Implementation of a Co-Design Strategy to Develop a Dashboard to Support Shared Decision Making in Advanced Cancer and Chronic Kidney Disease
Abstract
1. Background
2. Methods
2.1. Dashboard Co-Design Process
2.1.1. Proposed SDM Dashboard and Setting
2.1.2. Theoretical Model for Co-Production
2.1.3. Participants
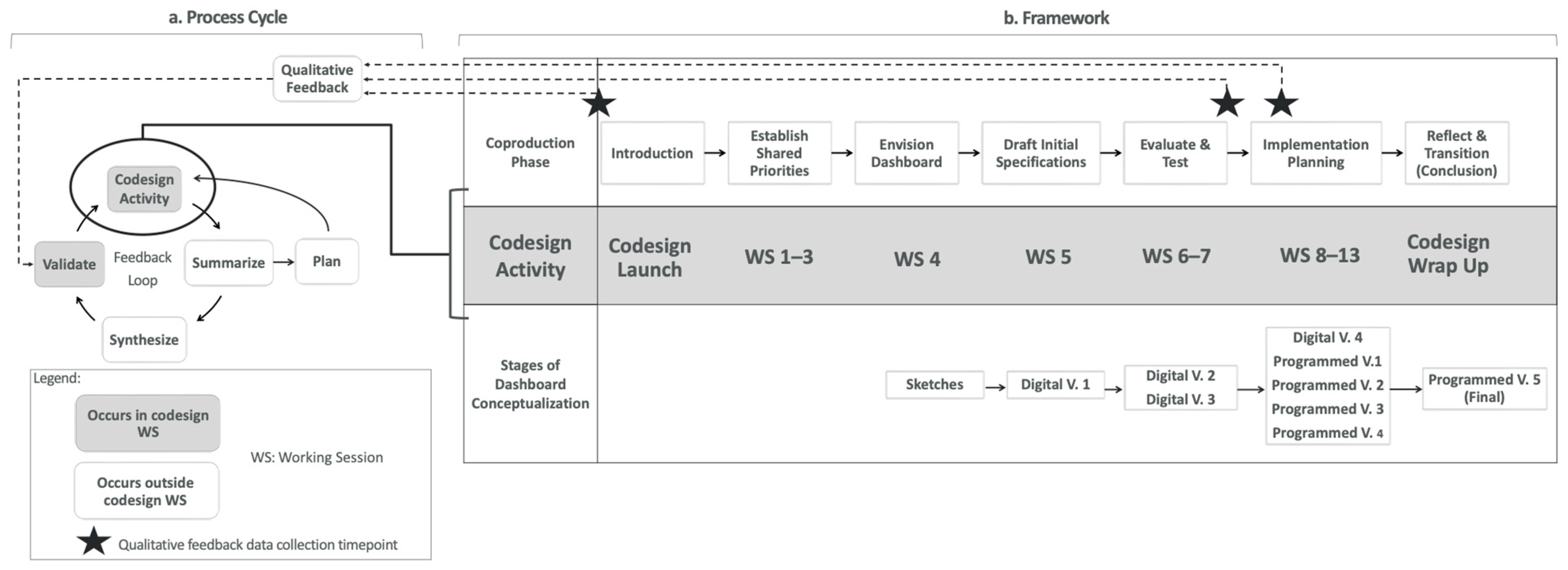
2.1.4. Dashboard Co-Design Process Cycle and Framework
2.1.5. Co-Design Strategies
2.2. Co-Design Implementation Outcomes
2.2.1. Fidelity to the Co-Design Process
2.2.2. Fidelity to Shared Decision Making within Co-Design Process
2.2.3. Acceptability
2.2.4. Feasibility
2.2.5. Adoption
2.2.6. Effectiveness
2.3. Data Analysis
3. Results
3.1. Characteristics of Co-Design Meetings
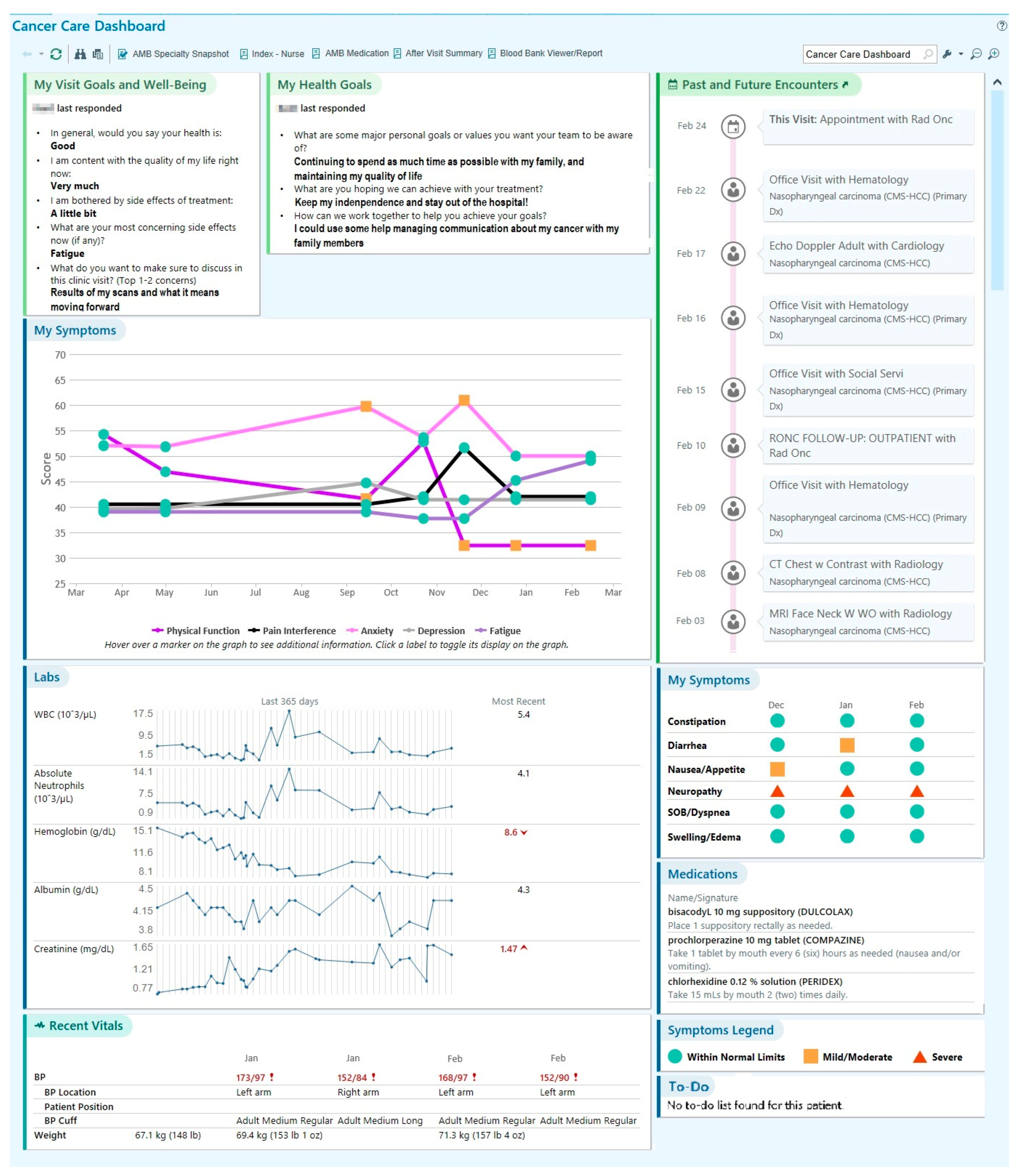
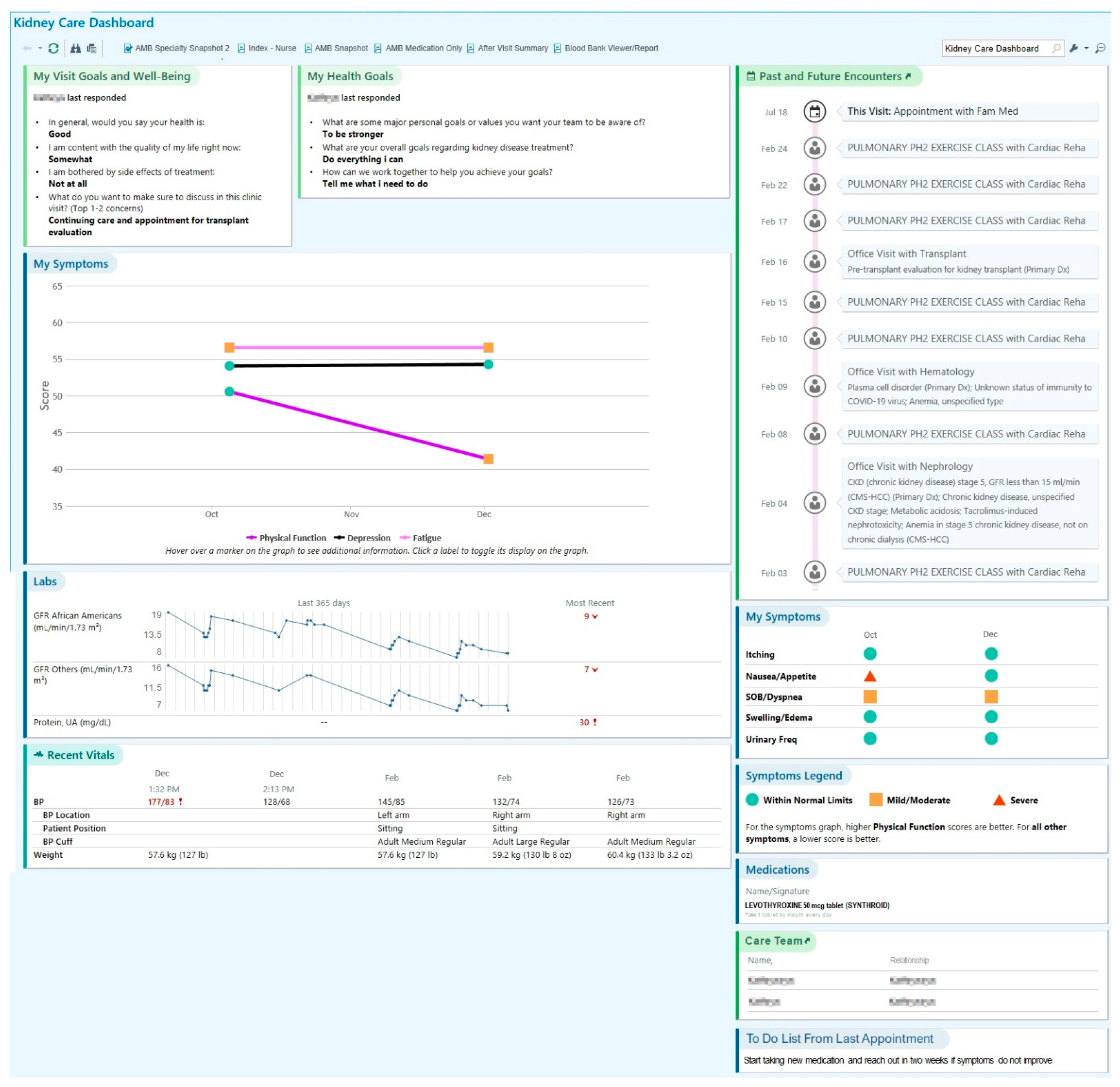
3.2. Content of SDM Dashboard
3.3. Facilitators and Barriers of Implementing Co-Design Strategies and Adaptations
3.4. Co-Design Implementation Outcomes
3.4.1. Fidelity and Adoption of Co-Design
3.4.2. Acceptability of Co-Design
3.4.3. Feasibility of Co-Design
3.4.4. Effectiveness
4. Discussion
4.1. Evaluation of the Co-Design Process
4.2. Co-Design Implementation Strategies and Learnings
5. Limitations
6. Implications and Future Directions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APP | Advanced Practice Professional (e.g., nurse practitioner, physician assistant) |
| CDIFM | Coproduction Design and Implementation Flow Model |
| CKD | Chronic Kidney Disease |
| EBP | Evidence-Based Practice |
| EHR | Electronic Health Record |
| GI | Gastrointestinal |
| HIT | Health Information Technology |
| IT | Information Technology |
| NM | Northwestern Medicine |
| NU | Northwestern University |
| PRO | Patient Reported Outcomes |
| PROM | Patient Report Outcome Measure |
| PROMIS | Patient Reported Outcome Measurement Information System |
| SDM | Shared decision making |
References
- National Quality Forum. The Care We Need: Driving Better Health Outcomes for People and Communities; National Quality Forum: Washington, DC, USA, 2020. [Google Scholar]
- Hughes, T.M.; Merath, K.; Chen, Q.; Sun, S.; Palmer, E.; Idrees, J.J.; Okunrintemi, V.; Squires, M.; Beal, E.W.; Pawlik, T.M. Association of shared decision-making on patient-reported health outcomes and healthcare utilization. Am. J. Surg. 2018, 216, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Elwyn, G.; Laitner, S.; Coulter, A.; Walker, E.; Watson, P.; Thomson, R. Implementing shared decision making in the NHS. BMJ 2010, 341, c5146. [Google Scholar] [CrossRef] [PubMed]
- Batalden, M.; Batalden, P.; Margolis, P.; Seid, M.; Armstrong, G.; Opipari-Arrigan, L.; Hartung, H. Coproduction of healthcare service. BMJ Qual. Saf. 2015, 25, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Elwyn, G.; Nelson, E.; Hager, A.; Price, A. Coproduction: When users define quality. BMJ Qual. Saf. 2019, 29, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Stiggelbout, A.M.; van der Weijden, T.; De Wit, M.P.T.; Frosch, D.; Légaré, F.; Montori, V.M.; Trevena, L.; Elwyn, G. Shared decision making: Really putting patients at the centre of healthcare. BMJ 2012, 344, e256. [Google Scholar] [CrossRef] [PubMed]
- Joseph-Williams, N.; Lloyd, A.; Edwards, A.; Stobbart, L.; Tomson, D.; Macphail, S.; Dodd, C.; Brain, K.; Elwyn, G.; Thomson, R. Implementing shared decision making in the NHS: Lessons from the MAGIC programme. BMJ 2017, 357, j1744. [Google Scholar] [CrossRef] [PubMed]
- Scholl, I.; LaRussa, A.; Hahlweg, P.; Kobrin, S.; Elwyn, G. Organizational-and system-level characteristics that influence implementation of shared decision-making and strategies to address them—A scoping review. Implement. Sci. 2018, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Pel-Littel, R.E.; Snaterse, M.; Teppich, N.M.; Buurman, B.M.; van Etten-Jamaludin, F.S.; van Weert, J.C.M.; Minkman, M.M.; Reimer, W.J.M.S.O. Barriers and facilitators for shared decision making in older patients with multiple chronic conditions: A systematic review. BMC Geriatr. 2021, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.M.-S.; Thavalathil, B.; Elwyn, G.; Nemeth, Z.; Dang, S. The promise of electronic health records to promote shared decision making: A narrative review and a look ahead. Med. Decis. Mak. 2018, 38, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.C.; Dixon-Woods, M.; Batalden, P.B.; Homa, K.; Van Citters, A.D.; Morgan, T.S.; Eftimovska, E.; Fisher, E.S.; Ovretveit, J.; Harrison, W.; et al. Patient focused registries can improve health, care, and science. BMJ 2016, 354, i3319. [Google Scholar] [CrossRef] [PubMed]
- Oliver, B.J.; Nelson, E.C.; Kerrigan, C.L. Turning feed-forward and feedback processes on patient-reported data into intelligent action and informed decision-making: Case studies and principles. Med. Care 2019, 57, S31–S37. [Google Scholar] [CrossRef] [PubMed]
- Van Citters, A.D.; Holthoff, M.M.; Kennedy, A.M.; Melmed, G.Y.; Oberai, R.; Siegel, C.A.; Weaver, A.; Nelson, E.C. Point-of-care dashboards promote coproduction of healthcare services for patients with inflammatory bowel disease. Int. J. Qual. Health Care 2021, 33 (Suppl. S2), ii40–ii47. [Google Scholar] [CrossRef] [PubMed]
- Van Citters, A.D.; Gifford, A.H.; Brady, C.; Dunitz, J.M.; Elmhirst, M.; Flath, J.; Laguna, T.A.; Moore, B.; Prickett, M.L.; Riordan, M.; et al. Formative evaluation of a dashboard to support coproduction of healthcare services in cystic fibrosis. J. Cyst. Fibros. 2020, 19, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Dixon-Woods, M.; Campbell, A.; Chang, T.; Martin, G.; Georgiadis, A.; Heney, V.; Chew, S.; Van Citters, A.; Sabadosa, K.A.; Nelson, E.C. A qualitative study of design stakeholders’ views of developing and implementing a registry-based learning health system. Implement. Sci. 2020, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Rutland, S.B.; Bergquist, R.P.; Hager, A.; Geurs, R.; Mims, C.; Gutierrez, H.H.; Oates, G.R. A mobile health platform for self-management of pediatric cystic fibrosis: Qualitative study of adaptation to stakeholder needs and integration in clinical settings. JMIR Form. Res. 2021, 5, e19413. [Google Scholar] [CrossRef] [PubMed]
- Taxter, A.; Johnson, L.; Tabussi, D.; Kimura, Y.; Donaldson, B.; Lawson, E.; Del Gaizo, V.; Vitelli, D.; Pinter, C.; Van Citters, A.; et al. Co-design of an electronic dashboard to support the coproduction of Care in Pediatric Rheumatic Disease: Human-centered design and usability testing. J. Particip. Med. 2022, 14, e34735. [Google Scholar] [CrossRef] [PubMed]
- Dolan, J.G.; Veazie, P.J.; Russ, A.J. Development and initial evaluation of a treatment decision dashboard. BMC Med. Inform. Decis. Mak. 2013, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Van der Horst, D.; van Uden-Kraan, C.; Parent, E.; Bart, J.; Waverijn, G.; Verberk-Jonkers, I.; Dorpel, M.v.D.; Pieterse, A.; Bos, W. Optimizing the use of patients’ individual outcome information–Development and usability tests of a Chronic Kidney Disease dashboard. Int. J. Med. Inform. 2022, 166, 104838. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Van Citters, A.; Khayal, I.; Saunders, C.; Ross, C.; Tomlin, S.; Cullinan, A.; MacMartin, M.; Holthoff, M.; Hort, S.; et al. Designing an electronic point-of-care dashboard to support serious illness clinical visits: A multi-stakeholder coproduction project (TH341B). J. Pain Symptom Manag. 2020, 59, 430–431. [Google Scholar] [CrossRef]
- Makhni, S. Co-creation in health systems design. AMA J. Ethics 2017, 19, 1070–1072. [Google Scholar]
- NWS Council of Social Service. Principles of Co-Design. Available online: https://www.ncoss.org.au/wp-content/uploads/2017/06/Codesign-principles.pdf (accessed on 26 May 2024).
- Bauer, M.S.; Damschroder, L.; Hagedorn, H.; Smith, J.; Kilbourne, A.M. An introduction to implementation science for the non-specialist. BMC Psychol. 2015, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.; Bhave, N.; Bragg-Gresham, J.; Balkrishnan, R.; Dietrich, X.; Eckard, A.; Eggers, P.W.; et al. US renal data system 2017 annual data report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2017, 71, A7. [Google Scholar] [CrossRef] [PubMed]
- Kaye, D.R.; Min, H.S.; Norton, E.C.; Ye, Z.; Li, J.; Dupree, J.M.; Ellimoottil, C.; Miller, D.C.; Herrel, L.A. System-level health-care integration and the costs of cancer care across the disease continuum. J. Oncol. Pract. 2018, 14, e149–e157. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.M.; Morken, V.; Peipert, J.D.; Yanez, B.; Garcia, S.F.; Barnard, C.; Hirschhorn, L.R.; Linder, J.A.; Jordan, N.; Ackermann, R.T.; et al. Patient-Reported Outcome Dashboards within the Electronic Health Record to Support Shared Decision-making: Protocol for Co-design and Clinical Evaluation with Patients with Advanced Cancer and Chronic Kidney Disease. JMIR Res. Protoc. 2022, 11, e38461. [Google Scholar] [CrossRef] [PubMed]
- Van Citters, A. Coproduction design and implementation flow. 2018. [Google Scholar]
- Van Citters, A.D.; Taxter, A.J.; Mathew, S.D.; Lawson, E.; Eseddi, J.; Del Gaizo, V.; Ahmad, J.; Bajaj, P.; Courtnay, S.; Davila, L.; et al. Enhancing Care Partnerships Using a Rheumatology Dashboard: Bringing Together What Matters Most to Both Patients and Clinicians. ACR Open Rheumatol. 2023, 5, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Brand, G.; Sheers, C.; Wise, S.; Seubert, L.; Clifford, R.; Griffiths, P.; Etherton-Beer, C. A research approach for co-designing education with healthcare consumers. Med. Educ. 2020, 55, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Mohindra, N.; Garcia, S.F.; Kircher, S. Development of an Electronic Health Record-integrated Patient-Reported Outcome-based Shared Decision-Making Dashboard in Oncology. JAMIA Open. in press.
- Green, T.; Bonner, A.; Teleni, L.; Bradford, N.; Purtell, L.; Douglas, C.; Yates, P.; MacAndrew, M.; Dao, H.Y.; Chan, R.J. Use and reporting of experience-based codesign studies in the healthcare setting: A systematic review. BMJ Qual. Saf. 2019, 29, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Rabin, B.; Glasgow, R.E. An implementation science perspective on psychological science and cancer: What is known and opportunities for research, policy, and practice. Am. Psychol. 2015, 70, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Eyles, H.; Jull, A.; Dobson, R.; Firestone, R.; Whittaker, R.; Morenga, L.T.; Goodwin, D.; Ni Mhurchu, C. Co-design of mHealth delivered interventions: A systematic review to assess key methods and processes. Curr. Nutr. Rep. 2016, 5, 160–167. [Google Scholar] [CrossRef]
- Slattery, P.; Saeri, A.K.; Bragge, P. Research co-design in health: A rapid overview of reviews. Health Res. Policy Syst. 2020, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Grindell, C.; Coates, E.; Croot, L.; O’cathain, A. The use of co-production, co-design and co-creation to mobilise knowledge in the management of health conditions: A systematic review. BMC Health Serv. Res. 2022, 22, 877. [Google Scholar] [CrossRef] [PubMed]
- Sligo, J.; Gauld, R.; Roberts, V.; Villa, L. A literature review for large-scale health information system project planning, implementation and evaluation. Int. J. Med. Inform. 2017, 97, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, S.; Ernestam, S.; Van Citters, A.; Lind, C.; Morgan, T.; Nelson, E. Creating a culture of health: Evolving healthcare systems and patient engagement. Qjm Int. J. Med. 2016, 110, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Iivanainen, S.; Ekstrom, J.; Virtanen, H.; Kataja, V.V.; Koivunen, J.P. Electronic patient-reported outcomes and machine learning in predicting immune-related adverse events of immune checkpoint inhibitor therapies. BMC Med. Inform. Decis. Mak. 2021, 21, 205. [Google Scholar] [CrossRef] [PubMed]
- Rodler, S.; Kopliku, R.; Ulrich, D.; Kaltenhauser, A.; Casuscelli, J.; Eismann, L.; Waidelich, R.; Buchner, A.; Butz, A.; Cacciamani, G.E.; et al. Patients’ Trust in Artificial Intelligence–based Decision-making for Localized Prostate Cancer: Results from a Prospective Trial. Trial. Eur. Urol. Focus 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Rivera, S.C.; Liu, X.; Hughes, S.E.; Dunster, H.; Manna, E.; Denniston, A.K.; Calvert, M.J. Embedding patient-reported outcomes at the heart of artificial intelligence health-care technologies. Lancet Digit. Health 2023, 5, e168–e173. [Google Scholar] [CrossRef] [PubMed]
| Cancer Team (N = 25) | CKD Team (N = 24) | Total (N = 35) | |
|---|---|---|---|
| Gender | |||
| Female | 20 (80%) | 14 (58.3%) | 24 (68.6%) |
| Male | 5 (20%) | 10 (41.7%) | 11 (31.4%) |
| Role | |||
| Patient | 2 (8%) | 3 (12.5%) | 5 (14.3%) |
| Care partner | 2 (8%) | 0 (0%) | 2 (5.7%) |
| Physician | 3 (12%) | 3 (12.5%) | 6 (17.1%) |
| Physician Assistant * | 1 (4%) | 1 (4.2%) | 2 (5.7%) |
| Nurse Practitioner * | 2 (8%) | 0 (0%) | 2 (5.7%) |
| Nurse * | 0 (0%) | 1 (4.2%) | 1 (2.9%) |
| Social Worker * | 1 (4%) | 1 (4.2%) | 2 (5.7%) |
| Clinical Psychologist ** | 3 (12%) | 3 (12.5%) | 3 (8.6%) |
| Dietician * | 0 (0%) | 1 (4.2%) | 1 (2.9%) |
| Health Informaticist | 3 (12%) | 3 (12.5%) | 3 (8.6%) |
| Health System Quality Leader ** | 4 (16%) | 4 (16.7%) | 4 (11.4%) |
| Researcher ** | 4 (16%) | 4 (16.7%) | 4 (11.4%) |
| Activity | Co-Design Phase | Objectives | Outcomes | CDIFM Phase (1–4) |
|---|---|---|---|---|
| Co-design Launch Meeting | Introduction |
|
| 1 |
| Working Session #1 | Establish Shared Priorities |
|
| 1, 2 |
| Working Session #2 | Establish Shared Priorities |
|
| 2 |
| Working Session #3 | Establish Shared Priorities |
|
| 2, 3 |
| Working Session #4 | Envision Dashboard |
|
| 1, 2, 3 |
| Working Session #5 | Draft initial Specifications |
|
| 2, 3, 4 |
| Working Session #6 | Evaluate and Test |
|
| 2, 3, 4 |
| Working Session #7 | Evaluate and Test |
|
| 2, 3 |
| Working Session #8 | Implementation Planning |
|
| 2, 3 |
| Working Session #9 | Implementation Planning |
|
| 2, 3 |
| Working Session #10 | Implementation Planning |
|
| 2, 3 |
| Working Session #11 | Implementation Planning |
|
| 3, 4 |
| Physician Champion Dashboard Implementation Survey | Implementation Planning |
|
| Not a CDIFM Phase |
| Working Session #12 | Implementation Planning |
|
| 2, 3 |
| Working Session #13 (Physician Champion Working Meeting) | Implementation Planning |
|
| 2, 3, 4 |
| Co-design Wrap Up Meeting | Reflect & Transition |
|
| 4 |
| Strategy | Inclusivity | Respect | Participation | Iteration | Outcomes Focused |
|---|---|---|---|---|---|
| Stakeholder feedback on evolving dashboard prototype | X | X | X | X | |
| Inclusion of HIT | X | X | X | X | |
| Design of sessions to accommodate stakeholders | X | X | X | ||
| Establish principles of equity in contribution of ideas | X | X | X | ||
| Establish agreed on goals of the dashboard | X | ||||
| External consultation from Dartmouth | X | X | |||
| Revisiting of CDFIM concepts throughout process | X | X | |||
| Incorporation of virtual participation options | X | X | X |
| Domain(s) | Description | Scoring Metric | Assessment Point(s) | Data Sources |
|---|---|---|---|---|
| Fidelity: the degree to which an intervention is implemented as planned | Three items measuring the perceived degree to which the following occurred during each co-design session met the criteria for SDM as part of co-design (from 0 to 4):
| Primary: Total scores of 0 to 12 (low SDM to high SDM) Secondary: Presence/absence of “Top Box” score (a 12 out of 12 on the total score) | Administered after each co-design session | Adapted CollaboRATE measure |
| Acceptability: the degree to which users find the intervention (co-design) satisfactory, agreeable | Three items measuring the perceived degree to which the following occurred during co-design process as a whole met the criteria for SDM as part of co-design (from 0 to 4):
| Primary: Total scores of 0 to 12 (low SDM to high SDM) Secondary: Presence/absence of “Top Box” score (a 12 out of 12 on the total score) | Administered after the last co-design session in a post-co-design reflection survey | Adapted CollaboRATE measure |
| Fidelity: degree to which 7 practices of codesign were met Adoption: Participation in codesign | 7 practices, rated from 1 (absent) to 3 (fulfilled):
| Total scores of 7 to 21 | Rated at each co-design session by outside observers | Observer-rated rubrics of co-design practices |
| Fidelity: degree to which the 5 core principles of codesign were met | 5 key principles of co-design:
| Scores from 5 to 20 | Administered after the last co-design session in a post-co-design reflection survey | Co-design participant-rated surveys of principles of co-design |
| Feasibility: ability of stakeholders to participate in the co-design process Adoption: actual participation of the stakeholder on the co-design process | Includes documentation of stakeholder representation and meeting modality across all | Mean scores of stakeholder representation from 0 to 1, indicating the proportion of stakeholder types represented across the co-design sessions. Frequency of attendance modalities from 0% to 100% | Calculated after the co-design process | Co-design session attendance logs (Supplementary Materials File S1) |
| Effectiveness: degree to which the co-design process resulted in a dashboard designed to meet the project goals | Poll of perceptions on whether the dashboard objectives were met after session 13. | Proportion of respondents selecting each response option (yes, partially, no) from 0% to 100% | Administered after the last co-design session in a post-co-design reflection survey | Co-design effectiveness poll |
| Implementation Outcome Measure | Respondents/Data Sources | Metric | Observed Range | Mean | Standard Deviation |
|---|---|---|---|---|---|
| Fidelity by session | Co-design team members | Adapted collaboRATE (see Table 4; Score 0–12) | 4–12 | 10.0 | 1.7 |
| Fidelity, adoption by session | Observers | Observer Rubric (Score 7–21) | 13–21 | 19.1 | 1.6 |
| Fidelity, rated after co-design process was complete | Co-design team members | Key principles of co-design (Score 5–20) | 15–20 | 18.5 | 1.4 |
| Acceptability, rated after co-design process was complete | Co-design team members | Adapted collaboRATE (see Table 4; Score 0–12) | 7–12 | 10.4 | 1.4 |
| Feasibility, adoption | Program data | Attendee representation from 0 to 1 (combined groups) | 0.71–1.00 | 0.90 | 0.07 |
| Attendee representation from 0 to 1 (cancer group) | 0.71–1.00 | 0.95 | 0.09 | ||
| Attendee representation from 0 to 1 (kidney disease group) | 0.71–1.00 | 0.85 | 0.07 | ||
| Frequency of sessions with live or remote participation | Group | Live | Remote | ||
| Cancer | 14/15 (93%) | 9/15 (60%) | |||
| Kidney Disease | 14/15 (93%) | 14/15 (93%) | |||
| Effectiveness | “Does the dashboard meet the intended (group defined) objective?” | Yes, partially, no | N = 10 Yes 50% (5) Partially 50% (5) No 0% (0) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morken, V.; Perry, L.M.; Coughlin, A.; O’Connor, M.; Chmiel, R.; Xinos, S.; Peipert, J.D.; Garcia, S.F.; Linder, J.A.; Ackermann, R.T.; et al. Implementation of a Co-Design Strategy to Develop a Dashboard to Support Shared Decision Making in Advanced Cancer and Chronic Kidney Disease. J. Clin. Med. 2024, 13, 4178. https://doi.org/10.3390/jcm13144178
Morken V, Perry LM, Coughlin A, O’Connor M, Chmiel R, Xinos S, Peipert JD, Garcia SF, Linder JA, Ackermann RT, et al. Implementation of a Co-Design Strategy to Develop a Dashboard to Support Shared Decision Making in Advanced Cancer and Chronic Kidney Disease. Journal of Clinical Medicine. 2024; 13(14):4178. https://doi.org/10.3390/jcm13144178
Chicago/Turabian StyleMorken, Victoria, Laura M. Perry, Ava Coughlin, Mary O’Connor, Ryan Chmiel, Stavroula Xinos, John Devin Peipert, Sofia F. Garcia, Jeffrey A. Linder, Ronald T. Ackermann, and et al. 2024. "Implementation of a Co-Design Strategy to Develop a Dashboard to Support Shared Decision Making in Advanced Cancer and Chronic Kidney Disease" Journal of Clinical Medicine 13, no. 14: 4178. https://doi.org/10.3390/jcm13144178
APA StyleMorken, V., Perry, L. M., Coughlin, A., O’Connor, M., Chmiel, R., Xinos, S., Peipert, J. D., Garcia, S. F., Linder, J. A., Ackermann, R. T., Kircher, S., Mohindra, N. A., Aggarwal, V., Weitzel, M., Nelson, E. C., Elwyn, G., Van Citters, A. D., Barnard, C., Cella, D., & Hirschhorn, L. R. (2024). Implementation of a Co-Design Strategy to Develop a Dashboard to Support Shared Decision Making in Advanced Cancer and Chronic Kidney Disease. Journal of Clinical Medicine, 13(14), 4178. https://doi.org/10.3390/jcm13144178






