Differences in Eotaxin Serum Levels between Polytraumatized Patients with and without Concomitant Traumatic Brain Injury—A Matched Pair Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Time Course of Median Protein Serum Levels
3.2. Spearman Correlations
3.3. Univariable Logistic Regression Analysis
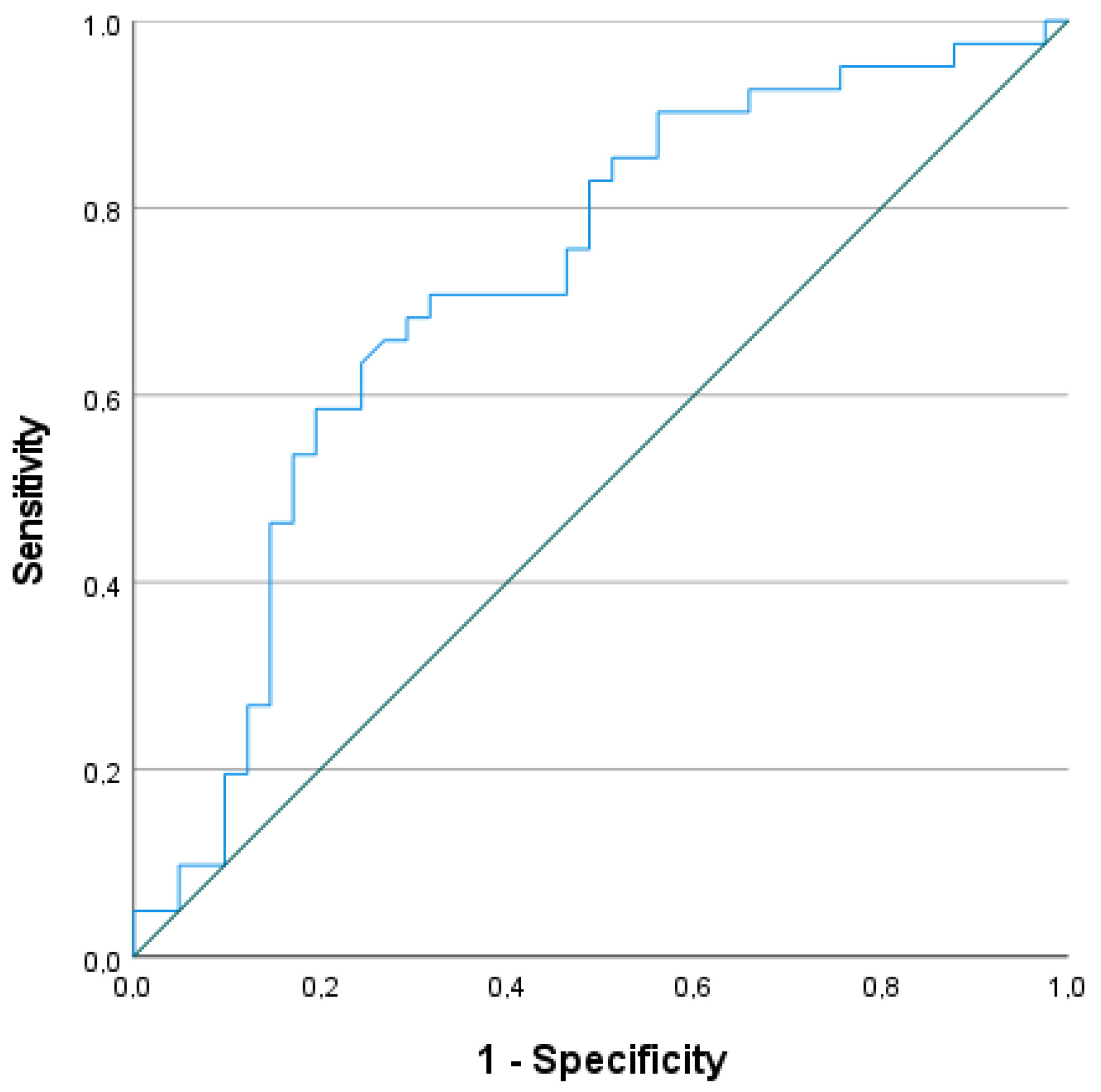
3.4. ROC Statistics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Åkerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic brain injury: Progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef]
- Johnson, L.W.; Diaz, I. Exploring the Social Determinants of Health and Health Disparities in Traumatic Brain Injury: A Scoping Review. Brain Sci. 2023, 13, 707. [Google Scholar] [CrossRef]
- Huang, X.-F.; Ma, S.-F.; Jiang, X.-H.; Song, R.-J.; Li, M.; Zhang, J.; Sun, T.-J.; Hu, Q.; Wang, W.-R.; Yu, A.-Y.; et al. Causes and global, regional, and national burdens of traumatic brain injury from 1990 to 2019. Chin. J. Traumatol. 2024, in press. [CrossRef]
- Guan, B.; Anderson, D.B.; Chen, L.; Feng, S.; Zhou, H. Global, regional and national burden of traumatic brain injury and spinal cord injury, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e075049. [Google Scholar] [CrossRef]
- Mair, O.; Greve, F.; Lefering, R.; Biberthaler, P.; Hanschen, M. The outcome of severely injured patients following traumatic brain injury is affected by gender-A retrospective, multicenter, matched-pair analysis utilizing data of the TraumaRegister DGU®. Front. Neurosci. 2022, 16, 974519. [Google Scholar] [CrossRef]
- Niemeyer, M.; Jochems, D.; Houwert, R.M.; van Es, M.A.; Leenen, L.; van Wessem, K. Mortality in polytrauma patients with moderate to severe TBI on par with isolated TBI patients: TBI as last frontier in polytrauma patients. Injury 2022, 53, 1443–1448. [Google Scholar] [CrossRef]
- Hardman, J.M.; Manoukian, A. Pathology of head trauma. Neuroimaging Clin. N. Am. 2002, 12, 175–187, vii. [Google Scholar] [CrossRef]
- Butterfield, M.; Bodnar, D.; Williamson, F.; Parker, L.; Ryan, G. Prevalence of secondary insults and outcomes of patients with traumatic brain injury intubated in the prehospital setting: A retrospective cohort study. Emerg. Med. J. 2023, 40, 167–174. [Google Scholar] [CrossRef]
- Howe, E.I.; Andelic, N.; Fure, S.C.R.; Røe, C.; Søberg, H.L.; Hellstrøm, T.; Spjelkavik, Ø.; Enehaug, H.; Lu, J.; Ugelstad, H.; et al. Cost-effectiveness analysis of combined cognitive and vocational rehabilitation in patients with mild-to-moderate TBI: Results from a randomized controlled trial. BMC Health Serv. Res. 2022, 22, 185. [Google Scholar] [CrossRef]
- Woodcock, T.; Morganti-Kossmann, M.C. The role of markers of inflammation in traumatic brain injury. Front. Neurol. 2013, 4, 18. [Google Scholar] [CrossRef]
- Ghaith, H.S.; Nawar, A.A.; Gabra, M.D.; Abdelrahman, M.E.; Nafady, M.H.; Bahbah, E.I.; Ebada, M.A.; Ashraf, G.M.; Negida, A.; Barreto, G.E. A Literature Review of Traumatic Brain Injury Biomarkers. Mol. Neurobiol. 2022, 59, 4141–4158. [Google Scholar] [CrossRef]
- Gerber, K.S.; Alvarez, G.; Alamian, A.; Behar-Zusman, V.; Downs, C.A. Biomarkers of Neuroinflammation in Traumatic Brain Injury. Clin. Nurs. Res. 2022, 31, 1203–1218. [Google Scholar] [CrossRef]
- Johnson, N.H.; Hadad, R.; Taylor, R.R.; Pilar, J.R.; Salazar, O.; Llompart-Pou, J.A.; Dietrich, W.D.; Keane, R.W.; Pérez-Bárcena, J.; Vaccari, J.P.d.R. Inflammatory Biomarkers of Traumatic Brain Injury. Pharmaceuticals 2022, 15, 660. [Google Scholar] [CrossRef] [PubMed]
- Chaban, V.; Clarke, G.J.; Skandsen, T.; Islam, R.; Einarsen, C.E.; Vik, A.; Damås, J.K.; Mollnes, T.E.; Håberg, A.K.; Pischke, S.E. Systemic Inflammation Persists the First Year after Mild Traumatic Brain Injury: Results from the Prospective Trondheim Mild Traumatic Brain Injury Study. J. Neurotrauma. 2020, 37, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Devoto, C.; Arcurio, L.; Fetta, J.; Ley, M.; Rodney, T.; Kanefsky, R.; Gill, J. Inflammation Relates to Chronic Behavioral and Neurological Symptoms in Military Personnel with Traumatic Brain Injuries. Cell Transplant. 2017, 26, 1169–1177. [Google Scholar] [CrossRef]
- Yue, J.K.; Kobeissy, F.H.; Jain, S.; Sun, X.; Phelps, R.R.; Korley, F.K.; Gardner, R.C.; Ferguson, A.R.; Huie, J.R.; Schneider, A.L.; et al. Neuroinflammatory Biomarkers for Traumatic Brain Injury Diagnosis and Prognosis: A TRACK-TBI Pilot Study. Neurotrauma Rep. 2023, 4, 171–183. [Google Scholar] [CrossRef]
- DiPiro, J.T.; Howdieshell, T.R.; Goddard, J.K.; Callaway, D.B.; Hamilton, R.G.; Mansberger, A.R., Jr. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch. Surg. 1995, 130, 1159–1162, discussion 62–63. [Google Scholar] [CrossRef]
- Miñambres, E.; Cemborain, A.; Sánchez-Velasco, P.; Gandarillas, M.; Díaz-Regañón, G.; Sánchez-González, U.; Leyva-Cobián, F. Correlation between transcranial interleukin-6 gradient and outcome in patients with acute brain injury. Crit. Care Med. 2003, 31, 933–938. [Google Scholar] [CrossRef]
- Tsitsipanis, C.; Miliaraki, M.; Paflioti, E.; Lazarioti, S.; Moustakis, N.; Ntotsikas, K.; Theofanopoulos, A.; Ilia, S.; Vakis, A.; Simos, P.; et al. Inflammation biomarkers IL-6 and IL-10 may improve the diagnostic and prognostic accuracy of currently authorized traumatic brain injury tools. Exp. Ther. Med. 2023, 26, 364. [Google Scholar] [CrossRef]
- Gebhard, F.; Pfetsch, H.; Steinbach, G.; Strecker, W.; Kinzl, L.; Brückner, U.B. Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch. Surg. 2000, 135, 291–295. [Google Scholar] [CrossRef]
- Di Battista, A.P.; Rhind, S.G.; Hutchison, M.G.; Hassan, S.; Shiu, M.Y.; Inaba, K.; Topolovec-Vranic, J.; Neto, A.C.; Rizoli, S.B.; Baker, A.J. Inflammatory cytokine and chemokine profiles are associated with patient outcome and the hyperadrenergic state following acute brain injury. J. Neuroinflammation 2016, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Seekamp, A.; van Griensven, M.; Lehmann, U.; Molituris, U.; Hildebrandt, F.; Pohlemann, T. Serum IL-6, IL-8 and IL-10 Levels in Multiple Trauma Compared to Traumatic Brain Injury and Combined Trauma. Eur. J. Trauma. 2002, 28, 183–189. [Google Scholar] [CrossRef]
- Rowland, B.; Savarraj, J.P.; Karri, J.; Zhang, X.; Cardenas, J.; Choi, H.A.; Holcomb, J.B.; Wade, C.E. Acute Inflammation in Traumatic Brain Injury and Polytrauma Patients Using Network Analysis. Shock 2020, 53, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Clausen, F.; Marklund, N.; Hillered, L. Acute Inflammatory Biomarker Responses to Diffuse Traumatic Brain Injury in the Rat Monitored by a Novel Microdialysis Technique. J. Neurotrauma. 2019, 36, 201–211. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Sokol, C.L.; Luster, A.D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.S.; Ley, K. Chemokines and chemokine receptors in leukocyte trafficking. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R7–R28. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Meng, Q.; Zeibecoglou, K.; Robinson, D.S.; Macfarlane, A.; Humbert, M.; Kay, A.B. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (Intrinsic) asthmatics. J. Immunol. 1999, 163, 6321–6329. [Google Scholar] [CrossRef]
- Filho, W.E.; Marcon, S.; Krupek, T.; Previdelli, I.; Pereira, O.; Silva, M.; Bazotte, R. Blood levels of pro-inflammatory and anti-inflammatory cytokines during an oral glucose tolerance test in patients with symptoms suggesting reactive hypoglycemia. Braz. J. Med. Biol. Res. 2016, 49, e5195. [Google Scholar] [CrossRef]
- Kodali, R.B.; Kim, W.J.; Galaria, I.I.; Miller, C.; Schecter, A.D.; Lira, S.A.; Taubman, M.B. CCL11 (Eotaxin) induces CCR3-dependent smooth muscle cell migration. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1211–1216. [Google Scholar] [CrossRef]
- Kitaura, M.; Nakajima, T.; Imai, T.; Harada, S.; Combadiere, C.; Tiffany, H.L.; Murphy, P.M.; Yoshie, O. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J. Biol. Chem. 1996, 271, 7725–7730. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.D.; Marleau, S.; Griffiths-Johnson, D.A.; Jose, P.J.; Williams, T.J. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J. Exp. Med. 1995, 182, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Palframan, R.T.; Collins, P.D.; Williams, T.J.; Rankin, S.M. Eotaxin induces a rapid release of eosinophils and their progenitors from the bone marrow. Blood 1998, 91, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Ponath, P.D.; Qin, S.; Ringler, D.J.; Clark-Lewis, I.; Wang, J.; Kassam, N.; Smith, H.; Shi, X.; A Gonzalo, J.; Newman, W.; et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J. Clin. Investig. 1996, 97, 604–612. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Wells, T.N.; Lukacs, N.W.; E Proudfoot, A.; Kunkel, S.L.; Williams, T.J.; Hellewell, P.G. Chemokine-induced eosinophil recruitment. Evidence of a role for endogenous eotaxin in an in vivo allergy model in mouse skin. J. Clin. Investig. 1997, 100, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Zepeda, E.A.; Rothenberg, M.E.; Ownbey, R.T.; Celestin, J.; Leder, P.; Luster, A.D. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat. Med. 1996, 2, 449–456. [Google Scholar] [CrossRef]
- Forssmann, U.; Uguccioni, M.; Loetscher, P.; Dahinden, C.A.; Langen, H.; Thelen, M.; Baggiolini, M. Eotaxin-2, a novel CC chemokine that is selective for the chemokine receptor CCR3, and acts like eotaxin on human eosinophil and basophil leukocytes. J. Exp. Med. 1997, 185, 2171–2176. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Mackay, C.R.; Lanzavecchia, A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science 1997, 277, 2005–2007. [Google Scholar] [CrossRef]
- de Paulis, A.; Annunziato, F.; Di Gioia, L.; Romagnani, S.; Carfora, M.; Beltrame, C.; Marone, G.; Romagnani, P. Expression of the chemokine receptor CCR3 on human mast cells. Int. Arch. Allergy Immunol. 2001, 124, 146–150. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Ying, S.; Sabroe, I.; Stubbs, V.L.; Soler, D.; Williams, T.J.; Kay, A.B. Eotaxin (CCL11) and eotaxin-2 (CCL24) induce recruitment of eosinophils, basophils, neutrophils, and macrophages as well as features of early- and late-phase allergic reactions following cutaneous injection in human atopic and nonatopic volunteers. J. Immunol. 2002, 169, 2712–2718. [Google Scholar] [CrossRef]
- Hiskens, M.I.; Schneiders, A.G.; Angoa-Pérez, M.; Vella, R.K.; Fenning, A.S. Blood biomarkers for assessment of mild traumatic brain injury and chronic traumatic encephalopathy. Biomarkers 2020, 25, 213–227. [Google Scholar] [CrossRef]
- Adar, T.; Shteingart, S.; Ben Ya’acov, A.; Bar-Gil Shitrit, A.; Goldin, E. From airway inflammation to inflammatory bowel disease: Eotaxin-1, a key regulator of intestinal inflammation. Clin. Immunol. 2014, 153, 199–208. [Google Scholar] [CrossRef]
- Kumar, R.K.; Thomas, P.S.; Seetoo, D.-Q.; Herbert, C.; McKenzie, A.N.J.; Foster, P.S.; Lloyd, A.R. Eotaxin expression by epithelial cells and plasma cells in chronic asthma. Lab. Investig. 2002, 82, 495–504. [Google Scholar] [CrossRef][Green Version]
- Bartels, J.; Schlüter, C.; Richter, E.; Noso, N.; Kulke, R.; Christophers, E.; Schröder, J.-M. Human dermal fibroblasts express eotaxin: Molecular cloning, mRNA expression, and identification of eotaxin sequence variants. Biochem. Biophys. Res. Commun. 1996, 225, 1045–1051. [Google Scholar] [CrossRef]
- Boström, E.A.; Kindstedt, E.; Sulniute, R.; Palmqvist, P.; Majster, M.; Holm, C.K.; Zwicker, S.; Clark, R.; Önell, S.; Johansson, I.; et al. Increased Eotaxin and MCP-1 Levels in Serum from Individuals with Periodontitis and in Human Gingival Fibroblasts Exposed to Pro-Inflammatory Cytokines. PLoS ONE 2015, 10, e0134608. [Google Scholar] [CrossRef]
- Maarof, M.; Law, J.X.; Chowdhury, S.R.; Khairoji, K.A.; Saim, A.B.; Idrus, R.B. Secretion of wound healing mediators by single and bi-layer skin substitutes. Cytotechnology 2016, 68, 1873–1884. [Google Scholar] [CrossRef]
- Alblowi, J.; Tian, C.; Siqueira, M.F.; Kayal, R.A.; McKenzie, E.; Behl, Y.; Gerstenfeld, L.; Einhorn, T.A.; Graves, D.T. Chemokine expression is upregulated in chondrocytes in diabetic fracture healing. Bone 2013, 53, 294–300. [Google Scholar] [CrossRef]
- Pease, J.E.; Williams, T.J. Eotaxin and asthma. Curr. Opin. Pharmacol. 2001, 1, 248–253. [Google Scholar] [CrossRef]
- Paplińska, M.; Hermanowicz-Salamon, J.; Nejman-Gryz, P.; Białek-Gosk, K.; Rubinsztajn, R.; Arcimowicz, M.; Placha, G.; Góra, J.; Chazan, R.; Grubek-Jaworska, H. Expression of eotaxins in the material from nasal brushing in asthma, allergic rhinitis and COPD patients. Cytokine 2012, 60, 393–399. [Google Scholar] [CrossRef]
- Eperon, S.; Sauty, A.; Lanz, R.; Leimgruber, A.; Lurati, F.; Guex-Crosier, Y. Eotaxin-1 (CCL11) up-regulation in tears during seasonal allergic conjunctivitis. Graefes Arch. Clin. Exp. Ophthalmol. 2004, 242, 966–970. [Google Scholar] [CrossRef][Green Version]
- Owczarek, W.; Paplińska, M.; Targowski, T.; Jahnz-Różyk, K.; Paluchowska, E.; Kucharczyk, A.; Kasztalewicz, B. Analysis of eotaxin 1/CCL11, eotaxin 2/CCL24 and eotaxin 3/CCL26 expression in lesional and non-lesional skin of patients with atopic dermatitis. Cytokine 2010, 50, 181–185. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, J.; Bi, H.; Li, L.; Gao, W.; Huang, M.; Adcock, I.M.; Barnes, P.J.; Yao, X. CCL11 as a potential diagnostic marker for asthma? J. Asthma. 2014, 51, 847–854. [Google Scholar] [CrossRef]
- Tacke, F.; Trautwein, C.; Yagmur, E.; Hellerbrand, C.; Wiest, R.; A Brenner, D.; Schnabl, B. Up-regulated eotaxin plasma levels in chronic liver disease patients indicate hepatic inflammation, advanced fibrosis and adverse clinical course. J. Gastroenterol. Hepatol. 2007, 22, 1256–1264. [Google Scholar] [CrossRef]
- Pham, B.-N.; Bernuau, J.; Durand, F.; Sauvanet, A.; Degott, C.; Prin, L.; Janin, A. Eotaxin expression and eosinophil infiltrate in the liver of patients with drug-induced liver disease. J. Hepatol. 2001, 34, 537–547. [Google Scholar] [CrossRef]
- Kokkonen, H.; Söderström, I.; Rocklöv, J.; Hallmans, G.; Lejon, K.; Rantapää Dahlqvist, S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010, 62, 383–391. [Google Scholar] [CrossRef]
- Sørensen, T.L.; Tani, M.; Jensen, J.; Pierce, V.; Lucchinetti, C.; Folcik, V.A.; Qin, S.; Rottman, J.; Sellebjerg, F.; Strieter, R.M.; et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Investig. 1999, 103, 807–815. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Gama, C.S.; Rocha, N.P.; Teixeira, M.M. Revisiting the Role of Eotaxin-1/CCL11 in Psychiatric Disorders. Front. Psychiatry 2018, 9, 241. [Google Scholar] [CrossRef]
- Milenkovic, V.M.; Stanton, E.H.; Nothdurfter, C.; Rupprecht, R.; Wetzel, C.H. The Role of Chemokines in the Pathophysiology of Major Depressive Disorder. Int. J. Mol. Sci. 2019, 20, 2283. [Google Scholar] [CrossRef]
- Panizzutti, B.; Gubert, C.; Schuh, A.; Ferrari, P.; Bristot, G.; Fries, G.; Massuda, R.; Walz, J.; Rocha, N.; Berk, M.; et al. Increased serum levels of eotaxin/CCL11 in late-stage patients with bipolar disorder: An accelerated aging biomarker? J. Affect. Disord. 2015, 182, 64–69. [Google Scholar] [CrossRef]
- Czepielewski, L.S.; Massuda, R.; Panizzutti, B.; Grun, L.K.; Barbé-Tuana, F.M.; Teixeira, A.L.; Barch, D.M.; Gama, C.S. Telomere Length and CCL11 Levels are Associated With Gray Matter Volume and Episodic Memory Performance in Schizophrenia: Evidence of Pathological Accelerated Aging. Schizophr. Bull. 2018, 44, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Sirivichayakul, S.; Kanchanatawan, B.; Thika, S.; Carvalho, A.F.; Maes, M. Eotaxin, an Endogenous Cognitive Deteriorating Chemokine (ECDC), Is a Major Contributor to Cognitive Decline in Normal People and to Executive, Memory, and Sustained Attention Deficits, Formal Thought Disorders, and Psychopathology in Schizophrenia Patients. Neurotox. Res. 2019, 35, 122–138. [Google Scholar] [CrossRef]
- Agarwal, M.; He, C.; Siddiqui, J.; Wei, J.T.; Macoska, J.A. CCL11 (eotaxin-1): A new diagnostic serum marker for prostate cancer. Prostate 2013, 73, 573–581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, C.; Wang, M.; Bi, X.; Fan, Z.; Feng, D.; Cai, H.; Zhang, Y.; Xu, X.; Cai, Y.; Qi, J.; et al. Elevated serum eotaxin and IP-10 levels as potential biomarkers for the detection of esophageal squamous cell carcinoma. J. Clin. Lab. Anal. 2021, 35, e23904. [Google Scholar] [CrossRef]
- Heidegger, I.; Höfer, J.; Luger, M.; Pichler, R.; Klocker, H.; Horninger, W.; Steiner, E.; Jochberger, S.; Culig, Z. Is Eotaxin-1 a serum and urinary biomarker for prostate cancer detection and recurrence? Prostate 2015, 75, 1904–1909. [Google Scholar] [CrossRef]
- Koç, Ü.; Çetinkaya, E.; Bostanci, E.B.; Kemık, A.S.; Tez, M.; Gömceli, I.; Akoğlu, M. Diagnostic significance of serum eotaxin-1 level in gastric cancer patients. Dis. Markers. 2013, 35, 363–367. [Google Scholar] [CrossRef]
- Zajkowska, M.; Mroczko, B. From Allergy to Cancer-Clinical Usefulness of Eotaxins. Cancers 2021, 13, 128. [Google Scholar] [CrossRef]
- Targowski, T.; Jahnz-Rózyk, K.; Plusa, T.; Glodzinska-Wyszogrodzka, E. Influence of age and gender on serum eotaxin concentration in healthy and allergic people. J. Investig. Allergol. Clin. Immunol. 2005, 15, 277–282. [Google Scholar]
- Hoefer, J.; Luger, M.; Dal-Pont, C.; Culig, Z.; Schennach, H.; Jochberger, S. The “Aging Factor” Eotaxin-1 (CCL11) Is Detectable in Transfusion Blood Products and Increases with the Donor’s Age. Front. Aging Neurosci. 2017, 9, 402. [Google Scholar] [CrossRef]
- Wallen, T.E.; Hanseman, D.; Caldwell, C.C.; Wang, Y.-W.W.; Wade, C.E.; Holcomb, J.B.; Pritts, T.A.; Goodman, M.D. Survival analysis by inflammatory biomarkers in severely injured patients undergoing damage control resuscitation. Surgery 2022, 171, 818–824. [Google Scholar] [CrossRef]
- Vicente, D.A.; Schobel, S.A.; Anfossi, S.; Hensman, H.; Lisboa, F.; Robertson, H.; Khatri, V.P.; Bradley, M.J.; Shimizu, M.B.; Buchman, T.G.; et al. Cellular microRNAs correlate with clinical parameters in multiple injury patients. J. Trauma. Acute Care Surg. 2022, 93, 427–438. [Google Scholar] [CrossRef]
- To, X.V.; Mohamed, A.Z.; Cumming, P.; Nasrallah, F.A. Diffusion tensor imaging and plasma immunological biomarker panel in a rat traumatic brain injury (TBI) model and in human clinical TBI. Front. Immunol. 2023, 14, 1293471. [Google Scholar] [CrossRef]
- Berger, R.P.; Ta’asan, S.; Rand, A.; Lokshin, A.; Kochanek, P. Multiplex assessment of serum biomarker concentrations in well-appearing children with inflicted traumatic brain injury. Pediatr. Res. 2009, 65, 97–102. [Google Scholar] [CrossRef]
- Shein, S.L.; Shellington, D.K.; Exo, J.L.; Jackson, T.C.; Wisniewski, S.R.; Jackson, E.K.; Vagni, V.A.; Bayır, H.; Clark, R.S.; Dixon, C.E.; et al. Hemorrhagic shock shifts the serum cytokine profile from pro- to anti-inflammatory after experimental traumatic brain injury in mice. J. Neurotrauma. 2014, 31, 1386–1395. [Google Scholar] [CrossRef]
- Ristl, R. Sample Size Calculator 2024. Available online: https://homepage.univie.ac.at/robin.ristl/samplesize.php (accessed on 16 July 2024).
- Baruch, K.; Ron-Harel, N.; Gal, H.; Deczkowska, A.; Shifrut, E.; Ndifon, W.; Mirlas-Neisberg, N.; Cardon, M.; Vaknin, I.; Cahalon, L.; et al. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc. Natl. Acad. Sci. USA 2013, 110, 2264–2269. [Google Scholar] [CrossRef]
- Erickson, M.A.; Morofuji, Y.; Owen, J.B.; Banks, W.A. Rapid transport of CCL11 across the blood-brain barrier: Regional variation and importance of blood cells. J. Pharmacol. Exp. Ther. 2014, 349, 497–507. [Google Scholar] [CrossRef]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef]
- Parajuli, B.; Horiuchi, H.; Mizuno, T.; Takeuchi, H.; Suzumura, A. CCL11 enhances excitotoxic neuronal death by producing reactive oxygen species in microglia. Glia 2015, 63, 2274–2284. [Google Scholar] [CrossRef]
- Kovac, A.; Erickson, M.A.; Banks, W.A. Brain microvascular pericytes are immunoactive in culture: Cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J. Neuroinflammation. 2011, 8, 139. [Google Scholar] [CrossRef]
- Yang, L.P.; Zhu, X.A.; Tso, M.O. Minocycline and sulforaphane inhibited lipopolysaccharide-mediated retinal microglial activation. Mol. Vis. 2007, 13, 1083–1093. [Google Scholar]
- Huber, A.K.; Giles, D.A.; Segal, B.M.; Irani, D.N. An emerging role for eotaxins in neurodegenerative disease. Clin. Immunol. 2018, 189, 29–33. [Google Scholar] [CrossRef]
- Cancelliere, C.; Verville, L.; Stubbs, J.L.; Yu, H.; Hincapié, C.A.A.; Hincapié, C.A.A. Post-Concussion Symptoms and Disability in Adults With Mild Traumatic Brain Injury: A Systematic Review and Meta-Analysis. J. Neurotrauma. 2023, 40, 1045–1059. [Google Scholar] [CrossRef]
- Babiloni, A.H.; Bouferguene, Y.; Exposto, F.G.; Beauregard, R.; Lavigne, G.J.; Moana-Filho, E.J.; Arbour, C. The prevalence of persistent post-traumatic headache in adult civilian traumatic brain injury: A systematic review and meta-analysis on the past 14 years. Pain. 2023, 164, 2627–2641. [Google Scholar] [CrossRef]
- Lambert, M.; Sheldrake, E.; Deneault, A.A.; Wheeler, A.; Burke, M.; Scratch, S. Depressive Symptoms in Individuals with Persistent Postconcussion Symptoms: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, e2248453. [Google Scholar] [CrossRef]
- Eggertsen, P.P.; Palmfeldt, J.; Pedersen, A.R.; Frederiksen, O.V.; Olsen, R.K.J.; Nielsen, J.F. Serum neurofilament light chain, inflammatory markers, and kynurenine metabolites in patients with persistent post-concussion symptoms: A cohort study. J. Neurol. Sci. 2024, 460, 123016. [Google Scholar] [CrossRef]
- Di Battista, A.P.; Churchill, N.; Rhind, S.G.; Richards, D.; Hutchison, M.G. The relationship between symptom burden and systemic inflammation differs between male and female athletes following concussion. BMC Immunol. 2020, 21, 11. [Google Scholar] [CrossRef]
- Cherry, J.D.; Stein, T.D.; Tripodis, Y.; Alvarez, V.E.; Huber, B.R.; Au, R.; Kiernan, P.T.; Daneshvar, D.H.; Mez, J.; Solomon, T.M.; et al. CCL11 is increased in the CNS in chronic traumatic encephalopathy but not in Alzheimer’s disease. PLoS ONE 2017, 12, e0185541. [Google Scholar] [CrossRef]
- Almahmoud, K.; Abboud, A.; Namas, R.A.; Zamora, R.; Sperry, J.; Peitzman, A.B.; Truitt, M.S.; Gaski, G.E.; McKinley, T.O.; Billiar, T.R.; et al. Computational evidence for an early, amplified systemic inflammation program in polytrauma patients with severe extremity injuries. PLoS ONE 2019, 14, e0217577. [Google Scholar] [CrossRef]
- Reikeras, O.; Borgen, P.; Reseland, J.E.; Lyngstadaas, S.P. Changes in serum cytokines in response to musculoskeletal surgical trauma. BMC Res. Notes. 2014, 7, 128. [Google Scholar] [CrossRef]
- Bastian, D.; Tamburstuen, M.V.; Lyngstadaas, S.P.; Reikerås, O. Local and systemic chemokine patterns in a human musculoskeletal trauma model. Inflamm. Res. 2009, 58, 483–489. [Google Scholar] [CrossRef]
- Çorbacıoğlu, Ş.K.; Aksel, G. Receiver operating characteristic curve analysis in diagnostic accuracy studies: A guide to interpreting the area under the curve value. Turk. J. Emerg. Med. 2023, 23, 195–198. [Google Scholar] [CrossRef]

| TBI | Non-TBI | p-Value | ||
|---|---|---|---|---|
| Number (n) | 41 | 41 | ||
| Age (years) | 40 [18, 81] | 41 [18, 84] | 0.930 | |
| Males:females (n) | 31:10 | 31:10 | 1.000 | |
| Fatalities (n) | 6 (14.6%) | 4 (9.8%) | 0.500 | |
| ISS | 34 [17, 66] | 29 [17, 59] | 0.357 | |
| At admission | Intubated (n) | 29 (70.7%) | 16 (39.0%) | 0.004 |
| GCS of non-intubated | 13 [3, 15] | 15 [3, 15] | 0.072 | |
| Heart rate (BPM) | 91 [46, 202] | 95 [45, 145] | 0.893 | |
| Hemoglobin (g/dL) | 11.6 [6.4, 16.2] | 10.4 [6.6, 18.1] | 0.133 | |
| Lactate (mmol/L) | 1.5 [0.4, 27.0] | 1.85 [0.7, 12.2] | 0.127 | |
| Oxygen saturation (%) | 98 [68, 100] | 99 [81, 100] | 0.856 | |
| pH value | 7.33 [6.55, 7.45] | 7.33 [6.81, 7.40] | 0.647 | |
| Shock index | 0.72 [0.36, 6.12] | 0.94 [0.43,2.08] | 0.144 | |
| Systolic blood pressure (mmHg) | 120 [33, 180] | 107 [51, 186] | 0.020 | |
| Blood alcohol concentration (‰) | 0 [0, 2.81] | 0 [0, 3.36] | 0.243 | |
| Abnormal pupils # (n) | 22 (53.7%) | 8 (19.5%) | 0.001 | |
| Hypothermia (n) | 4 (9.8%) | 3 (7.3%) | 0.693 | |
| AISHead | 4 [2, 5] | 0 [0, 5] | <0.001 | |
| AISFace | 2 [0, 4] | 0 [0, 3] | 0.013 | |
| AISChest | 3 [0, 5] | 3 [0, 5] | 0.246 | |
| AISAbdomen | 0 [0, 4] | 2 [0, 5] | 0.104 | |
| AISExtremitis | 2 [0, 4] | 3 [0, 5] | 0.179 | |
| AISExternal | 1 [0, 5] | 1 [0, 4] | 0.077 | |
| Subarachnoid hemorrhage (n) | 27 (65.9%) | 0 (0%) | <0.001 | |
| Epidural hematoma (n) | 5 (12.2%) | 0 (0%) | 0.019 | |
| Intracerebral hemorrhage (n) | 16 (30.0%) | 0 (0%) | <0.001 | |
| Subdural hematoma (n) | 24 (58.3%) | 0 (0%) | <0.001 | |
| Spine injury (n) | 18 (43.9%) | 31 (75.6%) | 0.003 | |
| Myelopathy (n) | 1 (2.4%) | 3 (7.3%) | 0.330 | |
| Injury causes | Traffic accident (n) | 20 (48.8%) | 14 (34.1%) | 0.109 |
| Hit by oncoming train/subway (n) | 3 (7.3%) | 4 (9.8%) | ||
| Pedestrian hit by vehicle (n) | 5 (12.2%) | 1 (2.4%) | ||
| Fall from height (n) | 12 (29.3%) | 22 (53.7%) | ||
| Hit by fallen tree branch (n) | 1 (2.4%) | 0 (0%) | ||
| Suicide attempt (n) | 7 (17.1%) | 18 (43.9%) | 0.008 | |
| Admitted under resuscitation conditions (n) | 3 (7.3%) | 1 (2.4%) | 0.305 | |
| Transport by rescue helicopter (n) | 8 (19.5%) | 7 (17.1%) | 0.775 | |
| Under drug influence (n) | 6 (14.6%) | 5 (12.2%) | 0.746 | |
| Acute emergency surgery necessary (n) | 32 (78.0%) | 38 (92.7%) | 0.061 | |
| Duration of intubation (days) | 6 [0, 62] | 1 [0, 75] | 0.076 | |
| Length of stay at the ICU (days) | 14 [0, 80] | 6 [0, 145] | 0.184 | |
| Length of stay at the hospital (days) | 34 [0, 206] | 44 [0, 263] | 0.450 | |
| Complications (n) | 21 (51.2%) | 13 (31.7%) | 0.073 | |
| Sepsis (n) | 8 (19.5%) | 5 (12.2%) | 0.364 | |
| ARDS (n) | 3 (7.3%) | 4 (9.8%) | 0.639 | |
| Pneumonia (n) | 14 (34.1%) | 8 (19.5%) | 0.135 | |
| Acute kidney injury (n) | 1 (2.4%) | 9 (22.0%) | 0.007 | |
| Hemofiltration (n) | 2 (4.9%) | 3 (7.3%) | 0.664 | |
| Urinary tract infiltration (n) | 6 (14.6%) | 8 (19.5%) | 0.557 | |
| Pancreatitis (n) | 1 (2.4%) | 2 (4.9%) | 0.556 | |
| Clostridium difficile infection (n) | 1 (2.4%) | 1 (2.4%) | 1.000 | |
| Day 0 | Day 1 | Day 3 | Day 5 | Day7 | Day 10 | ||
|---|---|---|---|---|---|---|---|
| IL-4 | TBI | 18.7 [4.0, 58.0] | 18.5 [1.8, 51.4] | 14.9 [1.8, 33.9} | 14.1 [3.0, 90.9] | 13.5 [1.8, 36.3] | 10.1 [3.0, 34.0] |
| Non-TBI | 21.3 [3.9, 98.9] | 19.8 [3.5, 60.8] | 16.9 [4.7, 49.8] | 17.5 [3.3, 46.4] | 17.1 [2.2, 43.3] | 13.1 [2.2, 48.5] | |
| p-value | 0.164 | 0.490 | 0.941 | 0.412 | 0.600 | 0.225 | |
| IL-6 | TBI | 59.5 [3.2, 21671,9] | 49.1 [5.4, 1539.4] | 34.3 [1.3, 1106.2] | 14.6 [1.1, 290.7] | 12.7 [1.3, 452.3] | 9.9 [1.6, 628.7] |
| Non-TBI | 99.3 [3,1, 766.6] | 49.3 [9.8, 564.6] | 23.2 [0.6, 967.7] | 14.8 [0.5, 664.4] | 15.9 [0.8, 213.5] | 10.8 [1.1, 262.4] | |
| p-value | 0.103 | 0.639 | 0.652 | 0.864 | 0.717 | 0.469 | |
| IL-7 | TBI | 7.6 [1.5, 18.2] | 7.8 [2.8, 17.8] | 7.4 [0.8, 21.5] | 9.3 [2.1, 25.6] | 10.3 [3.0, 31.1] | 15.0 [3.7, 30.2] |
| Non-TBI | 9.8 [1.7, 19.9] | 7.6 [1.3, 19.2] | 7.9 [2.2, 17.1] | 9.4 [3.8, 25.5] | 14.6 [4.7, 40.2] | 19.0 [2.6, 36.4] | |
| p-value | 0.101 | 0.625 | 0.810 | 0.267 | 0.071 | 0.032 | |
| IL-8 | TBI | 62.6 [14.6, 15897.0] | 42.5 [15.6, 403.5] | 34.2 [8.8, 287.6] | 37.6 [7.0, 125.3] | 31.0 [8.6, 34118.0] | 35.3 [12.4, 1763.4] |
| Non-TBI | 48.7 [11.7, 5374.1] | 50.1 [2.8, 816.10] | 23.7 [5.7, 311.1] | 38.3 [4.4, 474.5] | 37.2 [9.6, 404.9] | 36.9 [3.8, 1209.4] | |
| p-value | 0.985 | 0.706 | 0.447 | 0.800 | 0.462 | 0.651 | |
| IL-10 | TBI | 21.6 [0.9, 448.5] | 2.5 [0.0, 38.3] | 1.4 [0.1, 19.2] | 1.1 [0.3, 6.9] | 1.2 [0.0, 13.3] | 1.4 [0.0, 7.7] |
| Non-TBI | 34.5 [1.4, 273.8] | 3.5 [0.8, 54.2] | 1.3 [0.2, 12.1] | 1.3 [0.3, 7.7] | 1.5 [0.3, 17.5] | 1.2 [0.2, 19.8] | |
| p-value | 0.250 | 0.188 | 0.972 | 0.732 | 0.327 | 0.651 | |
| TNF | TBI | 4.9 [1.8, 130.9] | 6.6 [1.6, 16.1] | 5.8 [1.8, 27.6] | 6.8 [1.1, 18.1] | 4.8 [0.9, 19.2] | 5.6 [0.9, 50.2] |
| Non-TBI | 4.5 [0.1, 8.6] | 4.4 [0.3, 11.1] | 5.4 [1.6, 16.9] | 5.6 [0.4, 14.8] | 5.8 [0.4, 15.6] | 4.8 [0.6, 17.2] | |
| p-value | 0.120 | 0.079 | 0.201 | 0.256 | 0.897 | 0.611 | |
| Eotaxin | TBI | 182.4 [45.8, 2036.3] | 121.8 [36.0, 368.4] | 123.4 [48.9, 365.7] | 121.5 [24.5, 353.2] | 141.0 [32.4, 374.5] | 172.3 [57.6, 423.1] |
| Non-TBI | 118.7 [38.8, 478.3] | 94.5 [38.8, 313.9] | 85.1 [21.3, 342.6] | 102.8 [38.8, 258.7] | 107.9 [22.2, 281.9] | 141.3 [19.9, 442.4] | |
| p-value | <0.001 | 0.098 | 0.002 | 0.039 | 0.057 | 0.238 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negrin, L.L.; Ristl, R.; Wollner, G.; Hajdu, S. Differences in Eotaxin Serum Levels between Polytraumatized Patients with and without Concomitant Traumatic Brain Injury—A Matched Pair Analysis. J. Clin. Med. 2024, 13, 4218. https://doi.org/10.3390/jcm13144218
Negrin LL, Ristl R, Wollner G, Hajdu S. Differences in Eotaxin Serum Levels between Polytraumatized Patients with and without Concomitant Traumatic Brain Injury—A Matched Pair Analysis. Journal of Clinical Medicine. 2024; 13(14):4218. https://doi.org/10.3390/jcm13144218
Chicago/Turabian StyleNegrin, Lukas L., Robin Ristl, Gregor Wollner, and Stefan Hajdu. 2024. "Differences in Eotaxin Serum Levels between Polytraumatized Patients with and without Concomitant Traumatic Brain Injury—A Matched Pair Analysis" Journal of Clinical Medicine 13, no. 14: 4218. https://doi.org/10.3390/jcm13144218
APA StyleNegrin, L. L., Ristl, R., Wollner, G., & Hajdu, S. (2024). Differences in Eotaxin Serum Levels between Polytraumatized Patients with and without Concomitant Traumatic Brain Injury—A Matched Pair Analysis. Journal of Clinical Medicine, 13(14), 4218. https://doi.org/10.3390/jcm13144218






