectious pathogens Pseudomonas aeruginosa, Enterococcus faecalis and the family of Enterobacteriaceae showed a postoperative increase without being directly associated with a clinical infection. Streptococcus mitis showed a significant postoperative decrease on buccal mucosa and increase on the graft surface (oral dysbiosis) and was significantly reduced or displaced by other bacteria (e.g., Mycoplasma salivarium, positive selection) when treated with ampicillin/sulbactam. Conclusions: The cutaneous microbiome of the graft adapts to the local intraoral environment. Postoperative shifts in oral bacterial colonization and an increase in infection-relevant bacteria were observed. These perioperative changes in colonization are also influenced by the administration of ampicillin/sulbactam. Consequently, single doses of antibiotics appear to be more beneficial compared to longer-term preventive use.
1. Introduction
In oral and maxillofacial surgery, extensive tissue defects are often associated with severe loss of function and have esthetically disfiguring and stigmatizing effects [1,2,3,4,5,6]. If primary wound closure is not possible, reconstruction using microvascular grafts is the gold standard [3,4,7,8,9]. The anterolateral thigh (ALT), radial forearm, and latissimus dorsi muscle or myocutaneous flaps are the most commonly used donor sites [10].
Alternative flap donor sites such as the lateral arm or the medial sural artery perforator (MSAP) flap are also being used more and more frequently. They offer a better color match and less morbidity at the donor site and can be an alternative in cases of previous flap loss, recurrence or the need for multiple free flaps [11,12,13]. If bone reconstruction is required in addition to soft tissue reconstruction, virtual surgical planning (VSP) is used for complex cases [14,15,16,17]. The perioperative changes in microbial colonization of the surgical site and the oral cavity following intraoral microvascular grafts have been described to a limited extent in the literature. It has been demonstrated that an altered nutrient availability, oxygen concentration, host response or an altered pH value can lead to dysbiosis due to the proliferation of opportunistic bacteria [18,19]. It seems obvious that highly invasive procedures such as intraoral microvascular reconstructions lead to a distinct change in the oral milieu. Other factors that also indicate a disruption in the homeostatic balance between the host and the oral microflora include the following: difficult oral hygiene due to swelling or pain [20], long-term hospitalization [21], the use of antimicrobial mouth rinses [22,23], postoperative antibiotics [24], potential postoperative xerostomia [25], and the transfer of extraoral tissue into the oral cavity [26]. Durand and Eckert et al. were able to show that wound infections following oral microvascular transplantation are often triggered by pathogens that are not commonly part of the healthy oral flora [27,28]. The extent to which foreign pathogens can colonize and multiply in the oral cavity under certain conditions is currently being investigated [29,30,31]. The following factors have already been identified as possible influences on intraoral microbial colonization and the development of oral dysbiosis: long retention times (increased E. faecalis and E. faecium, as well as various representatives of the Enterobacteriaceae) [21]), nosocomial infections (increased S. aureus, A. baumannii, P. aeruginosa and various representatives of the Enterobacteriaceae) [32,33,34], intraoral reconstruction [27,28,35,36,37], and the severity of the disease (pharyngeal colonization of Gram-negative bacilli) [38].
Intraoral tumor diseases [39,40,41,42,43] and their surgical resection [44] have a significant impact on oral microbial colonization. Chan et al. (2021) investigated the postoperative long-term changes in the oral microbiome using next-generation sequencing. They observed a postoperative decrease (6 months) in some periodontopathogenic genera such as Fusobacterium, Capnocytophaga, Pophyromonas, Leptotrichia, Aggregatibacter and Treponema, while the commensals of the oral flora Streptococcus and Rothia increased. In addition, the authors showed a correlation between the postoperative shifts in the oral microflora and the patient-specific prognosis: the 3-year survival rate improved with a reduced relative frequency of the periodontopathogenic genera Capnocytophaga, Prevotella and Leptotrichia and with an increased relative frequency of the two commensal genera Streptococcus and Rothia six months postoperatively [44]. Dental and periodontal health also have an impact on the development of postoperative infections in the oral cavity. Sato et al. showed that professional dental cleaning and oral hygiene instructions significantly reduced the risk of wound infections after excision of oral squamous cell carcinoma [45]. Usubuchi et al. demonstrated that preoperative dental treatment (scaling, treatment of deep carious lesions and severe periodontitis) reduced the risk of postoperative infection after microvascular grafts in the head and neck region [46]. In addition, the activity of the immune system appears to change after extensive oral surgery. This was shown by Heimlich et al. with a significant drop in the total lymphocyte count, as well as CD4+ and CD8+ T lymphocytes after long operations lasting 7 h or more [47]. Kageyama et al. (2020) showed an influence of the surface composition of the oral epithelia on microbial colonization. They hypothesized a connection between the composition of the salivary microbiome and a postoperatively reduced tongue surface area. The authors used 16S rRNA gene sequencing and qPCR analysis to investigate the microbiome of stimulated saliva samples from patients with tumor resections in the tongue area. The analysis showed a significant postoperative decrease in microorganisms that are the predominant inhabitants of the dorsum of the tongue (S. salivarius, P. melaninogenica, P. histicola and Actinomyces spp.). In contrast, dental plaque bacteria such as L. mirabilis, N. flava, S. sanguinis and F. nucleatum increased significantly. This reflected a shift in the salivary microbiome in favor of plaque-associated bacteria (resident inhabitants of the oral flora) as part of the reduction in the tongue surface caused by the tumor resection. Only two of the patients included in the study underwent defect reconstruction by means of microvascular transplantation. The authors pointed out the need for further research in larger sample sizes in order to investigate the effect of microvascular grafts on oral microbial colonization, as an influence of the epithelial characteristics of the outer skin, which differ from the oral mucosa, on microbial colonization is obvious [48].
The aim of this study was to detect perioperative microbiological changes in patients following intraoral reconstructions using microvascular grafts. Other factors influencing microbial colonization, such as postoperative xerostomia, antibiotics administered, and the state of oral hygiene and oral health were also evaluated.
2. Materials and Methods
2.1. Study Population and Samples
Patients from the Department of Oral and Maxillofacial Plastic Surgery at the University Hospital Düsseldorf were included in the study if surgery was indicated for intraoral tissue defect reconstruction using microvascular transplantation. This study was approved by the local ethics committee at the University of Düsseldorf, Germany (Approval number 2021-1342). Alongside demographic data (age, gender, body mass index, and tobacco consumption), clinical parameters (such as laboratory tests and ASA classification) and patient-specific factors (including defect type, defect localization, type of graft, perioperative body temperature, and administration of antibiotics) were recorded. Preoperative swabs were collected from the cheek (W0) and the extraoral donor region (T0), while postoperative swabs were taken 1–3 days (cheek (W1), graft (T1), and intraoral suture (N1)) and 6–9 days (cheek (W2), graft (T2), and intraoral suture (N2)) after surgery. Cheek swabs were consistently obtained from the intraoral buccal mucosa on the contralateral side of the defect. The preoperative cheek swab (W0) served as a reference sample.
2.2. Genomic DNA Preparation and TaqMan Polymerase Chain Reaction (PCR)
Material attached to the swabs was resuspended by vortexing in 200 μL G2 storage buffer. The suspension was supplemented with 12.5 μL Proteinase K solution (1 mg/mL Proteinase K) and incubated for 30 min at 56 °C. (G2 storage buffer and Proteinase K were sourced from EZ1 DNA Tissue Kits, QIAGEN GmbH, Hilden, Germany). Total genomic DNA isolation was performed by a semiautomatic DNA preparation using BioRobot EZ1 and EZ1 DNA Tissue Kit according to the manufacturer’s instructions (protocol “Bact_200 µL”) with an elution volume of 50 μL. The eluate was stored at −20 °C until further use.
In-house TaqMan PCRs to quantify the selected pathogens, total eubacterial load and human GAPDH were carried out in a total volume of 25 μL consisting of 2× Takyon™ No Rox Probe MasterMix UNG (Eurogentec, Seraing, Belgium; containing Takyon™ DNA polymerase, 5.5 mM MgCl2, dNTPs (including dUTP), uracil-N-glycosylase and stabilizers), 300 nM each forward and reverse primer, 200 nM labeled probe, and 2.5 μL of template DNA. Amplicon-carrying plasmids were used as quantification standards in concentrations of 107, 105 and 104 copies/μL for detection of the total bacterial load and Enterobacteriaceae and 107, 105 and 102 copies/μL for the other bacterial species investigated and human GAPDH. Thermal cycling conditions were as follows: 1 cycle at 95 °C for 10min followed by 45 cycles at 95 °C for 15 s, and 60 °C for 1 min. CFX96 Real-Time Systems (BioRad, Shinagawa City, Tokyo, Japan) devices were used for all TaqMan PCRs performed. Data were analyzed using the BioRad CFX Manager 3.1 software.
2.3. Data Normalization
After TaqMan PCR analysis, the detected copies/PCR were converted into the number of genome equivalents (GE) per sample. To further normalize the results, numbers of total eubacterial loads and human cells removed from the swab were determined. The results were normalized by dividing the respective genome equivalents per sample by the corresponding total eubacterial load or human GAPDH. Thus, three normalized values were available for each species-specific PCR: the genome equivalents of the respective species (1) per sample, (2) per total eubacterial load and (3) per human GAPDH. A result was considered statistically significant in this study if two of the three data sets yielded statistically significant results.
2.4. Statistical Analysis
The minimum required sample size was determined using the G*Power 3.1 software. An analysis of variance (ANOVA) was used as a statistical test for the sample calculation for linked samples, as it was initially unclear whether the measured values were normally distributed. With an estimated effect size of 0.29, a test power of 95% and a significance level of 5%, the minimum number of study participants was 33. The statistical calculations were carried out using SPSS software version 28.0.0.0. A normal distribution of the measured values was tested using the Shapiro–Wilk test [49]. The Wilcoxon test was used for the comparative analysis of two metric measurements [50] The Friedman test was used for comparative analyses of more than two metric measurements [51]. The Spearman correlation was used for correlation analyses of ordinal-scale indices with metric measurement data (microbial colonization) [52]. The Mann–Whitney U test was used for the comparative analysis of two independent samples [52]. The Kruskal–Wallis test was used for comparative analyses of more than two independent samples [53].
3. Results
The 37 patients included in the study were characterized according to the following parameters (Table 1):

Table 1.
Overview of patient- and operation-specific factors.
3.1. Antibiotics
On the day of surgery, all study patients received an antibiotic in the form of intraoperative “single-shot” antibiotics, with n = 29 patients (78.4%) receiving intraoperative antibiotics with ampicillin/sulbactam and n = 8 patients (21.6%) with clindamycin due to penicillin allergy. Pre- and postoperative antibiotic medications varied.
Six patients received a single dose of 2 g ceftriaxone or 500 mg ciprofloxacin between the sixth and second preoperative day immediately prior to percutaneous endoscopic gastrostomy (PEG). Postoperatively, n = 15 patients (40.5%) received no further antibiotic treatment during the study period, while n = 15 patients (40.5%) received at least one more day of ampicillin/sulbactam and n = 6 patients (16.2%) received at least one more day of clindamycin. In individual cases, amoxicillin/clavulanic acid or piperacillin/tazobactam were used postoperatively.
3.2. Molecular Genetic Detections
Following TaqMan PCRs, the quantitative detection of the bacteria analyzed per swab was shown based on the data of all 37 study participants. The detected pathogen loads were to be analyzed in relation to a selected reference. As the focus of the comparative analysis was on intraoral perioperative changes in pathogen detection, the preoperative buccal swab W0 was selected as the reference swab. Figure 1 shows the difference between the detected bacterial load per swab and the corresponding reference swab W0 per patient:
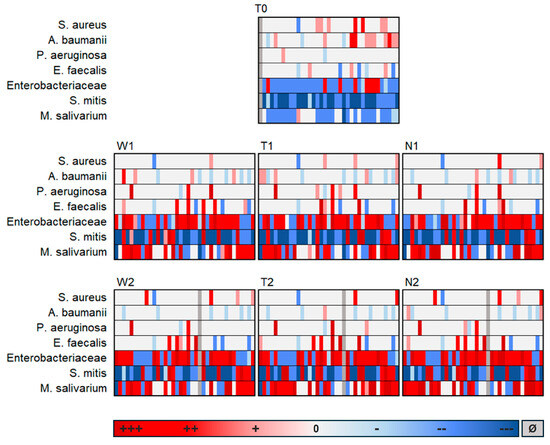
Figure 1.
Difference in the respective load of bacteria detection in relation to the reference swab W0; Shown are the swabs from the cheek (W), graft (T) and suture (N) at the preoperative time point (0), early postoperative time point (1) and late postoperative time point (2), +++ = Highly increased bacterial load (Δ [X] − [W0] > 1 × 106 GE/sample); ++ = Moderately increased bacterial load (Δ [X] − [W0] > 1 × 103 and < 1 × 106 GE/sample); + = Slightly increased bacterial load (Δ [X] − [W0] > 0 and < 1 × 103 GE/sample); 0 = No positive evidence; - = Slightly reduced bacterial load (Δ [X] − [W0] > −1 × 103 and <0 to GE/sample), -- = Moderately reduced bacterial load (Δ [X] − [W0] > −1 × 106 and < −1 × 103 GE/sample); --- Highly reduced bacterial load (Δ [X] − [W0] > −1 × 106 GE/sample); Ø = No data available.
It was revealed that the bacterial colonization examined on the preoperative graft surface (T0) differed fundamentally from all postoperative swabs, whereas the postoperative swabs (regardless of localization or postoperative time) hardly differed from each other. Therefore, it can be concluded that the composition of the analyzed species on the dermal graft after transfer to the intraoral environment resembled that of the buccal mucosa. This is remarkable considering the different surface characteristics of the oral mucosa and the external skin. Additionally, there appeared to be surprisingly little influence in the opposite direction, meaning hardly any impact of the transplanted external skin on the oral composition of the analyzed species.
It was also evident that S. mitis was reduced in the postoperative course compared to W0, while M. salivarium increased. Enterobacteriaceae, E. faecalis and P. aeruginosa tended to increase postoperatively, while A. baumannii tended to decrease.
Figure 2 shows the exemplary results of S. mitis, M. salivarium, Enterobacteriaceae and A. baumannii over time:
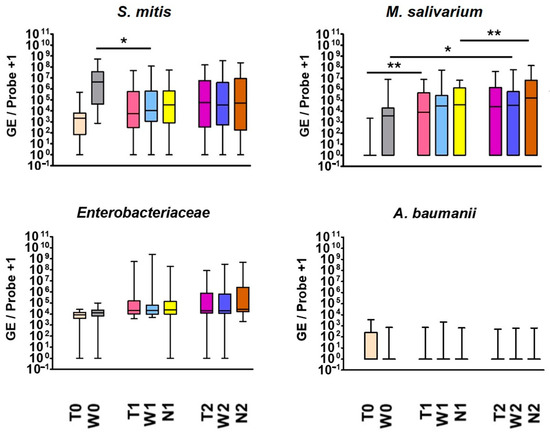
Figure 2.
Exemplary results of quantitative bacterial detections (GE/sample) of S. mitis, M. salivarium, Enterobacteriaceae and A. baumannii; the results of all 37 participants are shown on cheek (W), graft (T) and suture swab (N) at the preoperative time point (0), early postoperative time point (1) and late postoperative time point (2). * p <0.05; ** p <0.01.
Positive detections of S. mitis, M. salivarium and Enterobacteriaceae occurred regularly. A trend towards an increase in Enterobacteriaceae was observed during the perioperative course, while A. baumannii tended to decrease postoperatively, especially on the graft surface.
While M. salivarium was hardly detectable in the (dermal) donor region, it rose significantly on the graft postoperatively (p = 0.005/0.007/0.015). Consequently, the graft was colonized by M. salivarium, which is typically found in the oral cavity, after being transferred intraorally. However, the microorganism also increased in the purely intraorally localized examined regions during this study, which suggests that additional factors such as positive selection by administered beta-lactam antibiotics favored a postoperative increase. On the cheek, the microorganism increased significantly from preoperative to late postoperative time point (p = 0.203/0.024/0.005). Additionally, there was a significant increase on the suture in the postoperative course (p = 0.002/0.412/<0.001).
S. mitis exhibited a gradual increase on the graft surface until the colonization by this typical oral microorganism ultimately matched that of the cheek mucosa. The microorganism behaved oppositely when considering cheek swabs separately: It significantly decreased from preoperative to postoperative time point 1 (p = 0.01/0.034/<0.001). The postoperative reduction in S. mitis, a typical representative of healthy oral flora, may indicate postoperative dysbiosis.
3.3. Culture-Based Detections and Resistances
In addition to the molecular genetic analysis carried out as part of the current study, seven study patients underwent postoperative culture and resistance testing, which was commissioned by the Clinic for Oral and Maxillofacial Plastic Surgery for diagnostic purposes. The following table (Table 2) shows the detected species and respective resistances:

Table 2.
Species and resistances detected in the cultivation process per patient; the type, localization, and time of sampling per patient are also shown.
Although the samples for cultural detection were all taken extraorally and therefore the localization at no point corresponded to the samples for molecular genetic detection (only taken intraorally postoperatively), the cultural detections largely matched the molecular genetic detections: All positive culture results for K. aerogenes, E. coli, E. faecalis and P. aeruginosa were consistent with the results of the molecular genetic analysis. Only K. oxytoca and E. cloacae detected in the culture were not reliably reproduced in the molecular genetic analysis, which may be either due to the low sensitivity of the Enterobacteriaceae TaqMan PCR or the differing swab location. Positive detections of P. vulgaris were not included in the molecular genetic analysis.
3.4. Wound Complications and Inflammation Parameters
Three patients experienced graft complications in the postoperative course. While the microvascular graft had to be subsequently removed in one patient due to a thromboembolic complication, the graft was retained in two patients despite wound-healing disorders. In one patient, a wound dehiscence developed in the extraoral area of the graft during the postoperative course, which was subsequently covered by local flap plasty. The graft healed without irritation in the intraoral area. Although no signs of infection were clinically recognizable in the surgical area, molecular genetic analysis showed a strong postoperative increase in Enterobacteriaceae, which was present at high levels (>1 × 106 GE/sample) in five of the six postoperative swabs. S. aureus was detected in this patient almost throughout the entire course of the study. The molecular genetic evidence of Enterobacteriaceae and S. aureus corresponded with the results of the culture procedure carried out in parallel, which, in addition to positive evidence of P. vulgaris, also provided evidence of E. coli and S. aureus. All three species exhibited (in some cases multiple) antibiotic resistance (see Table 2).
In another patient, a clinically detectable infection (redness, swelling) developed in the postoperative course in the intraoral suture area. At the molecular genetic level, high levels of infection-relevant Enterobacteriaceae, as well as moderate or high levels of E. faecalis and P. aeruginosa, were detected in the current study. For this patient, no additional culture procedure was conducted.
In the postoperative course, 62% of patients had no fever, while 30% developed mild fever (38.0–38.5 °C) and 9% moderate-to-high fever (>38.5 °C). Mild fever mostly occurred within the first 2 days postoperatively. Moderate or high fever occurred exclusively within the first 2 days. A postoperative increase in body temperature was not statistically significant. Furthermore, no statistically significant correlation was found between the perioperative body temperature and the detection of the bacteria analyzed, nor between febrile temperatures and patient demographics or antibiotics administered.
The following boxplots (Figure 3) show the perioperative leucocyte counts and plasma levels of C-reactive protein for all 37 study participants:
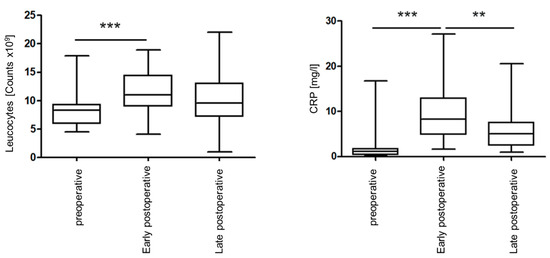
Figure 3.
Perioperative leukocyte counts (left) and perioperative C-reactive protein values (right) of all study participants. ** p <0.01; *** p <0.001.
Both leukocyte count and CRP values were elevated early postoperatively and recovered by the end of the postoperative phase. A statistically significant increase in the leukocyte counts from the preoperative to the early postoperative time point (p < 0.001) was observed with a slighter non-significant decrease later (p = 0.093). CRP values also showed a significant increase early postoperatively (p < 0.001), with a significant decrease later (p = 0.003). No statistically significant correlations were found between the perioperative detection of the microorganisms analyzed and the perioperative leukocyte counts, or CRP values determined. The duration of a postoperative leukocytosis or CRP increase was not significantly related to bacterial detection. Postoperative increases in CRP and leucocyte counts were associated with clinical signs of infection in individual cases. Both cases of wound complications showed early postoperative highly elevated CRP values, the highest in the study.
In summary, postoperative increases in pathogens only actually triggered a wound infection in individual cases. Similarly, increases in CRP, leukocyte count, and body temperature did not indicate wound infections. Overall, there were no significant correlations between inflammatory parameters and the detected perioperative bacterial colonization.
4. Discussion
4.1. Selection of the Microorganisms
The selection of pathogens was based on the current literature [27,28,36,37,54,55,56,57]. In the context of a wound infection that occurred in this study, E. faecalis, Enterobacteriaceae and P. aeruginosa were detected in all postoperative swabs in moderate or high loads. In accordance with the literature, this was therefore a mixed infection carried by classical nosocomial bacteria. However, it should be noted that the loads of the species detected for this patient only made up a small proportion of the total eubacterial load of the respective swabs. It can therefore be assumed that other microorganisms that were not detected may have been involved in the infection.
As a representative of healthy oral flora, the species S. mitis was selected, which is strongly associated with oral health in the literature. Some of the beneficial effects on oral health, such as the protection of the periodontium from tissue damage and the inhibition of pathogens, have been described [58,59,60,61,62]. It has been shown that the microorganism can be reduced in dysbiotic conditions and in association with diseases in the oral cavity and human body [62,63,64,65]. Therefore, a loss or decrease in the S. mitis load from the oral flora was considered a sign of dysbiosis in this study.
Furthermore, M. salivarium was included in the selection of detected microorganisms, which is generally considered a commensal of the oral flora, although there have also been occasional reports of associations with pathological processes [66,67,68,69,70,71,72,73]. M. salivarium was particularly well-suited for investigating the effect of antibiotic-associated dysbiosis, as M. salivarium, being a cell wall-less bacterium, does not respond to cell wall-acting and frequently administered beta-lactam antibiotics [74,75].
4.2. Patient Characteristics
The study patients were 51% female and 49% male. According to the literature, men are affected by oral cancer 1.5 times more frequently and two to three years earlier than women due to a higher exposure to risk factors [76,77]. On average, the study patients were 65.3 years old, which is in line with the average age in comparable studies [2,78]. The patients had an average BMI of 25.4, while slightly lower BMIs of between 21.6 and 24.6 were reported in comparable studies [2,37,46]. According to Bartella et al. (2018), an increased BMI is associated with an increased risk of postoperative infections after head and neck surgery [37], while according to Kruse et al. (2010), BMI has no influence on the survival of microvascular transplants in the head and neck region [79]. The ASA score I or II (62%) is in line with ASA scores of patients in comparable studies [2,55]. The literature provides evidence that an increased ASA score significantly increases the infection and mortality rates of microvascular grafts in the head and neck region [2,79]. In the current study, no correlation was found between postoperative graft survival or infections and the ASA score.
4.3. Complications and Inflammation
Postoperative infection occurred in one of the 37 patients and wound dehiscence in the extraoral area of the graft in another patient. This wound infection and dehiscence rate of 3% each is significantly lower than the values of 13.3 to 40.6% for infections described in the literature [2,27,36,78,80] and 14 to 29% for dehiscences [7,8]. This may be due to the overlap in sample collection from April 2021 to January 2023 with the additional hygiene protection measures introduced as part of the COVID-19 pandemic. A graft loss rate of 3% is slightly below the rates reported in the literature (5 to 8%) [8,81,82].
4.4. Perioperative Bacterial Colonization
The significantly reduced postoperative detection of S. mitis, which represents oral health, alongside increases in infectious pathogens like P. aeruginosa, E. faecalis, Enterobacteriaceae as well as M. salivarium, which is rarely associated with infection, strongly indicates postoperative dysbiosis. Typical pathogens of postoperative infections after intraoral microvascular transplantations are not part of the resident oral flora [27,28]. The microbiome of the external skin differs fundamentally from the oral microbiome [26]. The interactions of bacteria and body surfaces are based on the highly specific binding of adhesion molecules of the adhering microorganisms and certain receptors on the cell surface [42]. In the current study, there were hardly any differences between the postoperative colonization of the microorganisms examined on the cheek, suture, and graft. Very similar colonization patterns were found at the three localizations despite different epithelial and surface conditions. It was shown that M. salivarium can colonize the keratinized squamous epithelium to a similar extent as the buccal mucosa after the transfer of the extraoral skin surface into the mouth. The colonization of the graft was thus strongly influenced postoperatively by the surrounding oral flora, but conversely, the transfer of extraoral skin into the mouth had no significant influence on the oral colonization of the microorganisms examined. Consistent with this result, Kageyama et al. (2020) found no differences in oral microbial composition in patients who had received an oral microvascular graft compared to patients who had undergone primary closure of defects. Although the sample in this study was very small, the authors provided initial indications that the surface of the graft does not have as great an influence on the microbial colonization of the oral cavity, as might have been assumed [48]. One possible explanation for the postoperatively similar colonization patterns examined in the different localizations studied is the function of saliva as a fingerprint of the microbiome [83,84,85]. Although the microbial composition of saliva is not absolutely representative of the complete oral microbiome, saliva does contain microorganisms from various oral niches [19]. It is therefore conceivable that the saliva wetting the graft surface could transfer microorganisms from other oral niches such as the buccal mucosa to the graft.
4.5. Antibiotic Treatment
The bacteriostatic or bactericidal effect of an antibiotic inhibits sensitive species and chemically induced dysbiosis can occur. At the same time, non-sensitive species experience selection advantages, which can promote their proliferation [86,87,88].
The results showed that the microorganism of oral health S. mitis was widely represented in the preoperative buccal swab, whereas it had to give way to colonization by other microorganisms in the postoperative course. Longer-term administration of ampicillin/sulbactam for at least three days led to strong containment of the microorganism and drastic reductions in its proportion of the total bacterial count. Short-term antibiotics of ampicillin/sulbactam were also associated with significant reductions in the S. mitis load. Although medications with clindamycin also tended to reduce the bacterial load, this was less pronounced. Contrary to our results, viridans streptococci, which include S. mitis, can be inhibited similarly well by ampicillin/sulbactam and clindamycin according to the literature, although high resistance rates are reported in some cases [89]. In a large-scale review, Singh et al. (2022) examined the susceptibility rates of viridans streptococci to various antibiotics over the period from 2010 to 2020. The susceptibility rate of S. mitis to clindamycin was reported to be 83.8% on average over the period mentioned, while that to ampicillin was 81.0%, with no significant changes in susceptibility over the decade for either antibiotic [90]. While ampicillin/sulbactam medications of at least three days strongly reduced the colonization of S. mitis in our study, M. salivarium increased drastically; so, in some cases very high proportions of over 80% of the total bacterial load were achieved. The bacteria can therefore be regarded as antagonists after long-term administration of ampicillin/sulbactam. The fact that M. salivarium showed a strong increase after medication with ampicillin/sulbactam seems obvious, since this cell wall-less bacterium does not respond to the cell wall-active beta-lactam antibiotic [74,75,91]. Thus, a multiplication of the microorganism can be explained by a selection advantage over sensitive bacteria (such as staphylococci or streptococci like S. mitis).
Clindamycin exhibits limited efficacy against the entire Enterobacteriaceae family, while ampicillin/sulbactam and piperacillin/tazobactam are similarly ineffective against many members of this family. Additionally, P. aeruginosa is not effectively inhibited by either clindamycin or ampicillin/sulbactam. This lack of efficacy elucidates why, in the current study, increased detections of Enterobacteriaceae or P. aeruginosa were observed regardless of the antibiotic choice or duration of treatment. It can be inferred that the administered antibiotics further promoted postoperative increases in P. aeruginosa and representatives of Enterobacteriaceae by conferring a selective advantage over more sensitive species. Although antibiotic prophylaxis in head and neck surgery has been investigated in numerous studies, there are still controversial views regarding the choice and duration of antibiotic treatment. According to the literature, 30–80% of head and neck tumor surgery is covered with antibiotics, and antibiotic coverage can drastically reduce the risk of postoperative infection [54,92].
According to Durand et al. (2015), however, the choice of antibiotic for infection prophylaxis has a strong impact on the development of postoperative infections after microvascular transplants in the head and neck area: according to the authors, the risk of suffering a postoperative wound infection was more than doubled when clindamycin was used compared to ampicillin/sulbactam, cefazolin and other antibiotics. One of the reasons for this was the high incidence of Gram-negative rods after clindamycin medication. The authors identified clindamycin as a risk factor for the development of postoperative infections [27]. Other studies also reported an increased risk of postoperative wound infection after antibiotic prophylaxis with clindamycin [93,94]. Given the very low complication rates observed in our study, we are unable to determine the extent to which the choice or duration of antibiotic therapy influenced the development of postoperative wound infections. According to Bartella et al. (2018), the duration of antibiotic treatment did not appear to have any influence on the development of postoperative wound infections after oral surgery. Prolonged postoperative antibiotic administration as infection prophylaxis did not appear to offer any advantage over single-shot antibiotics administered perioperatively [37]. Vila et al. (2017) also described in their meta-analysis of 340 patients that a five-day postoperative antibiotic prophylaxis does not provide any advantage for infection prophylaxis compared to a one-day postoperative prophylaxis [92]. This is supported by the results of the current study: the fact that the oral colonization of the pathogens examined was not significantly reduced by prolonged antibiotic treatment compared to short-term antibiotic treatment suggests that prolonged postoperative antibiotic treatment does not offer the patient any advantage in terms of infection prophylaxis.
5. Conclusions
The colonization pattern of the examined bacteria on the postoperative graft surface adapts to the intraoral colonization. It can be assumed that the transplantation of extraoral skin surface into the oral cavity has a relatively minor influence on the development of postoperative dysbiosis following microvascular transplantation. However, the dysbiotic reduction in the S. mitis is significantly influenced by postoperative antibiotics like ampicillin/sulbactam. Therefore, standard antibiotics should be critically reviewed after microvascular transplantation in the oral cavity. It is recommended to exercise caution with the prolonged use of standard antibiotics following microvascular postoperative transplantation. This approach aims to minimize side effects, reduce the development of antibiotic resistance, prevent dysbiosis, and avoid the selection of non-sensitive and potentially infection-relevant species.
Author Contributions
Conceptualization H.L.M., N.K., K.K., B.H. and M.R.; methodology H.L.M., N.K., K.K. and B.H.; validation K.K. and B.H.; data collection H.L.M., K.K. and B.H., formal analysis K.K. and B.H.; writing—original draft preparation H.L.M. and K.K.—review and editing H.L.M. and B.H.; writing—revised the manuscript, H.L.M., K.K. and B.H.; visualization, K.K. and B.H.; supervision, K.P., B.H. and M.R.; project administration, H.L.M., N.K., B.H. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by departmental funds from the Institute of Medical Microbiology and Hospital Hygiene and the Department of Cranio-and-Maxillo Facial Surgery of the Medical Faculty of the Heinrich-Heine-University of Duesseldorf, Germany.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of University Hospital of Düsseldorf (2021-1342, 3 March 2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors acknowledge everyone who participated in this study. We especially thank Robert Langner (Statistical Consultancy Service, Institute of Systems Neuroscience, Heinrich Heine University, Düsseldorf) for statistical consulting and Dana Bäcker (Institute of Medical Microbiology and Hospital Hygiene, Heinrich Heine University, Düsseldorf) for support and assistance in DNA preparation and PCR.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lai, C.-S.; Chang, Y.-T.; Shen, C.-H.; Tsai, Y.-C.; Lu, C.-T.; Yen, J.-H.; Chen, I.-C.; Lin, Y.-L. The role of vein grafts in reconstructive head and neck microsurgery. Braz. J. Otorhinolaryngol. 2022, 88 (Suppl. S4), S81–S88. [Google Scholar] [CrossRef] [PubMed]
- Karakida, K.; Aoki, T.; Ota, Y.; Yamazaki, H.; Otsuru, M.; Takahashi, M.; Sakamoto, H.; Miyasaka, M. Analysis of risk factors for surgical-site infections in 276 oral cancer surgeries with microvascular free-flap reconstructions at a single university hospital. J. Infect. Chemother. 2010, 16, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, D.; Patel, R.S.; Goldstein, D.P.; Gullane, P.J. Salvage of failed free flaps used in head and neck reconstruction. Head Neck Oncol. 2009, 1, 33. [Google Scholar] [CrossRef]
- Wong, C.-H.; Wei, F.-C. Microsurgical free flap in head and neck reconstruction. Head Neck 2010, 32, 1236–1245. [Google Scholar] [CrossRef]
- Patel, S.Y.; Kim, D.D.; Ghali, G.E. Maxillofacial Reconstruction Using Vascularized Fibula Free Flaps and Endosseous Implants. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 259–284. [Google Scholar] [CrossRef] [PubMed]
- Mahieu, R.; Colletti, G.; Bonomo, P.; Parrinello, G.; Iavarone, A.; Dolivet, G.; Livi, L.; Deganello, A. Ricostruzioni del distretto testa collo con lembi peduncolati nell’era dei lembi liberi. Acta Otorhinolaryngol. Ital. 2016, 36, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, B.; Copelli, C.; Ferrari, S.; Ferri, A.; Sesenna, E. Free flaps: Outcomes and complications in head and neck reconstructions. J. Craniomaxillofac. Surg. 2009, 37, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Pohlenz, P.; Klatt, J.; Schön, G.; Blessmann, M.; Li, L.; Schmelzle, R. Microvascular free flaps in head and neck surgery: Complications and outcome of 1000 flaps. Int. J. Oral Maxillofac. Surg. 2012, 41, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.D.; Sercarz, J.A.; Abemayor, E.; Calcaterra, T.C.; Rawnsley, J.D.; Alam, D.; Blackwell, K.E. Analysis of outcome and complications in 400 cases of microvascular head and neck reconstruction. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 962–966. [Google Scholar] [CrossRef]
- Lee, Z.-H.; Ismail, T.; Shuck, J.W.; Chang, E.I. Innovative Strategies in Microvascular Head and Neck Reconstruction. Medicina 2023, 59, 1194. [Google Scholar] [CrossRef]
- Hurrell, M.J.L.; Clark, J.R.; Ch’ng, S.; Hubert Low, T.-H.; Nguyen, K.M.; Elliott, M.S.; Palme, C.E.; Wykes, J. Comparison between the radial forearm and superficial circumflex iliac artery perforator free flaps for oral soft tissue reconstruction. Int. J. Oral Maxillofac. Surg. 2023, 52, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Chim, H. Perforator mapping and clinical experience with the superthin profunda artery perforator flap for reconstruction in the upper and lower extremities. J. Plast. Reconstr. Aesthet. Surg. 2023, 81, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Al Omran, Y.; Evans, E.; Jordan, C.; Borg, T.-M.; AlOmran, S.; Sepehripour, S.; Akhavani, M.A. The Medial Sural Artery Perforator Flap versus Other Free Flaps in Head and Neck Reconstruction: A Systematic Review. Arch. Plast. Surg. 2023, 50, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Möllmann, H.L.; Apeltrath, L.; Karnatz, N.; Wilkat, M.; Riedel, E.; Singh, D.D.; Rana, M. Comparison of the Ac-curacy and Clinical Parameters of Patient-Specific and Conventionally Bended Plates for Mandibular Recon-struction. Front. Oncol. 2021, 11, 719028. [Google Scholar] [CrossRef] [PubMed]
- Karnatz, N.; Möllmann, H.L.; Wilkat, M.; Parviz, A.; Rana, M. Advances and Innovations in Ablative Head and Neck Oncologic Surgery Using Mixed Reality Technologies in Personalized Medicine. J. Clin. Med. 2022, 11, 4767. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-J.; Hanasono, M.M. Virtual Surgical Planning in Free Tissue Transfer for Orbito-Maxillary Reconstruc-tion. Semin. Plast. Surg. 2022, 36, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Manzie, T.; MacDougall, H.; Cheng, K.; Venchiarutti, R.; Fox, R.; Sharman, A.; Charters, E.; Seyfi, D.; Dunn, M.; Mukherjee, P.; et al. Virtual reality digital surgical planning for jaw reconstruction: A usability study. ANZ J. Surg. 2023, 93, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lückel, H. (Ed.) Karies: Wissenschaft und klinische Praxis; Thieme: Stuttgart, Germany, 2012; ISBN 9783131545411. [Google Scholar]
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef]
- Kilian, M. The oral microbiome—friend or foe? Eur. J. Oral Sci. 2018, 126 (Suppl. S1), 5–12. [Google Scholar] [CrossRef]
- Tranberg, A.; Samuelsson, C.; Klarin, B. Disturbance in the oropharyngeal microbiota in relation to antibiotic and proton pump inhibitor medication and length of hospital stay. APMIS 2021, 129, 14–22. [Google Scholar] [CrossRef]
- Brookes, Z.; Teoh, L.; Cieplik, F.; Kumar, P. Mouthwash Effects on the Oral Microbiome: Are They Good, Bad, or Balanced? Int. Dent. J. 2023, 73 (Suppl. S2), S74–S81. [Google Scholar] [CrossRef]
- Brookes, Z.L.S.; Belfield, L.A.; Ashworth, A.; Casas-Agustench, P.; Raja, M.; Pollard, A.J.; Bescos, R. Effects of chlorhexidine mouthwash on the oral microbiome. J. Dent. 2021, 113, 103768. [Google Scholar] [CrossRef]
- Ferrer, M.; Méndez-García, C.; Rojo, D.; Barbas, C.; Moya, A. Antibiotic use and microbiome function. Biochem. Pharmacol. 2017, 134, 114–126. [Google Scholar] [CrossRef]
- Hayashi, Y.; Saito, T.; Ohshima, T.; Nakagawa, Y.; Maeda, N. Alterations of the oral microbiota and oral clinical findings in dry mouth. J. Oral Biosci. 2015, 57, 171–174. [Google Scholar] [CrossRef]
- Wertz, P.W.; de Szalay, S. Innate Antimicrobial Defense of Skin and Oral Mucosa. Antibiotics 2020, 9, 159. [Google Scholar] [CrossRef]
- Durand, M.L.; Yarlagadda, B.B.; Rich, D.L.; Lin, D.T.; Emerick, K.S.; Rocco, J.W.; Deschler, D.G. The time course and microbiology of surgical site infections after head and neck free flap surgery. Laryngoscope 2015, 125, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.W.; Maurer, P.; Wilhelms, D.; Schubert, J. Weichteilinfektionen in der Mund-, Kiefer- und Plastischen Gesichtschirurgie. Keimspektren und Antibiotika. Mund Kiefer Gesichtschir. 2005, 9, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Da Silva-Boghossian, C.M.; do Souto, R.M.; Luiz, R.R.; Colombo, A.P.V. Association of red complex, A. actinomycetemcomitans and non-oral bacteria with periodontal diseases. Arch. Oral Biol. 2011, 56, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Souto, R.; de Andrade, A.F.B.; Uzeda, M.; Colombo, A.P.V. Prevalence of “non-oral” pathogenic bacteria in subgingival biofilm of subjects with chronic periodontitis. Braz. J. Microbiol. 2006, 37, 208–215. [Google Scholar] [CrossRef]
- Reddy, M.S. Reaching a better understanding of non-oral disease and the implication of periodontal infections. Periodontology 2000 2007, 44, 9–14. [Google Scholar] [CrossRef]
- Vincent, J.L.; Bihari, D.J.; Suter, P.M.; Bruining, H.A.; White, J.; Nicolas-Chanoin, M.H.; Wolff, M.; Spencer, R.C.; Hemmer, M. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA 1995, 274, 639–644. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Belusic-Gobic, M.; Car, M.; Juretic, M.; Cerovic, R.; Gobic, D.; Golubovic, V. Risk factors for wound infection after oral cancer surgery. Oral Oncol. 2007, 43, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Kamizono, K.; Sakuraba, M.; Nagamatsu, S.; Miyamoto, S.; Hayashi, R. Statistical analysis of surgical site infection after head and neck reconstructive surgery. Ann. Surg. Oncol. 2014, 21, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Bartella, A.K.; Lemmen, S.; Burnic, A.; Kloss-Brandstätter, A.; Kamal, M.; Breisach, T.; Hölzle, F.; Lethaus, B. Influence of a strictly perioperative antibiotic prophylaxis vs a prolonged postoperative prophylaxis on surgical site infections in maxillofacial surgery. Infection 2018, 46, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Johanson, W.G.; Pierce, A.K.; Sanford, J.P. Changing pharyngeal bacterial flora of hospitalized patients. Emergence of gram-negative bacilli. N. Engl. J. Med. 1969, 281, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.L.; Kuczynski, J.; Bhattacharya, A.; Huey, B.; Corby, P.M.; Queiroz, E.L.S.; Nightingale, K.; Kerr, A.R.; DeLacure, M.D.; Veeramachaneni, R.; et al. Changes in abundance of oral microbiota associated with oral cancer. PLoS ONE 2014, 9, e98741. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Godoy-Vitorino, F.; Jedlicka, A.; Rodríguez-Hilario, A.; González, H.; Bondy, J.; Lawson, F.; Folawiyo, O.; Michailidi, C.; Dziedzic, A.; et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget 2016, 7, 51320–51334. [Google Scholar] [CrossRef]
- Bolz, J.; Dosá, E.; Schubert, J.; Eckert, A.W. Bacterial colonization of microbial biofilms in oral squamous cell carcinoma. Clin. Oral Investig. 2014, 18, 409–414. [Google Scholar] [CrossRef]
- Mager, D.L.; Haffajee, A.D.; Devlin, P.M.; Norris, C.M.; Posner, M.R.; Goodson, J.M. The salivary microbiota as a diagnostic indicator of oral cancer: A descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J. Transl. Med. 2005, 3, 27. [Google Scholar] [CrossRef]
- Metgud, R.; Gupta, K.; Gupta, J. Exploring bacterial flora in oral squamous cell carcinoma: A microbiological study. Biotech. Histochem. 2014, 89, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.K.; Ng, C.W.K.; Lan, L.; Fung, S.; Li, J.-W.; Cai, L.; Lei, P.; Mou, Q.; Meehan, K.; Lau, E.H.L.; et al. Restoration of the Oral Microbiota After Surgery for Head and Neck Squamous Cell Carcinoma Is Associated with Patient Outcomes. Front. Oncol. 2021, 11, 737843. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Goto, J.; Harahashi, A.; Murata, T.; Hata, H.; Yamazaki, Y.; Satoh, A.; Notani, K.; Kitagawa, Y. Oral health care reduces the risk of postoperative surgical site infection in inpatients with oral squamous cell carcinoma. Support. Care Cancer 2011, 19, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Usubuchi, M.; Matsuura, K.; Goto, T.; Asada, Y.; Imai, T.; Ogawa, T.; Kato, K.; Saijo, S. Professional Oral Health Care at General Dental Clinic Reduces Postoperative Complications of Head and Neck Free-Flap Reconstruction Surgery. J. Cancer 2019, 10, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Heimlich, F.; Dietz, A.; Daniel, V.; Maier, H. Einfluss tumorchirurgischer Eingriffe im Kopf-Hals-Bereich auf das Immunsystem. HNO 1999, 47, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Nagao, Y.; Ma, J.; Asakawa, M.; Yoshida, R.; Takeshita, T.; Hirosue, A.; Yamashita, Y.; Nakayama, H. Compositional Shift of Oral Microbiota Following Surgical Resection of Tongue Cancer. Front. Cell. Infect. Microbiol. 2020, 10, 600884. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual Comparisons by Ranking Methods. Biom. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Friedman, M. A correction: The use of ranks to avoid the assumption of normality implicit in the analysis of variance. J. Am. Stat. Assoc. 1939, 34, 109. [Google Scholar] [CrossRef]
- du Prel, J.B.; Röhrig, B.; Hommel, G.; Blettner, M. Choosing statistical tests: Part 12 of a series on evaluation of scientific publications. Dtsch. Arztebl. Int. 2010, 107, 343. [Google Scholar] [PubMed]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Penel, N.; Fournier, C.; Lefebvre, D.; Lefebvre, J.-L. Multivariate analysis of risk factors for wound infection in head and neck squamous cell carcinoma surgery with opening of mucosa. Study of 260 surgical procedures. Oral Oncol. Extra 2005, 41, 35–44. [Google Scholar] [CrossRef][Green Version]
- Belusic-Gobic, M.; Zubovic, A.; Predrijevac, A.; Harmicar, D.; Cerovic, R.; Udovic Gobic, S.; Zubovic, L. Microbiology of wound infection after oral cancer surgery. J. Craniomaxillofac. Surg. 2020, 48, 700–705. [Google Scholar] [CrossRef]
- Rodrigo, J.P.; Sŭrez, C.; Bernaldez, R.; Collado, D. Efficacy of piperacillin-tazobactam in the treatment of surgical wound infection after clean-contaminated head and neck oncologic surgery. Head Neck 2004, 26, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Chew, K.-Y.; Solomkin, J.S.; Lin, P.-Y.; Chiang, Y.-C.; Kuo, Y.-R. Surgical site infections among high-risk patients in clean-contaminated head and neck reconstructive surgery: Concordance with preoperative oral flora. Ann. Plast. Surg. 2013, 71 (Suppl. S1), S55–S60. [Google Scholar] [CrossRef]
- Engen, S.A.; Schreurs, O.; Petersen, F.; Blix, I.J.S.; Baekkevold, E.S.; Schenck, K. The Regulatory Role of the Oral Commensal Streptococcus mitis on Human Monocytes. Scand. J. Immunol. 2018, 87, 80–87. [Google Scholar] [CrossRef]
- Eberhard, J.; Pietschmann, R.; Falk, W.; Jepsen, S.; Dommisch, H. The immune response of oral epithelial cells induced by single-species and complex naturally formed biofilms. Oral Microbiol. Immunol. 2009, 24, 325–330. [Google Scholar] [CrossRef]
- Teughels, W.; Kinder Haake, S.; Sliepen, I.; Pauwels, M.; van Eldere, J.; Cassiman, J.-J.; Quirynen, M. Bacteria interfere with A. actinomycetemcomitans colonization. J. Dent. Res. 2007, 86, 611–617. [Google Scholar] [CrossRef]
- Sliepen, I.; van Damme, J.; van Essche, M.; Loozen, G.; Quirynen, M.; Teughels, W. Microbial interactions influence inflammatory host cell responses. J. Dent. Res. 2009, 88, 1026–1030. [Google Scholar] [CrossRef]
- Gross, E.L.; Leys, E.J.; Gasparovich, S.R.; Firestone, N.D.; Schwartzbaum, J.A.; Janies, D.A.; Asnani, K.; Griffen, A.L. Bacterial 16S sequence analysis of severe caries in young permanent teeth. J. Clin. Microbiol. 2010, 48, 4121–4128. [Google Scholar] [CrossRef] [PubMed]
- Decsi, G.; Soki, J.; Pap, B.; Dobra, G.; Harmati, M.; Kormondi, S.; Pankotai, T.; Braunitzer, G.; Minarovits, J.; Sonkodi, I.; et al. Chicken or the Egg: Microbial Alterations in Biopsy Samples of Patients with Oral Potentially Malignant Disorders. Pathol. Oncol. Res. 2019, 25, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Galvin, S.; Healy, C.M.; Moran, G.P. The Microbiome of Potentially Malignant Oral Leukoplakia Exhibits Enrichment for Fusobacterium, Leptotrichia, Campylobacter, and Rothia Species. Front. Microbiol. 2017, 8, 2391. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T.W. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Baracaldo, R.; Foltzer, M.; Patel, R.; Bourbeau, P. Empyema caused by Mycoplasma salivarium. J. Clin. Microbiol. 2012, 50, 1805–1806. [Google Scholar] [CrossRef] [PubMed]
- Grisold, A.J.; Hoenigl, M.; Leitner, E.; Jakse, K.; Feierl, G.; Raggam, R.B.; Marth, E. Submasseteric abscess caused by Mycoplasma salivarium infection. J. Clin. Microbiol. 2008, 46, 3860–3862. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watanabe, T.; Matsuura, M.; Seto, K. Enumeration, isolation, and species identification of mycoplasmas in saliva sampled from the normal and pathological human oral cavity and antibody response to an oral mycoplasma (Mycoplasma salivarium). J. Clin. Microbiol. 1986, 23, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Engel, L.D.; Kenny, G.E. Mycoplasma salivarium in human gingival sulci. J. Periodontal Res. 1970, 5, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Ørsted, I.; Gertsen, J.B.; Schønheyder, H.C.; Jensen, J.S.; Nielsen, H. Mycoplasma salivarium isolated from brain abscesses. Clin. Microbiol. Infect. 2011, 17, 1047–1049. [Google Scholar] [CrossRef][Green Version]
- Kawamoto, D.; Borges, R.; Ribeiro, R.A.; de Souza, R.F.; Amado, P.P.P.; Saraiva, L.; Horliana, A.C.R.T.; Faveri, M.; Mayer, M.P.A. Oral Dysbiosis in Severe Forms of Periodontitis Is Associated with Gut Dysbiosis and Correlated with Salivary Inflammatory Mediators: A Preliminary Study. Front. Oral Health 2021, 2, 722495. [Google Scholar] [CrossRef]
- Henrich, B.; Rumming, M.; Sczyrba, A.; Velleuer, E.; Dietrich, R.; Gerlach, W.; Gombert, M.; Rahn, S.; Stoye, J.; Borkhardt, A.; et al. Mycoplasma salivarium as a dominant coloniser of Fanconi anaemia associated oral carcinoma. PLoS ONE 2014, 9, e92297. [Google Scholar] [CrossRef] [PubMed]
- So, A.K.; Furr, P.M.; Taylor-Robinson, D.; Webster, A.D. Arthritis caused by Mycoplasma salivarium in hypogammaglobulinaemia. Br. Med. J. (Clin. Res. Ed.) 1983, 286, 762–763. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bébéar, C.M.; Pereyre, S. Mechanisms of drug resistance in Mycoplasma pneumoniae. Curr. Drug Targets Infect. Disord. 2005, 5, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Razin, S.; Yogev, D.; Naot, Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 1998, 62, 1094–1156. [Google Scholar] [CrossRef]
- Robert Koch-Institut. Krebs in Deutschland für 2017/2018. Available online: https://edoc.rki.de/handle/176904/9042 (accessed on 9 June 2024).
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Makiguchi, T.; Yokoo, S.; Kanno, Y.; Kurihara, J.; Suzuki, K. Risk Factors for Surgical Site Infection in Patients Undergoing Free and Pedicled Myocutaneous Flap Reconstruction After Oral Cancer Resection. J. Oral Maxillofac. Surg. 2019, 77, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.L.D.; Luebbers, H.T.; Grätz, K.W.; Obwegeser, J.A. Factors influencing survival of free-flap in reconstruction for cancer of the head and neck: A literature review. Microsurgery 2010, 30, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-W.; Liu, Y.-H.; Su, H.-H. Bacteriology of peritonsillar abscess: The changing trend and predisposing factors. Braz. J. Otorhinolaryngol. 2018, 84, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.; Lin, G.-T.; Fu, Y.-C.; Shieh, T.-Y.; Huang, I.-Y.; Shen, Y.-S.; Chen, C.-H. Complications of free radial forearm flap transfers for head and neck reconstruction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Gabr, E.M.; Kobayashi, M.R.; Salibian, A.H.; Armstrong, W.B.; Sundine, M.; Calvert, J.W.; Evans, G.R.D. Oromandibular reconstruction with vascularized free flaps: A review of 50 cases. Microsurgery 2004, 24, 374–377. [Google Scholar] [CrossRef]
- Simon-Soro, A.; Ren, Z.; Krom, B.P.; Hoogenkamp, M.A.; Cabello-Yeves, P.J.; Daniel, S.G.; Bittinger, K.; Tomas, I.; Koo, H.; Mira, A. Polymicrobial Aggregates in Human Saliva Build the Oral Biofilm. mBio 2022, 13, e0013122. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhang, Q.; Liu, X.; Zheng, S.; Ma, L.; Chen, F.; Xu, T.; Xu, B. Exploring the oral microflora of preschool children. J. Microbiol. 2017, 55, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Tian, J.; Xu, H.; Zhou, Q.; Qin, M. Distinctions and associations between the microbiota of saliva and supragingival plaque of permanent and deciduous teeth. PLoS ONE 2018, 13, e0200337. [Google Scholar] [CrossRef]
- Rebelo, M.B.; Oliveira, C.S.; Tavaria, F.K. Novel Strategies for Preventing Dysbiosis in the Oral Cavity. Front. Biosci.-Elite 2023, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Cugini, C.; Ramasubbu, N.; Tsiagbe, V.K.; Fine, D.H. Dysbiosis from a Microbial and Host Perspective Relative to Oral Health and Disease. Front. Microbiol. 2021, 12, 617485. [Google Scholar] [CrossRef]
- Adler, N.; Balzer, F.; Bondzik, K.; Straff, W. Antibiotika und Antibiotikaresistenzen in der Umwelt: Hintergrund, Herausfordrungen und Handlungsoptionen; Umweltbundesamt: Dessau, Germany, 2018. [Google Scholar]
- Süzük, S.; Kaşkatepe, B.; Çetin, M. Antimicrobial susceptibility against penicillin, ampicillin and vancomycin of viridans group Streptococcus in oral microbiota of patients at risk of infective endocarditis. Infez. Med. 2016, 24, 190–193. [Google Scholar] [PubMed]
- Singh, N.; Poggensee, L.; Huang, Y.; Evans, C.T.; Suda, K.J.; Bulman, Z.P. Antibiotic susceptibility patterns of viridans group streptococci isolates in the United States from 2010 to 2020. JAC Antimicrob. Resist. 2022, 4, dlac049. [Google Scholar] [CrossRef] [PubMed]
- Chernova, O.A.; Medvedeva, E.S.; Mouzykantov, A.A.; Baranova, N.B.; Chernov, V.M. Mycoplasmas and Their Antibiotic Resistance: The Problems and Prospects in Controlling Infections. Acta Naturae 2016, 8, 24–34. [Google Scholar] [CrossRef]
- Vila, P.M.; Zenga, J.; Jackson, R.S. Antibiotic Prophylaxis in Clean-Contaminated Head and Neck Surgery: A Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2017, 157, 580–588. [Google Scholar] [CrossRef]
- Pool, C.; Kass, J.; Spivack, J.; Nahumi, N.; Khan, M.; Babus, L.; Teng, M.S.; Genden, E.M.; Miles, B.A. Increased Surgical Site Infection Rates following Clindamycin Use in Head and Neck Free Tissue Transfer. Otolaryngol.–Head Neck Surg. 2016, 154, 272–278. [Google Scholar] [CrossRef]
- Langerman, A.; Thisted, R.; Hohmann, S.; Howell, M. Antibiotic and Duration of Perioperative Prophylaxis Predicts Surgical Site Infection in Head and Neck Surgery. Otolaryngol.–Head Neck Surg. 2016, 154, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).