Prognostic Significance of Phenylalanine in Heart Failure: Clinical Insights and Inter-Organ Crosstalk Snapshot
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Phase 1: Retrospective Cohort Study
2.3. Phase 2: Inter-Organic Crosstalk Study
2.4. Plasma Phenylalanine Measurement
2.5. Ethical Considerations
2.6. Statistical Analysis
3. Results
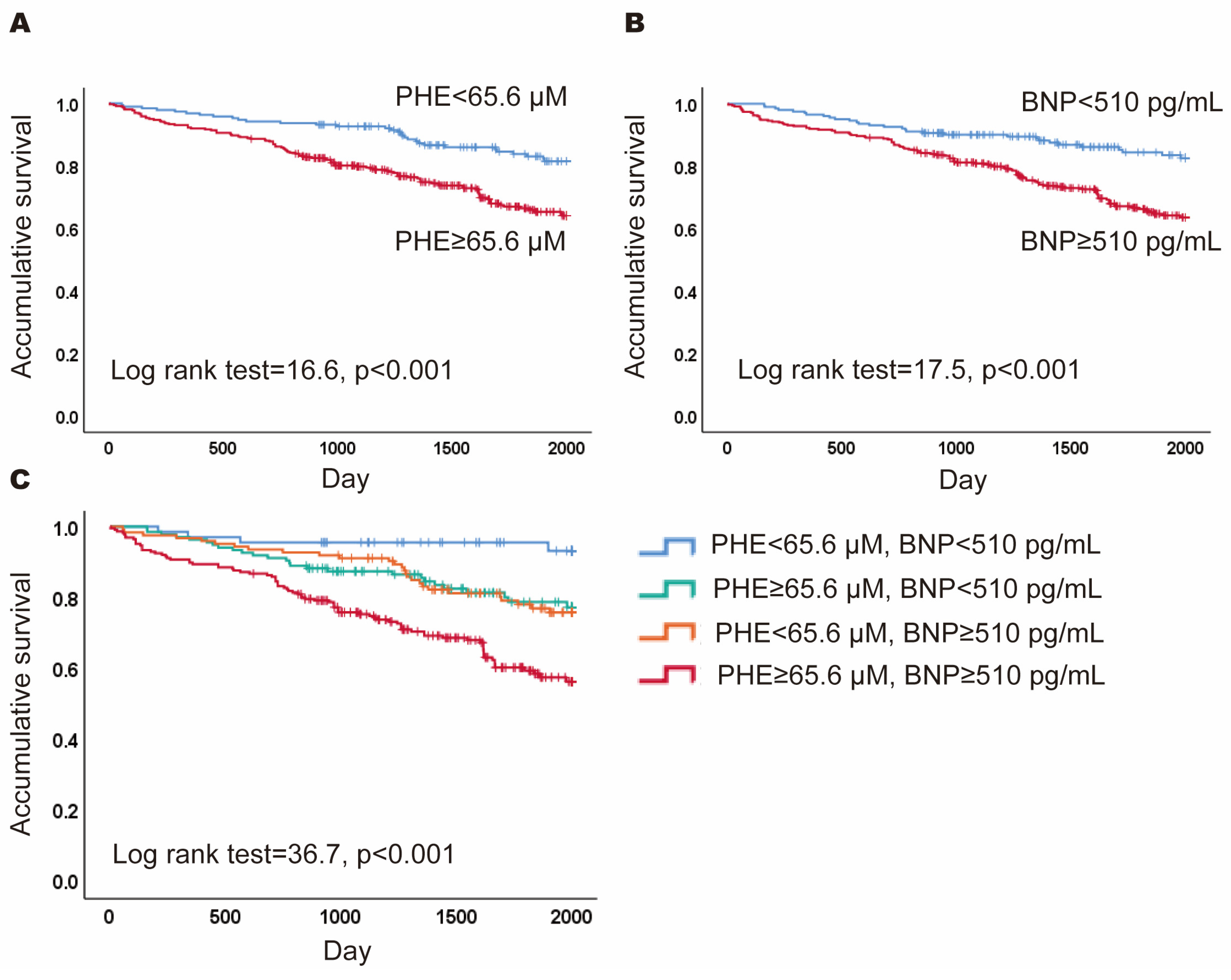
3.1. Prognostic Value of PHE and BNP: Survival Differences
3.2. Differences between Subgroups Defined by PHE and BNP Levels
3.3. Baseline Characteristics of Patients for the Inter-Organ Crosstalk Study
3.4. Differences in PHE Levels between Inlet and Outlet Vessels of Organs
3.5. Factors Associated with the Gradients of PHE Level across Different Organs
4. Discussion
4.1. Future Perspectives
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Njoroge, J.N.; Teerlink, J.R. Pathophysiology and Therapeutic Approaches to Acute Decompensated Heart Failure. Circ. Res. 2021, 128, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Pfeffer, M.A. Heart failure. Lancet 2005, 365, 1877–1889. [Google Scholar] [CrossRef] [PubMed]
- Yap, J. Risk stratification in heart failure: Existing challenges and potential promise. Int. J. Cardiol. 2020, 313, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Drazner, M.H. Risk Stratification of Patients With Decompensated Heart Failure by Echocardiographic Assessment of Hemodynamics. Am. J. Cardiol. 2023, 207, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Verdu-Rotellar, J.; Abellana, R.; Vaillant-Roussel, H.; Jevsek, L.G.; Assenova, R.; Lazic, D.K.; Torsza, P.; Glynn, L.G.; Lingner, H.; Demurtas, J.; et al. Risk stratification in heart failure decompensation in the community: HEFESTOS score. ESC Heart Fail. 2022, 9, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Nakao, M.; Anzai, T. Risk Stratification Towards Precision Medicine in Heart Failure—Current Progress and Future Perspectives. Circ. J. 2021, 85, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Li, D.Y.; Hazen, S.L. Dietary metabolism, the gut microbiome, and heart failure. Nat. Rev. Cardiol. 2019, 16, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhang, B.; Xie, M.; Li, T. Circulating metabolic signatures of heart failure in precision cardiology. Precis. Clin. Med. 2023, 6, pbad005. [Google Scholar] [CrossRef] [PubMed]
- Würtz, P.; Havulinna, A.S.; Soininen, P.; Tynkkynen, T.; Prieto-Merino, D.; Tillin, T.; Ghorbani, A.; Artati, A.; Wang, Q.; Tiainen, M.; et al. Metabolite profiling and cardiovascular event risk: A prospective study of 3 population-based cohorts. Circulation 2015, 131, 774–785. [Google Scholar] [CrossRef]
- Delles, C.; Rankin, N.J.; Boachie, C.; McConnachie, A.; Ford, I.; Kangas, A.; Soininen, P.; Trompet, S.; Mooijaart, S.P.; Jukema, J.W.; et al. Nuclear magnetic resonance-based metabolomics identifies phenylalanine as a novel predictor of incident heart failure hospitalisation: Results from PROSPER and FINRISK 1997. Eur. J. Heart Fail. 2018, 20, 663–673. [Google Scholar] [CrossRef]
- Chen, W.; Wang, C.; Cheng, C.; Liu, M.; Chu, C.; Wu, H.; Huang, P.; Lin, Y.; Ko, T.; Chen, W.; et al. Elevated plasma phenylalanine predicts mortality in critical patients with heart failure. ESC Heart Fail. 2020, 7, 2884–2893. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Mullens, W. Cardiorenal syndrome in decompensated heart failure. Heart 2010, 96, 255–260. [Google Scholar] [CrossRef]
- Laribi, S.; Mebazaa, A. Cardiohepatic syndrome: Liver injury in decompensated heart failure. Curr. Heart Fail. Rep. 2014, 11, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef] [PubMed]
- Hakuno, D.; Hamba, Y.; Toya, T.; Adachi, T. Plasma amino acid profiling identifies specific amino acid associations with cardiovascular function in patients with systolic heart failure. PLoS ONE 2015, 10, e0117325. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Chen, W.-S.; Liu, M.-H.R.; Lee, C.-Y.M.; Wang, M.-Y.; Liang, C.-Y.; Chu, C.-M.; Wu, H.-P.; Chen, W.-H. Stress Hyperphenylalaninemia Is Associated With Mortality in Cardiac ICU: Clinical Factors, Genetic Variants, and Pteridines. Crit. Care Med. 2022, 50, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Hiraiwa, H.; Okumura, T.; Kondo, T.; Kato, T.; Kazama, S.; Ishihara, T.; Iwata, E.; Shimojo, M.; Kondo, S.; Aoki, S.; et al. Usefulness of the plasma branched-chain amino acid/aromatic amino acid ratio for predicting future cardiac events in patients with heart failure. J. Cardiol. 2020, 75, 689–696. [Google Scholar] [CrossRef]
- Czibik, G.; Mezdari, Z.; Altintas, D.M.; Bréhat, J.; Pini, M.; D’humières, T.; Delmont, T.; Radu, C.; Breau, M.; Liang, H.; et al. Dysregulated Phenylalanine Catabolism Plays a Key Role in the Trajectory of Cardiac Aging. Circulation 2021, 144, 559–574. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, M.; Han, J.; Huang, H.; Xu, S.; Zhang, S.; Jing, Q.; Wang, H.; Bu, H.; Kou, Y.; et al. A tyrosine catabolic intermediate 4-hydroxyphenylpyruate attenuates murine endotoxic shock by blocking NLRP3 inflammasome activation. Int. Immunopharmacol. 2022, 111, 109098. [Google Scholar] [CrossRef]
- Lv, D.; Cao, X.; Zhong, L.; Dong, Y.; Xu, Z.; Rong, Y.; Xu, H.; Wang, Z.; Yang, H.; Yin, R.; et al. Targeting phenylpyruvate restrains excessive NLRP3 inflammasome activation and pathological inflammation in diabetic wound healing. Cell Rep. Med. 2023, 4, 101129. [Google Scholar] [CrossRef]
- Hiraiwa, H.; Okumura, T.; Kondo, T.; Kato, T.; Kazama, S.; Kimura, Y.; Ishihara, T.; Iwata, E.; Shimojo, M.; Kondo, S.; et al. Prognostic value of leucine/phenylalanine ratio as an amino acid profile of heart failure. Heart Vessels 2021, 36, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, R.; Liu, Y.; Li, Z.; Sun, Y.; Yin, P.; Huang, R. Characteristics of Blood Metabolic Profile in Coronary Heart Disease, Dilated Cardiomyopathy and Valvular Heart Disease Induced Heart Failure. Front. Cardiovasc. Med. 2020, 7, 622236. [Google Scholar] [CrossRef] [PubMed]
| All | Survival | Death | ||
|---|---|---|---|---|
| n = 550 | n = 410 | n = 140 | p Value | |
| Age (years) | 60.4 ± 12.8 | 59.5 ± 12.3 | 62.8 ± 13.7 | 0.008 |
| Male (%) | 391 (71.1) | 291 (71.0) | 100 (71.4) | 1.000 |
| LVEF (%) | 30.1 ± 9.9 | 30.1 ± 9.8 | 30.0 ± 10.4 | 0.902 |
| Blood pressure (mm Hg) | ||||
| Systolic | 121 ± 64.7 | 122 ± 73.8 | 117 ± 20.5 | 0.437 |
| Diastolic | 75.1 ± 12.5 | 75.5 ± 12.2 | 74.1 ± 13.5 | 0.274 |
| Heart rate, beats/min | 84.8 ± 19.7 | 84.6 ± 19.9 | 85.4 ± 19.4 | 0.674 |
| Body mass index (kg/m2) | 25.5 ± 5.0 | 25.7 ± 5.0 | 25.0 ± 4.9 | 0.154 |
| Co-morbidity | ||||
| Diabetes mellitus (%) | 163 (29.6) | 116 (28.3) | 47 (33.6) | 0.240 |
| Hypertension (%) | 321 (58.4) | 230 (56.1) | 91 (65.0) | 0.074 |
| COPD (%) | 112 (20.4) | 80 (19.5) | 32 (22.9) | 0.397 |
| Atrial fibrillation (%) | 136 (24.7) | 99 (24.1) | 37 (26.4) | 0.650 |
| Coronary artery disease (%) | 289 (52.5) | 222 (54.1) | 67 (47.9) | 0.204 |
| Laboratory data | ||||
| Phenylalanine (μM) | 73.6 ± 20.5 | 71.4 ± 16.3 | 80.1 ± 28.8 | 0.008 |
| B-type natriuretic peptide (pg/mL) | 714 (315–1410) | 644 (277–1395) | 1085 (541–2489) | <0.001 |
| Hemoglobin (g/dL) | 13.7 ± 5.3 | 13.6 ± 2.2 | 13.9 ± 9.8 | 0.659 |
| C-reactive protein (mg/L) | 10.0 (3.23–30.64) | 8.63 (3.0–26.0) | 14.1 (5.0–40.39) | 0.006 |
| eGFR (ml/min/1.73 m2) | 69.7 ± 29.7 | 71.5 ± 27.1 | 64.4 ± 35.9 | 0.016 |
| Uric acid (mg/dL) | 7.5 ± 2.6 | 7.4 ± 2.5 | 8.0 ± 2.8 | 0.016 |
| Total cholesterol (mg/dL) | 172 ± 47.1 | 175 ± 47.2 | 164 ± 46.0 | 0.012 |
| Triglyceride (mg/dL) | 133 ± 87.3 | 134 ± 89.7 | 130 ± 80.0 | 0.588 |
| Sodium (mEq/L) | 139 ± 5.7 | 139 ± 6.0 | 140 ± 4.3 | 0.306 |
| Albumin (g/dL) | 3.8 ± 0.7 | 3.9 ± 0.7 | 3.6 ± 0.5 | 0.006 |
| ALT (U/L) | 31.0 (20.0–52.0) | 32.0 (21.0–55.0) | 27.0 (17.0–40.0) | 0.002 |
| Bilirubin, total (mg/dL) | 0.8 (0.5–1.2) | 0.8 (0.6–1.2) | 0.8 (0.5–1.4) | 0.182 |
| QRS complex (msec) | 103 ± 24.7 | 100 ± 23.3 | 110 ± 27.1 | <0.001 |
| LPLB | HPLB | LPHB | HPHB | ||
|---|---|---|---|---|---|
| Variable | (n = 67) | (n = 135) | (n = 123) | (n = 225) | p Value |
| Age (years) | 60.5 ± 11.9 | 59.3 ± 12.7 | 60.2 ± 12.7 | 61.1 ± 13.1 | 0.634 |
| Male (%) | 38 (56.7) | 117 (86.7) † | 73 (59.3) # | 163 (72.4) #,§ | <0.001 |
| LVEF (%) | 32.6 ± 9.5 | 33.1 ± 10.5 | 28.8 ± 8.9 # | 28.2 ± 9.7 †,# | <0.001 |
| Blood pressure (mm Hg) | |||||
| Systolic | 117 ± 17.6 | 119 ± 17.2 | 119 ± 18.1 | 125 ± 98.7 | 0.741 |
| Diastolic | 73.6 ± 10.4 | 75.0 ± 12.6 | 75.4 ± 12.4 | 75.5 ± 13.2 | 0.735 |
| Heart rate, beats/min | 84.5 ± 19.0 | 82.6 ± 21.8 | 85.3 ± 18.2 | 85.9 ± 19.5 | 0.477 |
| Body mass index (kg/m2) | 24.1 ± 3.4 | 27.4 ± 5.5 † | 24.7 ± 4.8 # | 25.2 ± 4.9 # | <0.001 |
| Co-morbidity | |||||
| Diabetes mellitus (%) | 13 (19.4) | 31 (23.0) | 38 (30.9) | 81 (36.0) *,‡ | 0.013 |
| Hypertension (%) | 33 (49.3) | 80 (59.3) | 78 (63.4) | 130 (57.8) | 0.301 |
| COPD (%) | 15 (22.4) | 32 (23.7) | 29 (23.6) | 36 (16.0) | 0.210 |
| Atrial fibrillation (%) | 15 (22.4) | 34 (25.2) | 26 (21.1) | 61 (27.1) | 0.626 |
| Coronary artery disease (%) | 37 (55.2) | 87 (64.4) | 54 (43.9) # | 111 (49.3) ‡ | 0.006 |
| Laboratory data | |||||
| PHE (μM) | 57.1 ± 6.0 | 79.2 ± 12.5 † | 57.4 ± 6.5 # | 83.0 ± 23.8 †,ϕ | <0.001 |
| BNP (pg/mL) | 257 ± 144 | 223 ± 144 | 1591 ± 1250 †,# | 1611 ± 1171 †,# | <0.001 |
| Hemoglobin (g/dL) | 13.4 ± 2.0 | 14.2 ± 2.2 | 13.0 ± 2.4 | 13.9 ± 7.8 | 0.333 |
| C-reactive protein (mg/L) | 7.00 (2.00–25.6) | 7.90 (2.94–32.7) | 10.7 (3.00–30.2) | 11.3 (4.06–32.3) | 0.301 |
| eGFR (mL/min/1.73 m2) | 81.0 ± 29.9 | 73.5 ± 25.7 | 67.9 ± 30.2 * | 65.0 ± 30.5 †,‡ | <0.001 |
| Uric acid (mg/dL) | 6.6 ± 2.0 | 7.0 ± 2.2 | 7.8 ± 2.7 * | 8.0 ± 2.8 †,# | <0.001 |
| Total cholesterol (mg/dL) | 181 ± 47.4 | 181 ± 46.6 | 174 ± 48.5 | 164 ± 45.4 # | 0.003 |
| Triglyceride (mg/dL) | 130 ± 72.2 | 136 ± 95.6 | 145 ± 99.0 | 125 ± 78.8 | 0.239 |
| Sodium (mEq/L) | 139 ± 2.8 | 139 ± 2.7 | 140 ± 5.5 | 139 ± 7.4 | 0.956 |
| Albumin (g/dL) | 3.9 ± 0.4 | 4.0 ± 1.2 | 3.7 ± 0.5 # | 3.7 ± 0.5 # | <0.001 |
| ALT (U/L) | 25.0 (19.0–39.0) | 31.0 (20.0–50.0) | 27.5 (18.0–41.3) | 34.5 (21.0–61.0) *,§ | 0.032 |
| Total bilirubin (mg/dL) | 0.70 (0.50–0.90) | 0.80 (0.50–1.10) | 0.80 (0.50–1.20) | 0.90 (0.60–1.40) *,§ | 0.046 |
| QRS complex (msec) | 105 ± 27.0 | 98.2 ± 21.4 | 102 ± 23.2 | 105 ± 26.3 | 0.078 |
| Variable | Total Sample (n = 24) |
|---|---|
| Age (years) | 52.5 (43.8–63.1) |
| Male (%) | 17 (70.8%) |
| Left ventricular ejection fraction (%) | 33 (22–61) |
| Heart failure (%) | 15 (62.5%) |
| Acute heart failure (%) | 10 (41.7%) |
| Acute or Old Myocardial Infarction (%) | 3 (12.5%) |
| NYHA functional class III-IV (%) | 14 (58.3%) |
| Co-morbidity | |
| Diabetes mellitus (%) | 11 (45.8%) |
| Hypertension (%) | 14 (58.3%) |
| Chronic Kidney Disease (%) | 2 (8.3%) |
| Chronic Obstructive Pulmonary Disease (%) | 2 (8.3%) |
| Coronary Artery Disease (%) | 8 (33.3%) |
| Hyperlipidemia (%) | 7 (29.2%) |
| Atrial fibrillation (%) | 2 (8.3%) |
| Laboratory data | |
| White Blood Cells (/mm3) | 7600 (6000–9300) |
| Hemoglobin (g/dL) | 13.5 (11.4–15.1) |
| B-type Natriuretic Peptide (pg/mL) | 478.7 (210.0–897.9) |
| Albumin (g/dL) | 3.75 (3.48–4.06) |
| Creatinine (mg/dL) | 0.83 (0.65–1.06) |
| Uric acid (mg/dL) | 6.2 (4.8–7.9) |
| Total cholesterol (mg/dL) | 147 (122–160) |
| Triglycerides (mg/dL) | 107 (72–135) |
| C-reactive Protein (mg/L) | 1.36 (0.59–12.4) |
| Alanine aminotransferase (U/L) | 28 (10–44) |
| Total bilirubin (mg/dL) | 1.2 (0.7–1.7) |
| Organs | (μM) | p Value |
|---|---|---|
| Skeletal muscle | ||
| Femoral artery | 67.5 ± 14.1 | |
| Femoral vein | 72.8 ± 16.8 | |
| Δ(Femoral vein–femoral artery) | 5.3 ± 6.1 | <0.001 |
| Liver | ||
| Superior mesentery artery | 67.1 ± 14.8 | |
| Hepatic vein | 64.0 ± 13.2 | |
| Δ(Hepatic vein–superior mesentery artery) | −3.1 ± 6.1 | 0.022 |
| Heart | ||
| Aorta | 68.5 ± 15.6 | |
| Coronary sinus | 66.2 ± 15.2 | |
| Δ(Coronary sinus–aorta) | −2.3 ± 4.6 | 0.024 |
| Kidney | ||
| Renal artery | 65.5 ± 14.8 | |
| Renal vein | 65.9 ± 12.6 | |
| Δ(Renal vein–renal artery) | 0.4 ± 4.9 | 0.725 |
| Lung | ||
| Pulmonary artery | 67.0 ± 14.9 | |
| Left ventricle | 68.1 ± 15.5 | |
| Δ(Left ventricle–pulmonary artery) | 1.1 ± 3.5 | 0.155 |
| Skeletal Muscle | Heart | Liver | ||||||
|---|---|---|---|---|---|---|---|---|
| Δ(FV–FA) | Δ(AO–CS) | Δ(SMA–HV) | PHE (AO) | |||||
| Variable | r | p Value | r | p Value | r | p Value | r | p Value |
| LVEF | −0.296 | 0.160 | −0.023 | 0.915 | −0.142 | 0.507 | −0.186 | 0.385 |
| NYHA Fc | 0.446 | 0.029 | 0.041 | 0.850 | 0.312 | 0.138 | 0.258 | 0.224 |
| Acute heart failure | 0.455 | 0.025 | 0.062 | 0.772 | 0.183 | 0.392 | 0.422 | 0.040 |
| Acute MI | 0.216 | 0.312 | −0.112 | 0.604 | 0.093 | 0.664 | 0.524 | 0.009 |
| Atrial fibrillation | 0.012 | 0.956 | 0.467 | 0.021 | 0.042 | 0.845 | 0.029 | 0.893 |
| B-natriuretic peptide | 0.585 | 0.036 | 0.014 | 0.963 | 0.262 | 0.388 | 0.049 | 0.875 |
| CRP | 0.414 | 0.044 | −0.447 | 0.029 | −0.241 | 0.257 | 0.036 | 0.867 |
| Creatitine | 0.323 | 0.123 | 0.408 | 0.048 | 0.454 | 0.026 | 0.637 | 0.001 |
| Uric acid | 0.522 | 0.009 | 0.425 | 0.039 | 0.470 | 0.021 | 0.660 | <0.001 |
| ALT | 0.075 | 0.728 | 0.157 | 0.464 | −0.029 | 0.891 | 0.162 | 0.448 |
| Total bilirubin | 0.217 | 0.522 | 0.096 | 0.780 | 0.054 | 0.876 | 0.679 | <0.001 |
| Δ(FV–FA) | - | - | 0.013 | 0.953 | 0.106 | 0.622 | 0.393 | 0.057 |
| Δ(AO–CS) | −0.013 | 0.953 | - | - | 0.139 | 0.518 | 0.430 | 0.036 |
| Δ(SMA–HV) | −0.106 | 0.622 | 0.139 | 0.518 | - | - | 0.458 | 0.025 |
| PHE (AO) | 0.393 | 0.057 | 0.430 | 0.036 | 0.458 | 0.025 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, J.-K.; Tsou, Y.-L.; Liu, M.-H.; Chen, W.-S.; Cheng, C.-I.; Pan, K.-L.; Wang, C.-H.; Hsieh, I.-C. Prognostic Significance of Phenylalanine in Heart Failure: Clinical Insights and Inter-Organ Crosstalk Snapshot. J. Clin. Med. 2024, 13, 4251. https://doi.org/10.3390/jcm13144251
Yeh J-K, Tsou Y-L, Liu M-H, Chen W-S, Cheng C-I, Pan K-L, Wang C-H, Hsieh I-C. Prognostic Significance of Phenylalanine in Heart Failure: Clinical Insights and Inter-Organ Crosstalk Snapshot. Journal of Clinical Medicine. 2024; 13(14):4251. https://doi.org/10.3390/jcm13144251
Chicago/Turabian StyleYeh, Jih-Kai, Yi-Liang Tsou, Min-Hui Liu, Wei-Siang Chen, Cheng-I Cheng, Kuo-Li Pan, Chao-Hung Wang, and I-Chang Hsieh. 2024. "Prognostic Significance of Phenylalanine in Heart Failure: Clinical Insights and Inter-Organ Crosstalk Snapshot" Journal of Clinical Medicine 13, no. 14: 4251. https://doi.org/10.3390/jcm13144251
APA StyleYeh, J.-K., Tsou, Y.-L., Liu, M.-H., Chen, W.-S., Cheng, C.-I., Pan, K.-L., Wang, C.-H., & Hsieh, I.-C. (2024). Prognostic Significance of Phenylalanine in Heart Failure: Clinical Insights and Inter-Organ Crosstalk Snapshot. Journal of Clinical Medicine, 13(14), 4251. https://doi.org/10.3390/jcm13144251







