Oral Reconstruction with Locoregional Flaps after Cancer Ablation: A Systematic Review of the Literature
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Data Source and Study Searching
2.3. Infrahyoid Flap
2.4. Nasolabial Flap
2.5. Platysma Flap
2.6. Submental Flap
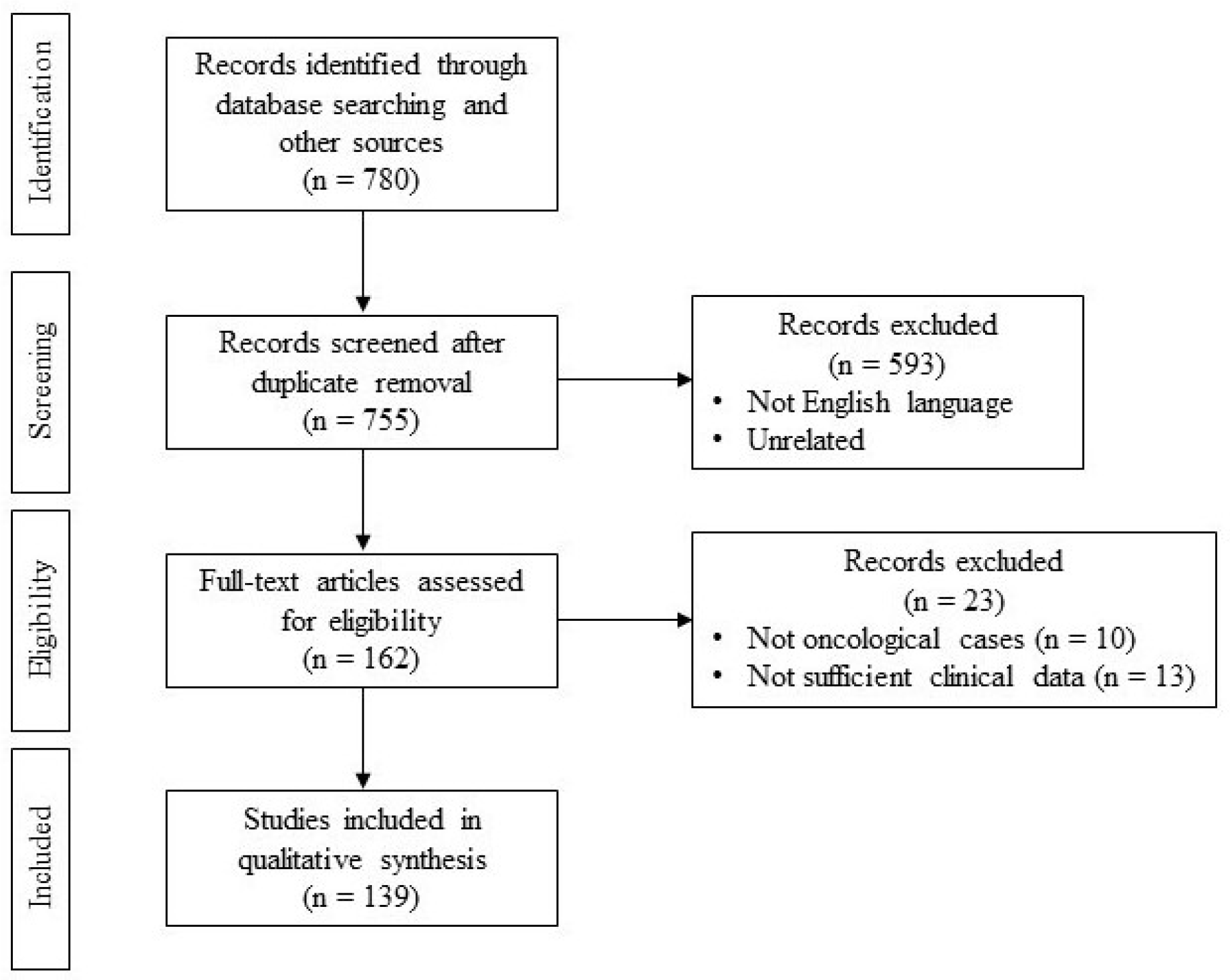
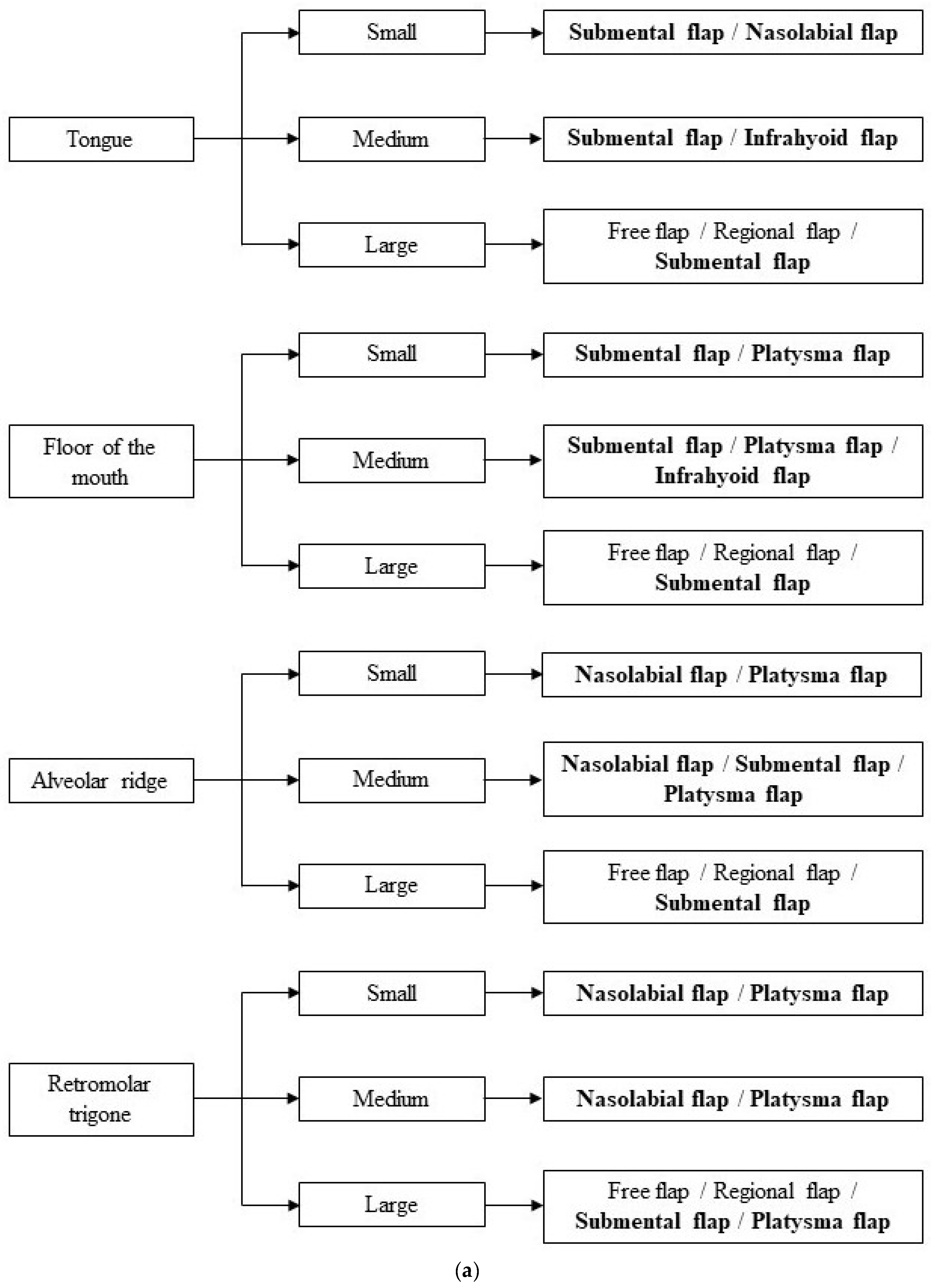
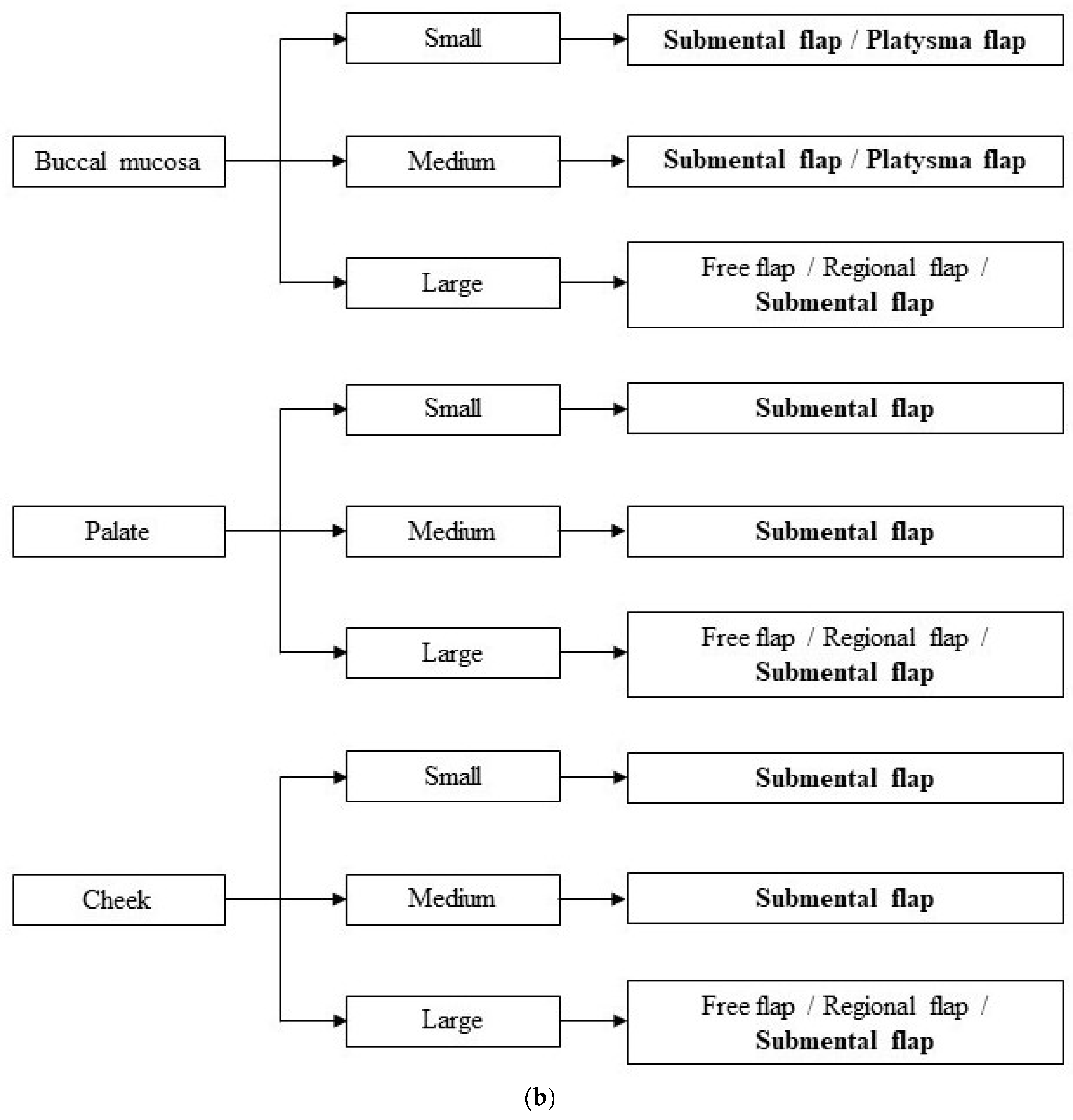
2.7. Dimensional Criteria
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashtiani, A.K.; Emami, S.A.; Rasti, M. Closure of palatal fistula with facial artery musculomucosal flap. Plast. Reconstr. Surg. 2005, 116, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Rocha, D.; Arnaut, M.; Freitas, R.; Alonso, N. Versatiliy of the buccinator myomucosal flap in atypical palate reconstructions. J. Cranio-Maxillofac. Surg. 2014, 42, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, J.P.; Brady, C.M.; Burstein, F.D. Primary Abbe flap for median cleft lip deformity: New trends on an old concept. J. Craniofacial Surg. 2016, 27, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Zareem, H.A.; Greer, D.M. Tongue flap for reconstruction of the lips after electrical burns. Plast. Reconstr. Surg. 1974, 53, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Ferri, A.; Bianchi, B.; Varazzani, A.; Giovacchini, F.; Sesenna, E. Oncologic safety of facial artery myomucosal flaps in oral cavity reconstruction. Head Neck 2015, 38 (Suppl. S1), E1200–E1202. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Datakar, A.; Rai, M. Is buccal fat pad a better option than nasolabial flap or reconstruction of intraoral defects after surgical release of fibrous bands in patients with oral submucous fibrosis? A pilot study: A protocol for the management of oral submucous fibrosis. J. Cranio-Maxillofac. Surg. 2014, 42, e111–e116. [Google Scholar] [CrossRef] [PubMed]
- Lambade, P.; Dawane, P.; Thorat, A. Efficacy of buccal fat pad in the surgical management of oral submucous fibrosis: A prospective study. Oral Maxillofac. Surg. 2016, 20, 167–170. [Google Scholar] [CrossRef]
- Dhara, V.; Kudva, A.; Chithra, A.; Rajan, J.; Singh, A. Reconstruction of buccal mucosa: A minimalist symbiotic approach with local flaps. Oral Oncol. 2021, 114, 105081. [Google Scholar] [CrossRef]
- Shetty, R.K.; Pradhan, S.; Kannan, R.; Doctor, A.; Surnare, K.; Jondhale, M.; Patil, D.; Shetty, N. Clinical Profile and Quality of Life Assessment of oral cancer patients following nasolabial flap reconstruction surgery. Indian J. Otolaryngol. Head Neck Surg. 2020, 72, 200–207. [Google Scholar] [CrossRef]
- Boyapati, R.P.; Shah, K.C.; Flood, V.; Stassen, L.F. Quality of life outcome measures using UW-QOL questionnaire v4 in early oral cancer/squamous cell cancer resections of the tongue and floor of mouth with reconstruction solely using local methods. Br. J. Oral Maxillofac. Surg. 2013, 51, 502–507. [Google Scholar] [CrossRef]
- Comini, L.V.; Spinelli, G.; Mannelli, G. Algorithm for the treatment of oral and peri-oral defects through local flaps. J. Cranio-Maxillofac. Surg. 2018, 46, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Deganello, A.; Manciocco, V.; Dolivet, G.; Leemans, C.R.; Spriano, G. Infrahyoid fascio-myocutaneous flap as an alternative to free radial forearm flap in head and neck reconstruction. Head Neck 2007, 29, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Shen, J.W. Preliminary report on a new approach to the reconstruction of tongue. Acta Acad. Med. Prim. Shanghai 1980, 7, 256–259. [Google Scholar]
- Wang, H.S.; Shen, J.W.; Ma, D.B.; Wang, J.D.; Tian, A.L. The infrahyoid myocutaneous flap for reconstruction after resection of head and neck cancer. Cancer 1986, 57, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Deganello, A.; Leemans, C.R. The infrahyoid flap: A comprehensive review of ana often overlooked reconstructive method. Oral Oncol. 2014, 50, 70410. [Google Scholar] [CrossRef] [PubMed]
- Magrin, J.; Kowalski, L.P.; Santo, G.E.; Waksmann, G.; DiPaula, R.A. Infrahyoid myocutaneous flap in head and neck reconstruction. Head Neck 1993, 15, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Islek, A.; Balci, M.K.; Yuksel, O.; Onal, K.; Arslanoglu, S.; Eren, E. Infrahyoid flap, a convenient alternative for reconstruction of tongue and floor of mouth defects: Case series. Turk. Arch. Otorhinolaringol. 2018, 56, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, S.J.; Yang, X.; Guo, H.; Liu, M. Infrahyoid myocutaneous flap for medium-sized head and neck defects: Surgical outcome and technique modification. Otolaryngol. Head Neck Surg. 2013, 148, 47–53. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Zhang, W.F.; Zhao, J.H. Reconstruction of intraoral defects after cancer surgery using cervical pedicle flaps. J. Oral Maxillofac. Surg. 2001, 59, 1142–1146. [Google Scholar] [CrossRef]
- Gewirtz, H.S.; Eilber, F.R.; Zarem, H.A. Use of the nasolabial flap for reconstruction of the floor of the mouth. Am. J. Surg. 1978, 136, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Nishibuko, S.; Matsuda, H.; Watanabe, S.; Tamura, H.; Tonogi, M. Reconstruction of maxillary palatal defects after partial maxillectomy using a pedicled buccal fat pad and a nasolabial flap. Clin. Case Rep. 2021, 9, e04442. [Google Scholar]
- Kallappa, S.; Shah, N. Outcome of nasolabial flap in the reconstruction of head and neck defects. Indian J. Surg. Oncol. 2019, 10, 77–581. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, S.E.; Nueangkhota, P.; Liang, Y.J.; Su, Y.X.; Liao, G.Q. Assessment of the contralateral facial artery pedicle nasolabial island flap for buccal defect repair. Int. J. Oral Maxillofac. Surg. 2020, 49, 862–866. [Google Scholar] [CrossRef]
- Nueangkhota, P.; Liang, Y.J.; Zheng, G.S.; Su, Y.X.; Yang, W.F.; Liao, G.Q. Reconstruction of tongue defects with the contralateral nasolabial island flap. J. Oral Maxillofac. Surg. 2016, 74, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Farr, H.W.; Jean-Gilles, B.; Die, A. Cervical island skin flap repair of oral and pharyngeal defects in the composite operation for cancer. Am. J. Surg. 1969, 18, 759–763. [Google Scholar] [CrossRef]
- Saito, H.; Tsuda, G.; Ohtsubo, T.; Noda, I.; Fujieda, S. Platysma myocutaneous flap including the external jugular vein with special reference to neck dissection. ORL J. Otorhinolaryngol. Relat. Spec. 1998, 60, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Futrell, J.W.; Johns, M.E.; Edgerton, M.T.; Cantrell, W.; Fitz-Hugh, G.S. Platysma myocutaneous flap for intra-oral reconstruction. Am. J. Surg. 1978, 136, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.J.; Nahai, F.; Mathes, S.J. Platysma musculocutaneous flap: Clinical and anatomic considerations in head and neck reconstruction. Am. J. Surg. 1982, 144, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Baur, D.A.; Helman, J.I. The posteriorly based platysma flap in oral and facial reconstruction: A case series. J. Oral Maxillofac. Surg. 2002, 60, 1147–1150. [Google Scholar] [CrossRef]
- Hurwitz, D.J.; Rabson, J.A.; Futrell, J.W. The anatomic basis for the platysma skin flap. Plast. Reconstr. Surg. 1983, 72, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Uehara, M.; Helman, J.I.; Lillie, J.H.; Brooks, S.L. Blood supply to the platysma muscle flap: An anatomic study with clinical correlation. J. Oral Maxillofac. Surg. 2001, 59, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Baur, D.A.; Williams, J.; Alakaily, X. The platysma myocutaneous flap. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.; Accorona, R.; Gazzini, L.; Giorgetti, G.; Tagliabue, M.; Bruschini, R.; Pietrobon, G.; Ansarin, M. Platysma myocutaneous flap revised in the free flaps era: Clinical experience in 61 patients. Acta Otorhinolaryngol. Ital. 2020, 40, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Gao, X.; Su, T.; Jiang, C.H.; Jian, X.C. Vertical platysma myocutaneous flap reconstruction for oral defects using three different incision designs: Experience with 68 cases. Int. J. Oral Maxillofac. Surg. 2018, 47, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Pascal, J.F.; Baudet, J.; Mondie, J.M.; Farhat, J.B.; Athoum, A.; Le Gaillard, P.; Peri, G. The submental island flap: A new donor site. Anatomy and clinical applications as a free or pedicled flap. Plast. Reconstr. Surg. 1993, 92, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.A.; Bayles, S.W.; Hayden, R.E. The submental flap: A modified technique for resident training. Laryngoscope 2007, 117, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; He, J.; Wang, F.; Liu, J.; Sun, B.; Zhang, W. Reconstruction of anterior mandibular defect using submental island flap pedicled with mental artery. Ear Nose Throat J. 2021, 8, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Faltaous, A.A.; Yetman, R.J. The submental artery flap: A anatomic study. Plast. Reconstr. Surg. 1996, 97, 56–60. [Google Scholar] [CrossRef]
- Magden, O.; Edizer, M.; Tayfur, V.; Atabey, A. Anatomic study of the vasculature of the submental artery flap. Plast. Reconstr. Surg. 2004, 114, 1719–1723. [Google Scholar] [CrossRef]
- Amin, A.A.; Jamali, O.M.; Ibrahim, A.S.; Rifaat, M.A.; Zedan, M.H. The contralateral based submental island flap for reconstruction of tongue and floor of mouth defects: Reliability and oncological outcome. Head Neck 2020, 42, 2920–2930. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.A.; Hartig, G.K.; Hanasono, M.M.; Lin, D.T.; Richmon, J.D. Locoregional flaps for oral cavity reconstruction: A review of modern options. Otolaryngol. Head Neck Surg. 2017, 157, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Mahieu, R.; Colletti, G.; Bonomo, P.; Parrinello, G.; Iavarone, A.; Dolivet, G.; Livi, L.; Deganello, A. Head and neck reconstruction with pedicle flaps in the free flap era. Acta Otorhinolaryngol. Ital. 2016, 36, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, B.; Ferri, A.; Ferrari, S.; Copelli, C.; Sesenna, E. Myomucosal cheek flaps: Applications in intraoral reconstruction using three different techniques. Oral Surg. Oral Med. Oral Pathol. Radiol. Endod. 2009, 108, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Braasch, D.C.; Lam, D.; Oh, E.S. Maxillofacial reconstruction with nasolabial and facial artery musculomucosal flaps. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Massarelli, O.; Baj, A.; Gobbi, R.; Soma, D.; Marelli, S.; De Riu, G.; Tullio, A.; Giannì, A.B. Cheek mucosa: A versatile donor site of myomucosal flaps. Technical and functional considerations. Head Neck 2013, 35, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Hanasono, M.M.; Matros, E.; Disa, J.J. Important aspects of head and neck reconstruction. Plast. Reconstr. Surg. 2014, 134, 968e–980e. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.R.; Schusterman, M.A.; Kroll, S.S.; Miller, M.J.; Reece, G.P.; Robb, G.L.; Ainslie, N. The radial forearm free flap for head and neck reconstruction: A review. Am. J. Surg. 1994, 168, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.F.; Johnson, J.T.; Shestak, K.C.; Myers, E.N.; Swartz, W.M. Microsurgical reconstruction of the head and neck: Interdisciplinary collaboration between head and neck surgeons and plastic surgeons in 305 cases. Ann. Plast. Surg. 1996, 36, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Amarante, J.; Malheiro, E.; Santa-Comba, A.; Costa-Ferreira, A.; Barroso, M.L. Free flaps in the reconstruction of head and neck. Clinical experience. Acta Med. Port. 1998, 11, 103–110. [Google Scholar]
- Issing, P.R.; Kempf, H.G.; Heppt, W.; Schonermark, M.; Lenarz, T. Reconstructive surgery in the head-neck area with regional and free tissue transfer. Laryngorhinootologie 1996, 75, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Liu, S.; Zheng, L.; Huang, M.; Zhang, J.; Zhang, J. New approach to an overlooked flap: Technique to augment venous drainage of the infrahyoid myocutaneous flap. Head Neck 2021, 43, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Tincani, A.J.; Del Negro, A.; Araújo, P.P.; Akashi, H.K.; Neves Fda, S.; Martins, A.S. Head and neck reconstruction using infrahyoid myocutaneous flaps. Sao Paulo Med. J. 2006, 124, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Rojananin, S.; Suphaphongs, N.; Ballantyne, A.J. The infrahyoid musculocutaneous flap in head and neck reconstruction. Am. J. Surg. 1991, 162, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Windfuhr, J.P.; Remmert, S. Infrahyoid myofascial flap for tongue reconstruction. Eur. Arch. Otorhinolaryngol. 2006, 263, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, R.; Menard, P.; Chout, P.; Favre-Dauvergne, E.; Berard, P.; Bertrand, J.C. Infrahyoid myocutaneous flap in reconstructive maxillofacial cancer and trauma surgery. Int. J. Oral Maxillofac. Surg. 1998, 27, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Mitra, G.V.; Bajaj, S.S.; Rajmohan, S.; Motiwale, T. Versatility of Modified Nasolabial Flap in Oral and Maxillofacial Surgery. Arch. Craniofacial Surg. 2017, 18, 243–248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qayyum, M.U.; Janjua, O.S.; Ul Haq, E.; Zahra, R. Nasolabial and extended nasolabial flaps for reconstruction in oral submucous fibrosis. J. Korean Assoc. Oral Maxillofac. Surg. 2018, 44, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, N. Unilateral subcutaneous pedicled nasolabial island flap for anterior mouth floor reconstruction. J. Oral Maxillofac. Surg. 2003, 61, 182–190. [Google Scholar] [CrossRef]
- Lazaridis, N.; Tilaveridis, I.; Karakasis, D. Superiorly or inferiorly based “islanded” nasolabial flap for buccal mucosa defects reconstruction. J. Oral Maxillofac. Surg. 2008, 66, 7–15. [Google Scholar] [CrossRef]
- Rökenes, H.K.; Bretteville, G.; Lövdal, O.; Boysen, M. The nasolabial skinflap in intraoral reconstruction. ORL J. Otorhinolaryngol. Relat. Spec. 1991, 53, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Uglesić, V.; Virag, M. Musculomucosal nasolabial island flaps for floor of mouth reconstruction. Br. J. Plast. Surg. 1995, 48, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Bande, C.; Joshi, A.; Gawande, M.; Tiwari, M.; Rode, V. Utility of superiorly based platysma myocutaneous flap for reconstruction of intraoral surgical defects: Our experience. Oral Maxillofac. Surg. 2018, 22, 45–51. [Google Scholar] [CrossRef]
- Puxeddu, R.; Dennis, S.; Ferreli, C.; Caldera, S.; Brennan, P.A. Platysma myocutaneous flap for reconstruction of skin defects in the head and neck. Br. J. Oral Maxillofac. Surg. 2008, 46, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Tosco, P.; Garzino-Demo, P.; Ramieri, G.; Tanteri, G.; Pecorari, G.; Caldarelli, C.; Garzaro, M.; Giordano, C.; Berrone, S. The platysma myocutaneous flap (PMF) for head and neck reconstruction: A retrospective and multicentric analysis of 91 T1-T2 patients. J. Cranio-Maxillofac. Surg. 2012, 40, e415–e418. [Google Scholar] [CrossRef] [PubMed]
- Pegan, A.; Rašić, I.; Bedeković, V.; Ivkić, M. Cheek reconstruction following facial malignant melanoma surgery with the platysma myocutaneous flap. Int. J. Oral Maxillofac. Surg. 2015, 44, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Künzel, J.; Iro, H.; Psychogios, G.; Zenk, J.; Koch, M. Closure of defects after resection of tumors of the oral cavity and the pharynx: Medium- to long-term oncologic and functional results with the myocutaneous platysma flap. Eur. Arch. Otorhinolaryngol. 2013, 270, 2537–2545. [Google Scholar] [CrossRef] [PubMed]
- Esclamado, R.M.; Burkey, B.B.; Carroll, W.R.; Bradford, C.R. The platysma myocutaneous flap. Indications and caveats. Arch. Otolaryngol. Head Neck Surg. 1994, 120, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Manni, J.J.; Bruaset, I. Reconstruction of the anterior oral cavity using the platysma myocutaneous island flap. Laryngoscope 1986, 96, 564–567. [Google Scholar] [CrossRef]
- Kummoona, R. Use of lateral cervical flap in reconstructive surgery of the orofacial region. Int. J. Oral Maxillofac. Surg. 1994, 23, 85–89. [Google Scholar] [CrossRef]
- Shen, Z.Z.; Lu, C.; Huang, L.; Li, N.; Wang, W.; Jiang, C. Assessment of surgical outcomes and oncological safety for submental artery perforator flap reconstruction after ablation of oral cancer. Br. J. Oral Maxillofac. Surg. 2021, 59, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Mishra, N.; Pati, D.; Samal, D.; Kar, I.B.; Mohapatra, D.; Sarkar, D.F. Oncologic safety of submental island flap reconstruction in clinically node-negative oral cancer patients: A prospective comparative study. Int. J. Oral Maxillofac. Surg. 2022, 51, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Rahpeyma, A.; Khajehahmadi, S. Oral reconstruction with submental flap. Ann. Maxillofac. Surg. 2013, 3, 144–147. [Google Scholar] [PubMed]
- Sittitrai, P.; Reunmakkaew, D.; Srivanitchapoom, C. Submental island flap versus radial forearm free flap for oral tongue reconstruction: A comparison of complications and functional outcomes. J. Laryngol. Otol. 2019, 133, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.A. The submental flap for head and neck reconstruction: Comparison of outcomes to the radial forearm free flap. Laryngoscope 2020, 130 (Suppl. S2), S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Aslam-Pervez, N.; Caldroney, S.J.; Isaiah, A.; Lubek, J.E. A Retrospective Volume Matched Analysis of the Submental Artery Island Pedicled Flap as Compared to the Forearm Free Flap: Is It a Good Alternative Choice for the Reconstruction of Defects of the Oral Cavity and Oropharynx? J. Oral Maxillofac. Surg. 2018, 76, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Forner, D.; Phillips, T.; Rigby, M.; Hart, R.; Taylor, M.; Trites, J. Submental island flap reconstruction reduces cost in oral cancer reconstruction compared to radial forearm free flap reconstruction: A case series and cost analysis. J. Otolaryngol. Head Neck Surg. 2016, 45, 11. [Google Scholar] [CrossRef]
- Paydarfar, J.A.; Patel, U.A. Submental island pedicled flap vs. radial forearm free flap for oral reconstruction: Comparison of outcomes. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Han, X.; Chen, H. Comparison of Clinical Results and Quality-of-Life in Tongue Cancer Patients Undergoing Submental Island Flap and Radial Forearm Free Flap Reconstruction. J. Oral Maxillofac. Surg. 2020, 78, 1639–1644. [Google Scholar] [CrossRef]
- Chitlangia, P.; Kumuran, E.; Sabitha, K.S. Use of nasolabial flap in intra and extraoral reconstruction: Our experience with 40 cases. J. Maxillofac. Oral Surg. 2012, 11, 451–454. [Google Scholar] [CrossRef]
- Bartal, N.; Temkin, D. Platysma myocutaneous flap reconstruction. Laryngoscope 1997, 107, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.A.; Sakkary, M.A.; Khalil, A.A.; Rifaat, M.A.; Zayed, S.B. The submental flap for oral cavity reconstruction: Extended indications and technical refinements. Head Neck Oncol. 2011, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Chotipanich, A.; Wongmanee, S. The infrahyoid myocutaneous flap for reconstruction after oral cancer resection: A retrospective single-surgeon study. World J. Otorhinolaryngol. Head Neck Surg. 2018, 4, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.A.; Ngo, T.X.; Nguyen, C.Q.; Wein, R.O. Vein preservation strategies to improve the survival rate of the infrahyoid musculocutaneous flap. Laryngoscope Investig. Otolaryngol. 2021, 6, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Minni, A.; Mascelli, A.; Suriano, M. The infrahyoid myocutaneous flap in intra-oral reconstruction as an alternative to free flaps. Acta Otolaryngol. 2010, 130, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramaniyan, M.; Rajappa, S.K.; Agarwal, M.; Chopra, A.; Singh, A.; Paul, R. Infrahyoid flap revisited—A head and neck surgical perspective in the Indian setting. Indian J. Cancer 2020, 57, 62–69. [Google Scholar] [PubMed]
- Bhambar, R.S.; Baliga, M.; Kumar, A.; Jagannathan, S.; Kumar, H.; Kumar, R.; Pokhrel, P.; Singh, H.P. Revisit of Nasolabial Flap in the Reconstruction of Defects Involving the Oral Floor. Niger. J. Surg. 2016, 22, 21–25. [Google Scholar] [PubMed]
- Chakrabarti, S.; Gupta, D.K.; Gupta, M.; Daga, D.; Mishra, A.; Sharma, S.S.; Chugh, R.; Singh, S.K. Versatility and Reliability of Islanded Pedicled Nasolabial Flap in Head and Neck Cancer Reconstruction. Laryngoscope 2020, 130, 1967–1972. [Google Scholar] [CrossRef]
- Rengifo, D.; Lian, T.T. Radial Forearm Tissue Transfer. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar] [PubMed]
- Otsuki, N.; Furukawa, T.; Avinçsal, M.O.; Teshima, M.; Shinomiya, H.; Oshikiri, T.; Nakamura, T.; Nomura, T.; Hashikawa, K.; Nibu, K.I. Results of free flap reconstruction for patients aged 80 years or older with head and neck cancer. Auris Nasus Larynx 2020, 47, 123–127. [Google Scholar] [CrossRef]
| Infrahyoid Flap | Nasolabial Flap | Plastysma Flap | Submental Flap | |
|---|---|---|---|---|
| Tongue | 380 (52.9%) | 219 (21.4%) | 199 (18.5%) | 1205 (39%) |
| Floor of the mouth | 275 (38.3%) | 372 (36.3%) | 437 (40.7%) | 702 (22.8%) |
| Alveolar ridge | 16 (2.2%) | 95 (9.3%) | 45 (41.9%) | 61 (2%) |
| Retromolar trigone | 3 (0.4%) | 95 (9.3%) | 60 (5.7%) | 29 (1%) |
| Buccal mucosa | 44 (6.1%) | 220 (21.5%) | 278 (25.9%) | 439 (14.2%) |
| Palate | 0 (0%) | 21 (2%) | 7 (0.6%) | 274 (8.9%) |
| Cheek | 0 (0%) | 0 (0%) | 48 (4.5%) | 374 (12.1%) |
| Infrahyoid Flap | Nasolabial Flap | Platysma Flap | Submental Flap | |
|---|---|---|---|---|
| Small | 103 (14.3%) | 380 (37.2%) | 369 (34.4%) | 204 (6.6%) |
| Medium | 615 (85.7%) | 605 (59.2%) | 659 (61.4%) | 2628 (85.2%) |
| Large | 0 | 37 (3.6%) | 46 (4.2%) | 252 (8.2%) |
| Infrahyoid Flap | Nasolabial Flap | Plastysma Flap | Submental Flap | |
|---|---|---|---|---|
| Flap necrosis (0.57%) | 6 | 4 | 11 | 13 |
| Flap loss (0.45%) | 1 | 6 | 6 | 14 |
| Dehiscence of the donor site (0.39%) | 0 | 9 | 5 | 9 |
| Orocutaneous fistulas (0.35%) | 1 | 4 | 6 | 10 |
| Skin necrosis (0.29%) | 6 | 0 | 7 | 4 |
| Oral incompetence (0.03%) | 0 | 2 | 0 | 0 |
| Trismus | 0 | 1 | 0 | 0 |
| Infrahyoid Flap | Nasolabial Flap | Plastysma Flap | Submental Flap | |
|---|---|---|---|---|
| Viable oral diet (1.12%) | 12 | 18 | 12 | 24 |
| Non-viable oral diet (0.05%) | 0 | 2 | 1 | 0 |
| Good cosmetic results (0.97%) | 2 | 18 | 12 | 25 |
| Bad cosmetic results (0.10%) | 0 | 3 | 1 | 2 |
| Adequate verbalization (0.63%) | 2 | 9 | 4 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Accorona, R.; Di Furia, D.; Cremasco, A.; Gazzini, L.; Mevio, N.; Pilolli, F.; Achena, A.; Iftikhar, H.; Awny, S.; Ormellese, G.L.; et al. Oral Reconstruction with Locoregional Flaps after Cancer Ablation: A Systematic Review of the Literature. J. Clin. Med. 2024, 13, 4181. https://doi.org/10.3390/jcm13144181
Accorona R, Di Furia D, Cremasco A, Gazzini L, Mevio N, Pilolli F, Achena A, Iftikhar H, Awny S, Ormellese GL, et al. Oral Reconstruction with Locoregional Flaps after Cancer Ablation: A Systematic Review of the Literature. Journal of Clinical Medicine. 2024; 13(14):4181. https://doi.org/10.3390/jcm13144181
Chicago/Turabian StyleAccorona, Remo, Domenico Di Furia, Alice Cremasco, Luca Gazzini, Niccolò Mevio, Francesco Pilolli, Andrea Achena, Haissan Iftikhar, Shadi Awny, Giorgio Luigi Ormellese, and et al. 2024. "Oral Reconstruction with Locoregional Flaps after Cancer Ablation: A Systematic Review of the Literature" Journal of Clinical Medicine 13, no. 14: 4181. https://doi.org/10.3390/jcm13144181
APA StyleAccorona, R., Di Furia, D., Cremasco, A., Gazzini, L., Mevio, N., Pilolli, F., Achena, A., Iftikhar, H., Awny, S., Ormellese, G. L., Dragonetti, A. G., & De Virgilio, A. (2024). Oral Reconstruction with Locoregional Flaps after Cancer Ablation: A Systematic Review of the Literature. Journal of Clinical Medicine, 13(14), 4181. https://doi.org/10.3390/jcm13144181






