Settlement Is at the End—Common Trauma Scores Require a Critical Reassessment Due to the Possible Dynamics of Traumatic Brain Injuries in Patients’ Clinical Course
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic Data
3.2. Mechanism of Injury
3.3. Classification of Injury Severity According to the Glasgow Coma Scale
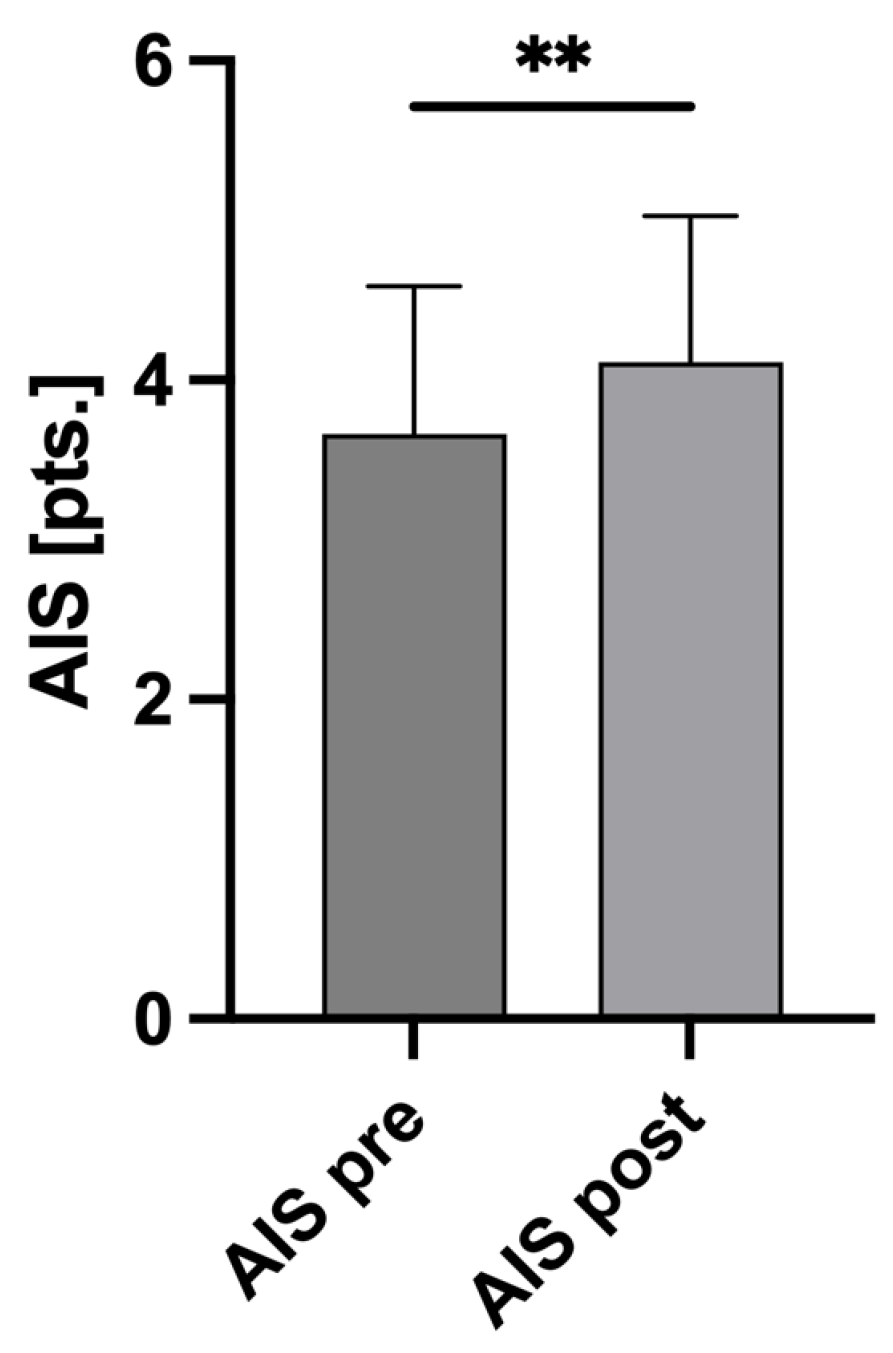
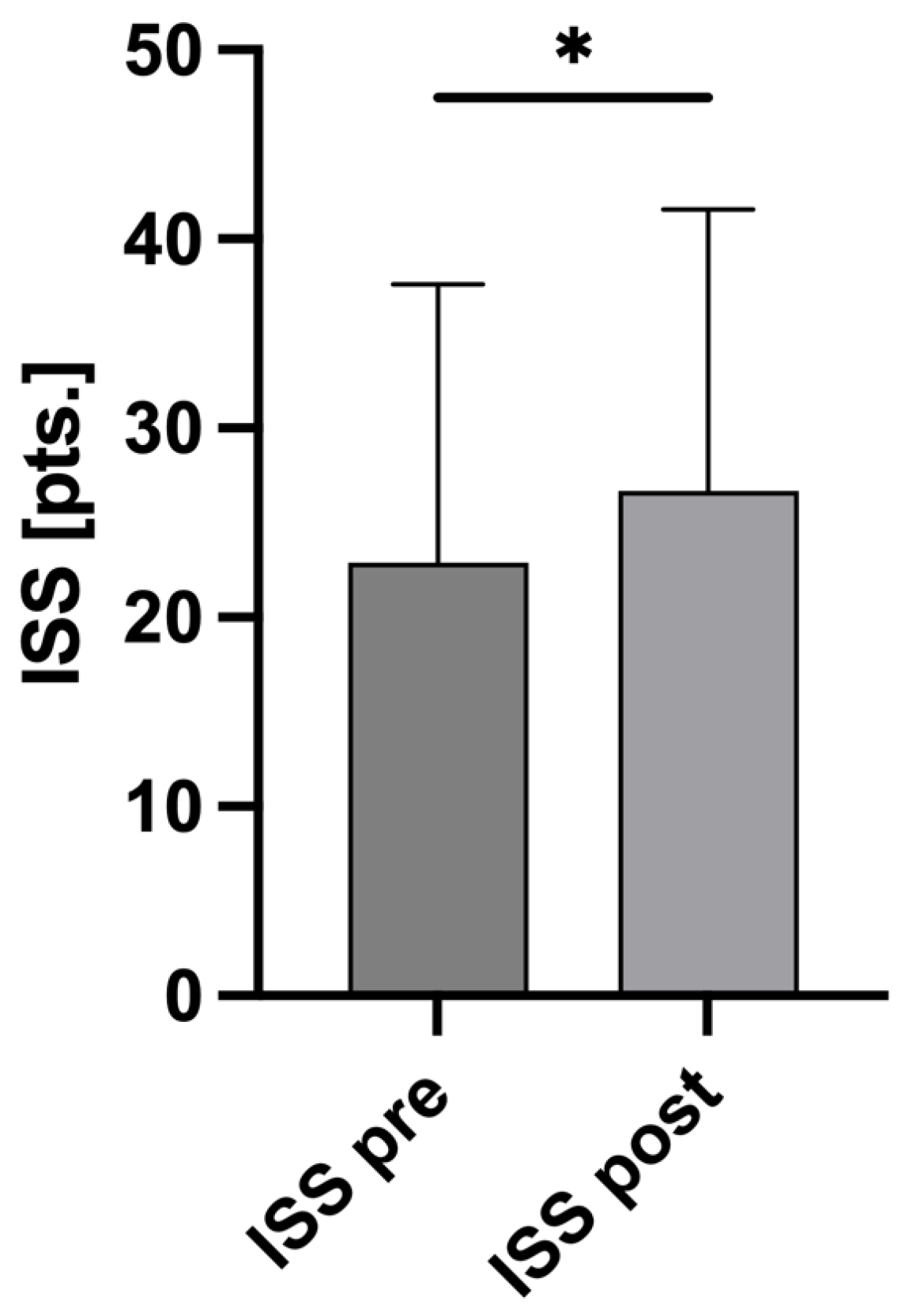
3.4. AIS and ISS Changes
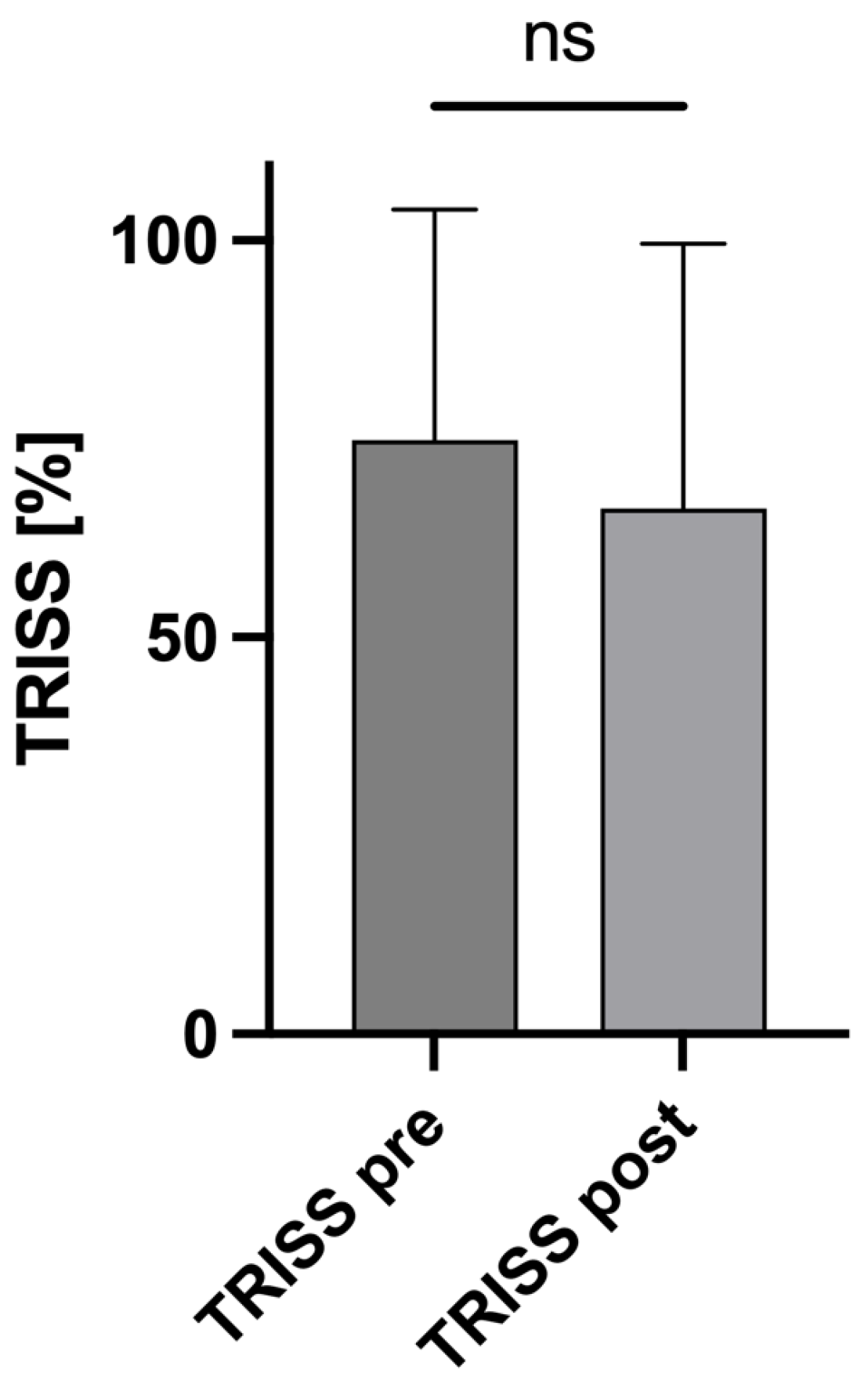
3.5. Influence of the Altered AIS Scores on the TRISS
3.6. Influence of the Altered AIS/ISS Values on Clinical Outcome
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andriessen, T.M.; Horn, J.; Franschman, G.; van der Naalt, J.; Haitsma, I.; Jacobs, B.; Steyerberg, E.W.; Vos, P.E. Epidemiology, Severity Classification, and Outcome of Moderate and Severe Traumatic Brain Injury: A Prospective Multicenter Study. J. Neurotrauma 2011, 28, 2019–2031. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2019, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, G.; Jennett, B. Assessment of Coma and Impaired Consciousness. Lancet 1974, 304, 81–84. [Google Scholar] [CrossRef]
- Maegele, M.; Lefering, R.; Sakowitz, O.; Kopp, M.A.; Schwab, J.M.; Steudel, W.-I.; Unterberg, A.; Hoffmann, R.; Uhl, E.; Marzi, I. The Incidence and Management of Moderate to Severe Head Injury. Dtsch. Arztebl. Int. 2019, 116, 167–173. [Google Scholar] [CrossRef]
- Baker, S.P.; O’Neill, B.; Haddon, W.; Long, W.B. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 1974, 14, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Gennarelli, T.A.; Wodzin, E. AIS 2005: A contemporary injury scale. Injury 2006, 37, 1083–1091. [Google Scholar] [CrossRef]
- Maeda, Y.; Ichikawa, R.; Misawa, J.; Shibuya, A.; Hishiki, T.; Maeda, T.; Yoshino, A.; Kondo, Y. External validation of the TRISS, CRASH, and IMPACT prognostic models in severe traumatic brain injury in Japan. PLoS ONE 2019, 14, e0221791. [Google Scholar] [CrossRef]
- Domingues, C.D.A.; Coimbra, R.; Poggetti, R.S.; Nogueira, L.D.S.; De Sousa, R.M.C. New Trauma and Injury Severity Score (TRISS) adjustments for survival prediction. World J. Emerg. Surg. 2018, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, R.; Barghi, A.; Mehrabi, S.; Salaminia, S.; Tobeh, P. Prognosis of the Trauma Patients According to the Trauma and Injury Severity Score (TRISS); A Diagnostic Accuracy Study. Bull. Emerg. Trauma 2020, 8, 148–155. [Google Scholar]
- Abujaber, A.; Fadlalla, A.; Gammoh, D.; Abdelrahman, H.; Mollazehi, M.; El-Menyar, A. Prediction of in-hospital mortality in patients with post traumatic brain injury using National Trauma Registry and Machine Learning Approach. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 44. [Google Scholar] [CrossRef]
- TraumaRegister DGU®. 20 years TraumaRegister DGU®: Development, aims and structure. Injury 2014, 45, S6–S13. [Google Scholar] [CrossRef] [PubMed]
- Maegele, M.; Schöchl, H.; Menovsky, T.; Maréchal, H.; Marklund, N.; Buki, A.; Stanworth, S. Coagulopathy and haemorrhagic progression in traumatic brain injury: Advances in mechanisms, diagnosis, and management. Lancet Neurol. 2017, 16, 630–647. [Google Scholar] [CrossRef] [PubMed]
- Kurland, D.; Hong, C.; Aarabi, B.; Gerzanich, V.; Simard, J.M. Hemorrhagic Progression of a Contusion after Traumatic Brain Injury: A Review. J. Neurotrauma 2012, 29, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Chauny, J.-M.; Marquis, M.; Bernard, F.; Williamson, D.; Albert, M.; Laroche, M.; Daoust, R. Risk of Delayed Intracranial Hemorrhage in Anticoagulated Patients with Mild Traumatic Brain Injury: Systematic Review and Meta-Analysis. J. Emerg. Med. 2016, 51, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Champion, H.R.; Sacco, W.J.; Copes, W.S.; Gann, D.S.; Gennarelli, T.A.; Flanagan, M.E. A Revision of the Trauma Score. J. Trauma Inj. Infect. Crit. Care 1989, 29, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.R.; Tolson, M.A.; Copes, W.S. Evaluating trauma care: The TRISS method. Trauma Score and the Injury Severity Score. J. Trauma 1987, 27, 370–378. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; von Elm, E.; Langan, S.M.; RECORD Working Committee. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med. 2015, 12, e1001885. [Google Scholar] [CrossRef] [PubMed]
- Lefering, R. Trauma scoring systems. Curr. Opin. Crit. Care 2012, 18, 637–640. [Google Scholar] [CrossRef]
- Lefering, R.; Huber-Wagner, S.; Nienaber, U.; Maegele, M.; Bouillon, B. Update of the trauma risk adjustment model of the TraumaRegister DGUTM: The Revised Injury Severity Classification, version II. Crit. Care 2014, 18, 476. [Google Scholar] [CrossRef]
- Paffrath, T.; Lefering, R.; Flohé, S. How to define severely injured patients?—An Injury Severity Score (ISS) based approach alone is not sufficient. Injury 2014, 45, S64–S69. [Google Scholar] [CrossRef] [PubMed]
- Huber-Wagner, S.; Stegmaier, J.; Mathonia, P.; Paffrath, T.; Euler, E.; Mutschler, W.; Kanz, K.G.; Lefering, R.; Working Group on Polytrauma (NIS) of the German Trauma Society (DGU). The sequential trauma score—A new instrument for the sequential mortality prediction in major trauma. Eur. J. Med. Res. 2010, 15, 185. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Sharma, S. Recent Advances in Pathophysiology of Traumatic Brain Injury. Curr. Neuropharmacol. 2018, 16, 1224–1238. [Google Scholar] [CrossRef] [PubMed]
- Grote, S.; Böcker, W.; Mutschler, W.; Bouillon, B.; Lefering, R. Diagnostic Value of the Glasgow Coma Scale for Traumatic Brain Injury in 18,002 Patients with Severe Multiple Injuries. J. Neurotrauma 2011, 28, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Reith, F.C.; Synnot, A.; van den Brande, R.; Gruen, R.L.; Maas, A.I. Factors Influencing the Reliability of the Glasgow Coma Scale: A Systematic Review. Neurosurgery 2017, 80, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.F.; Marshall, S.B.; Klauber, M.R.; Clark, M.v.B.; Eisenberg, H.M.; Jane, J.A.; Luerssen, T.G.; Marmarou, A.; Foulkes, M.A. A new classification of head injury based on computerized tomography. J. Neurosurg. 1991, 75, S14–S20. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Hukkelhoven, C.W.P.M.; Marshall, L.F.; Steyerberg, E.W. Prediction of Outcome in Traumatic Brain Injury with Computed Tomographic Characteristics: A Comparison between the Computed Tomographic Classification and Combinations of Computed Tomographic Predictors. Neurosurgery 2005, 57, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.R.; Lingsma, H.F.; Roozenbeek, B. Predicting outcome after traumatic brain injury. Handb. Clin. Neurol. 2015, 128, 455–474. [Google Scholar] [PubMed]
- Savitsky, B.; Givon, A.; Rozenfeld, M.; Radomislensky, I.; Peleg, K. Traumatic brain injury: It is all about definition. Brain Inj. 2016, 30, 1194–1200. [Google Scholar] [CrossRef]
- Pape, H.C.; Lefering, R.; Butcher, N.; Peitzman, A.; Leenen, L.; Marzi, I.; Lichte, P.; Josten, C.; Bouillon, B.; Schmucker, U.; et al. The definition of polytrauma revisited: An international consensus process and proposal of the new ‘Berlin definition’. J. Trauma Acute Care Surg. 2014, 77, 780–786. [Google Scholar] [CrossRef]
- Palmer, C. Major trauma and the injury severity score--where should we set the bar? Annu. Proc. Assoc. Adv. Automot. Med. 2007, 51, 13–29. [Google Scholar] [PubMed]
- Bendinelli, C.; Ku, D.; King, K.L.; Nebauer, S.; Balogh, Z.J. Trauma patients with prehospital Glasgow Coma Scale less than nine: Not a homogenous group. Eur. J. Trauma Emerg. Surg. 2020, 46, 873–878. [Google Scholar]
- Bossers, S.M.; Pol, K.M.; Ophuis, E.P.A.O.; Jacobs, B.; Visser, M.C.; Loer, S.A.; Boer, C.; van der Naalt, J.; Schober, P. Discrepancy between the initial assessment of injury severity and post hoc determination of injury severity in patients with apparently mild traumatic brain injury: A retrospective multicenter cohort analysis. Eur. J. Trauma Emerg. Surg. 2018, 44, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Relja, B.; Huber-Lang, M.; van Griensven, M.; Hildebrand, F.; Maegele, M.; Nienaber, U.; Brucker, D.P.; Sturm, R.; Marzi, I. A nationwide fluidics biobank of polytraumatized patients: Implemented by the Network “Trauma Research” (NTF) as an expansion to the TraumaRegister DGU® of the German Trauma Society (DGU). Eur. J. Trauma Emerg. Surg. 2020, 46, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Gesellschaft für Neurochirurgie, e.V. DGNC, S2e-Leitlinie Schädel-Hirn-Trauma im Erwachsenenalter, Version: 3.0, State: 2 December 2015. Available online: https://register.awmf.org/de/leitlinien/detail/008-001 (accessed on 1 December 2023).
- Polytrauma Guideline Update Group. Level 3 guideline on the treatment of patients with severe/multiple injuries: AWMF Register-Nr. 012/019. Eur. J. Trauma Emerg. Surg. 2018, 44, 3–271. [Google Scholar] [CrossRef] [PubMed]
- Picetti, E.; Maier, R.V.; Rossi, S.; Kirkpatrick, A.W.; Biffl, W.L.; Stahel, P.F.; Moore, E.E.; Kluger, Y.; Baiocchi, G.L.; Ansaloni, L.; et al. Preserve encephalus in surgery of trauma: Online survey. (P.E.S.T.O). World J. Emerg. Surg. 2019, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Picetti, E.; Rossi, S.; Abu-Zidan, F.M.; Ansaloni, L.; Armonda, R.; Baiocchi, G.L.; Bala, M.; Balogh, Z.J.; Berardino, M.; Biffl, W.L.; et al. WSES consensus conference guidelines: Monitoring and management of severe adult traumatic brain injury patients with polytrauma in the first 24 hours. World J. Emerg. Surg. 2019, 14, 53. [Google Scholar] [CrossRef]
- Giannoudi, M.; Harwood, P. Damage control resuscitation: Lessons learned. Eur. J. Trauma Emerg. Surg. 2016, 42, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Pape, H.C.; Giannoudis, P.; Krettek, C. The timing of fracture treatment in polytrauma patients: Relevance of damage control orthopedic surgery. Am. J. Surg. 2002, 183, 622–629. [Google Scholar]
- Gebhard, F.; Huber-Lang, M. Polytrauma—Pathophysiology and management principles. Langenbecks Arch. Surg. 2008, 393, 825–831. [Google Scholar] [CrossRef]
- Van Wessem, K.J.P.; Leenen, L.P.H.; Hietbrink, F. Physiology dictated treatment after severe trauma: Timing is everything. Eur. J. Trauma Emerg. Surg. 2022, 48, 3969–3979. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, R.; Kalbas, Y.; Coimbra, R.; Leenen, L.; Komadina, R.; Hildebrand, F.; Halvachizadeh, S.; Akhtar, M.; Peralta, R.; Fattori, L.; et al. Indications and interventions of damage control orthopedic surgeries: An expert opinion survey. Eur. J. Trauma Emerg. Surg. 2021, 47, 2081–2092. [Google Scholar] [CrossRef] [PubMed]
- Younsi, A.; Unterberg, A.; Marzi, I.; Steudel, W.-I.; Uhl, E.; Lemcke, J.; Berg, F.; Woschek, M.; Friedrich, M.; Clusmann, H.; et al. Development and first results of a national databank on care and treatment outcome after traumatic brain injury. Eur. J. Trauma Emerg. Surg. 2023, 49, 1171–1181. [Google Scholar] [CrossRef] [PubMed]

| n = 80 Patients | |
|---|---|
| age (years) ± SD | 54.6 ± 20.9 |
| sex (male) | 49 (61.3%) |
| GCSadmission (pts.) ± SD | 7.9 ± 5.1 |
| ICUstay (days) ± SD | 8.6 ± 7.4 |
| AISpre (pts.) ± SD | 3.66 ± 0.93 |
| AISpost (pts.) ± SD | 4.11 ± 0.91 |
| ISSpre (pts.) ± SD | 22.89 ± 14.73 |
| ISSpost (pts.) ± SD | 26.68 ± 14.89 |
| TRISSpre (%) ± SD | 74.82 ± 29.07 |
| TRISSpost (%) ± SD | 66.25 ± 33.3 |
| mortality (n) | 9 (11.3%) |
| Injury mechanism (n) | |
| fall below 3 m | 25 (31.3%) |
| traffic accident | 18 (22.5%) |
| bicycle fall | 14 (17.5%) |
| fall over 3 m | 14 (17.5%) |
| assault | 8 (10%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hörauf, J.-A.; Woschek, M.; Schindler, C.R.; Verboket, R.D.; Lustenberger, T.; Marzi, I.; Störmann, P. Settlement Is at the End—Common Trauma Scores Require a Critical Reassessment Due to the Possible Dynamics of Traumatic Brain Injuries in Patients’ Clinical Course. J. Clin. Med. 2024, 13, 3333. https://doi.org/10.3390/jcm13113333
Hörauf J-A, Woschek M, Schindler CR, Verboket RD, Lustenberger T, Marzi I, Störmann P. Settlement Is at the End—Common Trauma Scores Require a Critical Reassessment Due to the Possible Dynamics of Traumatic Brain Injuries in Patients’ Clinical Course. Journal of Clinical Medicine. 2024; 13(11):3333. https://doi.org/10.3390/jcm13113333
Chicago/Turabian StyleHörauf, Jason-Alexander, Mathias Woschek, Cora Rebecca Schindler, Rene Danilo Verboket, Thomas Lustenberger, Ingo Marzi, and Philipp Störmann. 2024. "Settlement Is at the End—Common Trauma Scores Require a Critical Reassessment Due to the Possible Dynamics of Traumatic Brain Injuries in Patients’ Clinical Course" Journal of Clinical Medicine 13, no. 11: 3333. https://doi.org/10.3390/jcm13113333
APA StyleHörauf, J.-A., Woschek, M., Schindler, C. R., Verboket, R. D., Lustenberger, T., Marzi, I., & Störmann, P. (2024). Settlement Is at the End—Common Trauma Scores Require a Critical Reassessment Due to the Possible Dynamics of Traumatic Brain Injuries in Patients’ Clinical Course. Journal of Clinical Medicine, 13(11), 3333. https://doi.org/10.3390/jcm13113333






