Association of Centre Quality Certification with Characteristics of Patients, Management, and Outcomes Following Carotid Endarterectomy or Carotid Artery Stenting
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Case Selection
2.3. Study Variables
2.4. Study Outcomes
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Diagnostic Procedures, Management, and Treatment
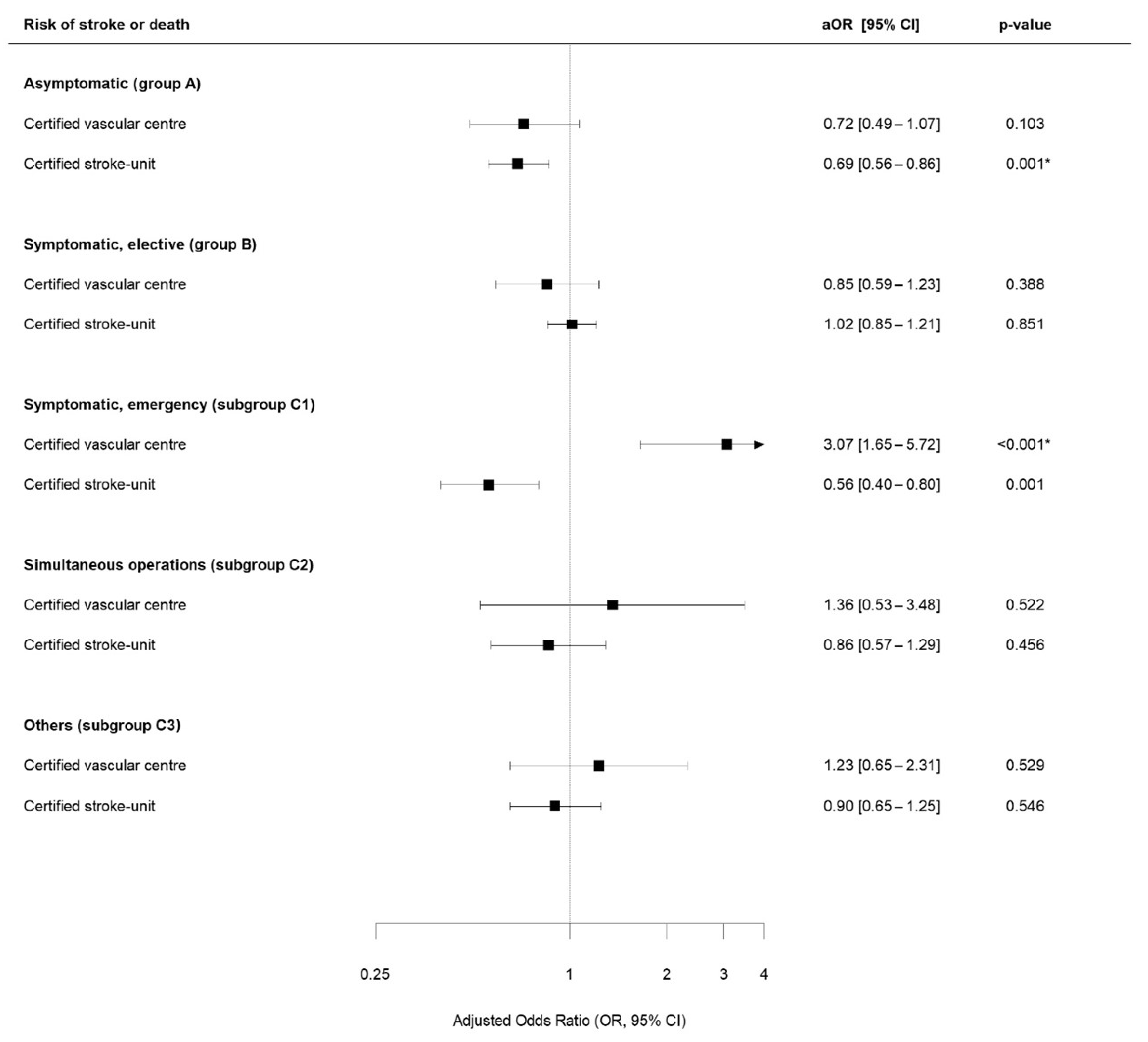
3.3. Study Outcomes
4. Discussion
5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Bjorck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef]
- Naylor, R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.H.; Sillesen, H.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Eckstein, H.H.; Kuhnl, A.; Berkefeld, J.; Lawall, H.; Storck, M.; Sander, D. Diagnosis, Treatment and Follow-up in Extracranial Carotid Stenosis. Dtsch. Arztebl. Int. 2020, 117, 801–807. [Google Scholar] [CrossRef]
- Bonati, L.H.; Kakkos, S.; Berkefeld, J.; de Borst, G.J.; Bulbulia, R.; Halliday, A.; van Herzeele, I.; Koncar, I.; McCabe, D.J.; Lal, A.; et al. European Stroke Organisation guideline on endarterectomy and stenting for carotid artery stenosis. Eur. Stroke J. 2021, 6, I–XLVII. [Google Scholar] [CrossRef]
- Reiff, T.; Eckstein, H.H.; Mansmann, U.; Jansen, O.; Fraedrich, G.; Mudra, H.; Bockler, D.; Bohm, M.; Bruckmann, H.; Debus, E.S.; et al. Angioplasty in asymptomatic carotid artery stenosis vs. endarterectomy compared to best medical treatment: One-year interim results of SPACE-2. Int. J. Stroke 2019, 15, 1747493019833017. [Google Scholar] [CrossRef]
- Nasr, B.; Crespy, V.; Penasse, E.; Gaudry, M.; Rosset, E.; Feugier, P.; Goueffic, Y.; Maurel, B.; Hostalrich, A.; Alric, P.; et al. Late Outcomes of Carotid Artery Stenting for Radiation Therapy-Induced Carotid Stenosis. J. Endovasc. Ther. 2022, 29, 921–928. [Google Scholar] [CrossRef]
- Bonati, L.H.; Gregson, J.; Dobson, J.; McCabe, D.J.H.; Nederkoorn, P.J.; van der Worp, H.B.; de Borst, G.J.; Richards, T.; Cleveland, T.; Muller, M.D.; et al. Restenosis and risk of stroke after stenting or endarterectomy for symptomatic carotid stenosis in the International Carotid Stenting Study (ICSS): Secondary analysis of a randomised trial. Lancet Neurol. 2018, 17, 587–596. [Google Scholar] [CrossRef]
- Cole, T.S.; Mezher, A.W.; Catapano, J.S.; Godzik, J.; Baranoski, J.F.; Nakaji, P.; Albuquerque, F.C.; Lawton, M.T.; Little, A.S.; Ducruet, A.F. Nationwide Trends in Carotid Endarterectomy and Carotid Artery Stenting in the Post-CREST Era. Stroke 2020, 51, 579–587. [Google Scholar] [CrossRef]
- Garg, K.; Chang, H.; Siracuse, J.J.; Jacobowitz, G.R.; Torres, J.; Veith, F.J.; Patel, V.I.; Maldonado, T.S.; Sadek, M.; Cayne, N.S.; et al. Severity of stenosis in symptomatic patients undergoing carotid interventions might influence perioperative neurologic events. J. Vasc. Surg. 2022, 76, 741–749.e1. [Google Scholar] [CrossRef]
- Silver, F.L.; Mackey, A.; Clark, W.M.; Brooks, W.; Timaran, C.H.; Chiu, D.; Goldstein, L.B.; Meschia, J.F.; Ferguson, R.D.; Moore, W.S.; et al. Safety of stenting and endarterectomy by symptomatic status in the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST). Stroke 2011, 42, 675–680. [Google Scholar] [CrossRef]
- Brott, T.G.; Hobson, R.W., 2nd; Howard, G.; Roubin, G.S.; Clark, W.M.; Brooks, W.; Mackey, A.; Hill, M.D.; Leimgruber, P.P.; Sheffet, A.J.; et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N. Engl. J. Med. 2010, 363, 11–23. [Google Scholar] [CrossRef]
- Rosenfield, K.; Matsumura, J.S.; Chaturvedi, S.; Riles, T.; Ansel, G.M.; Metzger, D.C.; Wechsler, L.; Jaff, M.R.; Gray, W.; Investigators, A.I. Randomized Trial of Stent versus Surgery for Asymptomatic Carotid Stenosis. N. Engl. J. Med. 2016, 374, 1011–1020. [Google Scholar] [CrossRef]
- Mas, J.L.; Chatellier, G.; Beyssen, B.; Branchereau, A.; Moulin, T.; Becquemin, J.P.; Larrue, V.; Lievre, M.; Leys, D.; Bonneville, J.F.; et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N. Engl. J. Med. 2006, 355, 1660–1671. [Google Scholar] [CrossRef]
- International Carotid Stenting Study Investigators. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): An interim analysis of a randomised controlled trial. Lancet 2010, 375, 985–997. [Google Scholar] [CrossRef]
- Yadav, J.S.; Wholey, M.H.; Kuntz, R.E.; Fayad, P.; Katzen, B.T.; Mishkel, G.J.; Bajwa, T.K.; Whitlow, P.; Strickman, N.E.; Jaff, M.R.; et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N. Engl. J. Med. 2004, 351, 1493–1501. [Google Scholar] [CrossRef]
- Halliday, A.; Bulbulia, R.; Bonati, L.H.; Chester, J.; Cradduck-Bamford, A.; Peto, R.; Pan, H.; Group, A.-C. Second asymptomatic carotid surgery trial (ACST-2): A randomised comparison of carotid artery stenting versus carotid endarterectomy. Lancet 2021, 398, 1065–1073. [Google Scholar] [CrossRef]
- Cui, L.; Han, Y.; Zhang, S.; Liu, X.; Zhang, J. Safety of Stenting and Endarterectomy for Asymptomatic Carotid Artery Stenosis: A Meta-Analysis of Randomised Controlled Trials. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 614–624. [Google Scholar] [CrossRef]
- Seretis, K.; Goudakos, I.; Vlachakis, I.; Anthimidis, G.; Papadimitriou, D. Carotid artery disease in octogenarians: Endarterectomy or stenting? Int. Angiol. 2007, 26, 353–360. [Google Scholar]
- Kallmayer, M.A.; Salvermoser, M.; Knappich, C.; Trenner, M.; Karlas, A.; Wein, F.; Eckstein, H.H.; Kuehnl, A. Quality appraisal of systematic reviews, and meta-analysis of the hospital/surgeon-linked volume-outcome relationship of carotid revascularization procedures. J. Cardiovasc. Surg. 2019, 60, 354–363. [Google Scholar] [CrossRef]
- Kuehnl, A.; Tsantilas, P.; Knappich, C.; Schmid, S.; Konig, T.; Breitkreuz, T.; Zimmermann, A.; Mansmann, U.; Eckstein, H.H. Significant Association of Annual Hospital Volume With the Risk of Inhospital Stroke or Death Following Carotid Endarterectomy but Likely Not After Carotid Stenting: Secondary Data Analysis of the Statutory German Carotid Quality Assurance Database. Circ. Cardiovasc. Interv. 2016, 9, e004171. [Google Scholar] [CrossRef] [PubMed]
- Poorthuis, M.H.F.; Brand, E.C.; Halliday, A.; Bulbulia, R.; Bots, M.L.; de Borst, G.J. High Operator and Hospital Volume Are Associated With a Decreased Risk of Death and Stroke After Carotid Revascularization: A Systematic Review and Meta-analysis. Ann. Surg. 2019, 269, 631–641. [Google Scholar] [CrossRef] [PubMed]
- AbuRahma, A.F.; Stone, P.A.; Srivastava, M.; Hass, S.M.; Mousa, A.Y.; Dean, L.S.; Campbell, J.E.; Chong, B.Y. The effect of surgeon’s specialty and volume on the perioperative outcome of carotid endarterectomy. J. Vasc. Surg. 2013, 58, 666–672. [Google Scholar] [CrossRef] [PubMed]
- German Vascular Society. Zertifizierung Gefaesszentren: Minimalanforderungen für die Zertifizierung zum Gefäßzentrum. Available online: https://www.dgg-akademie.de/anforderungsprofil-gefaesszentren/ (accessed on 12 December 2023).
- Neumann-Haefelin, T.; Busse, O.; Faiss, J.; Koennecke, H.C.; Ossenbrink, M.; Steinmetz, H.; Nabavi, D. Zertifizierungskriterien für Stroke-Units in Deutschland: Update 2022. DGNeurologie 2021, 4, 438–446. [Google Scholar] [CrossRef]
- Knappich, C.; Bohmann, B.; Kirchhoff, F.; Lohe, V.; Naher, S.; Kallmayer, M.; Eckstein, H.H.; Kuehnl, A. Intraoperative Completion Studies and their Associations with Carotid Endarterectomy Outcomes. Ann. Surg. 2024. online-ahead-of-print. [Google Scholar] [CrossRef] [PubMed]
- Kuehnl, A.; Kallmayer, M.; Bohmann, B.; Lohe, V.; Moser, R.; Naher, S.; Kirchhoff, F.; Eckstein, H.H.; Knappich, C. Association between hospital ownership and patient selection, management, and outcomes after carotid endarterectomy or carotid artery stenting—Secondary data analysis of the Bavarian statutory quality assurance database. BMC Surg. 2024, 24, 158. [Google Scholar] [CrossRef] [PubMed]
- LAG Bayern GbR. Verfahren 7—Karotis-Revaskularisation (QS KAROTIS). Available online: https://lag-by.de/qs-verfahren/deqs-richtlinie/qs-karotis/ (accessed on 17 July 2024).
- Bundesausschuss, G. Qualitätsförderungs- und Durchsetzungs-Richtlinie. 2019. Available online: https://www.g-ba.de/richtlinien/109/ (accessed on 17 July 2024).
- Swart, E.; Gothe, H.; Geyer, S.; Jaunzeme, J.; Maier, B.; Grobe, T.G.; Ihle, P. Good Practice of Secondary Data Analysis (GPS): Guidelines and recommendations. Gesundheitswesen 2015, 77, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Hemkens, L.G.; Moher, D.; Petersen, I.; Sorensen, H.T.; von Elm, E.; Langan, S.M.; et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. Z. Evid. Fortbild. Qual. Gesundhwes 2016, 115–116, 33–48. [Google Scholar] [CrossRef]
- Eckstein, H.H.; Tsantilas, P.; Kuhnl, A.; Haller, B.; Breitkreuz, T.; Zimmermann, A.; Kallmayer, M. Surgical and Endovascular Treatment of Extracranial Carotid Stenosis. Dtsch. Arztebl. Int. 2017, 114, 729–736. [Google Scholar] [CrossRef]
- Nimptsch, U.; Krautz, C.; Weber, G.F.; Mansky, T.; Grutzmann, R. Nationwide In-hospital Mortality Following Pancreatic Surgery in Germany is Higher than Anticipated. Ann. Surg. 2016, 264, 1082–1090. [Google Scholar] [CrossRef]
- Nimptsch, U.; Mansk, T. Deaths Following Cholecystectomy and Herniotomy: An Analysis of Nationwide German Hospital Discharge Data From 2009 to 2013. Dtsch. Arztebl. Int. 2015, 112, 535–543. [Google Scholar] [CrossRef][Green Version]
- Nimptsch, U.; Mansky, T. Trends in acute inpatient stroke care in Germany--an observational study using administrative hospital data from 2005–2010. Dtsch. Arztebl. Int. 2012, 109, 885–892. [Google Scholar] [CrossRef]
- R Foundation. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 20 July 2022).
- North American Symptomatic Carotid Endarterectomy Trial C; Barnett, H.J.M.; Taylor, D.W.; Haynes, R.B.; Sackett, D.L.; Peerless, S.J.; Ferguson, G.G.; Fox, A.J.; Rankin, R.N.; Hachinski, V.C.; et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991, 325, 445–453. [Google Scholar]
- Patel, P.D.; Khanna, O.; Lan, M.; Baldassari, M.; Momin, A.; Mouchtouris, N.; Tjoumakaris, S.; Gooch, R.; Rosenwasser, R.H.; Farrell, C.; et al. The effect of institutional case volume on post-operative outcomes after endarterectomy and stenting for symptomatic carotid stenosis. J. Stroke Cerebrovasc. Dis. 2024, 33, 107828. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Kobzeva-Herzog, A.J.; Yahn, C.; Farber, A.; King, E.G.; Hicks, C.; Eslami, M.H.; Patel, V.I.; Rybin, D.; Siracuse, J.J. Higher stroke risk after carotid endarterectomy and transcarotid artery revascularization is associated with relative surgeon volume ratio. J. Vasc. Surg. 2024. [Google Scholar] [CrossRef]
- Tsantilas, P.; Kuehnl, A.; Kallmayer, M.; Knappich, C.; Schmid, S.; Breitkreuz, T.; Zimmermann, A.; Eckstein, H.H. Risk of Stroke or Death Is Associated With the Timing of Carotid Artery Stenting for Symptomatic Carotid Stenosis: A Secondary Data Analysis of the German Statutory Quality Assurance Database. J. Am. Heart Assoc. 2018, 7, e007983. [Google Scholar] [CrossRef] [PubMed]
- Solomon, Y.; Conroy, P.D.; Rastogi, V.; Yadavalli, S.D.; Schneider, P.A.; Wang, G.J.; Malas, M.B.; de Borst, G.J.; Schermerhorn, M.L. Outcomes following carotid revascularization for stroke stratified by Modified Rankin Scale and time of intervention. J. Vasc. Surg. 2024, 79, 287–296.e1. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.; Peixoto, J.; Mansilha, A.; Naylor, A.R.; de Borst, G.J. Editor’s Choice—Timing of Carotid Intervention in Symptomatic Carotid Artery Stenosis: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 3–23. [Google Scholar] [CrossRef]
- Muller, M.D.; Lyrer, P.; Brown, M.M.; Bonati, L.H. Carotid artery stenting versus endarterectomy for treatment of carotid artery stenosis. Cochrane Database Syst. Rev. 2020, 2, CD000515. [Google Scholar] [CrossRef]
- Steg, P.G.; Feldman, L.J.; Omerovic, E. Observational studies play little role in guiding evidence-based medicine: Pros and cons. EuroIntervention 2024, 20, 29–31. [Google Scholar] [CrossRef]
- Shapiro, S. Confounding by indication? Epidemiology 1997, 8, 110–111. [Google Scholar] [PubMed]
- Winzer, S.; Rickmann, H.; Kitzler, H.; Abramyuk, A.; Krogias, C.; Strohm, H.; Barlinn, J.; Pallesen, L.P.; Siepmann, T.; Arnold, S.; et al. Ultrasonography Grading of Internal Carotid Artery Disease: Multiparametric German Society of Ultrasound in Medicine (DEGUM) versus Society of Radiologists in Ultrasound (SRU) Consensus Criteria. Ultraschall Med. 2022, 43, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Cassola, N.; Baptista-Silva, J.C.; Nakano, L.C.; Flumignan, C.D.; Sesso, R.; Vasconcelos, V.; Carvas Junior, N.; Flumignan, R.L. Duplex ultrasound for diagnosing symptomatic carotid stenosis in the extracranial segments. Cochrane Database Syst. Rev. 2022, 7, CD013172. [Google Scholar] [CrossRef] [PubMed]


| Certified Stroke-Unit | Certified Vascular Centre | |||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | |||||||
| Yes | No | Yes | No | |||||
| N (%) | 4969 | (16) | 3625 | (11) | 11,257 | (35) | 11,942 | (38) |
| Age (years, median, Q1–Q3) | 72 | (65–77) | 72 | (65–78) | 72 | (65–78) | 73 | (66–78) |
| Sex, Male | 3434 | (69) | 2443 | (67) | 7652 | (68) | 8120 | (68) |
| Right carotid artery treated | 2543 | (51) | 1795 | (50) | 5622 | (50) | 6115 | (51) |
| ASA stage | ||||||||
| Stage I + II | 1739 | (35) | 1224 | (34) | 3952 | (35) | 3734 | (31) |
| Stage III | 2937 | (59) | 2211 | (61) | 6675 | (59) | 7777 | (65) |
| Stage IV + V | 275 | (5.5) | 135 | (3.7) | 496 | (4.4) | 344 | (2.9) |
| Ipsilateral degree of stenosis | ||||||||
| Mild (<50%, NASCET) | 113 | (2.3) | 51 | (1.4) | 232 | (2.1) | 217 | (1.8) |
| Moderate (50–69%, NASCET) | 280 | (5.6) | 167 | (4.6) | 656 | (5.8) | 503 | (4.2) |
| Severe (70–99%, NASCET) | 4499 | (91) | 3333 | (92) | 10,041 | (89) | 11,094 | (93) |
| Occlusion (100%) | 77 | (1.5) | 74 | (2.0) | 328 | (2.9) | 128 | (1.1) |
| Contralateral degree of stenosis | ||||||||
| Mild (<50%, NASCET) | 3385 | (68) | 2350 | (65) | 7977 | (71) | 8012 | (67) |
| Moderate (50–69%, NASCET) | 683 | (14) | 645 | (18) | 1452 | (13) | 1860 | (16) |
| Severe (70–99%, NASCET) | 620 | (12) | 415 | (11) | 1194 | (11) | 1429 | (12) |
| Occlusion (100%) | 281 | (5.7) | 215 | (5.9) | 634 | (5.6) | 641 | (5.4) |
| Indication Group | ||||||||
| - Group A (asymptomatic) | 2812 | (57) | 2033 | (56) | 5478 | (49) | 7115 | (60) |
| - Group B (symptomatic, elective) | 1681 | (34) | 1068 | (29) | 4271 | (38) | 3712 | (31) |
| Amaurosis fugax | 258 | (5.2) | 214 | (5.9) | 576 | (5.1) | 579 | (4.8) |
| Transitory ischemic attack (TIA) | 589 | (12) | 429 | (12) | 1259 | (11) | 1404 | (12) |
| Stroke (Rankin 0–5) | 765 | (15) | 345 | (9.5) | 2245 | (20) | 1524 | (13) |
| Other symptoms | 69 | (1.4) | 80 | (2.2) | 191 | (1.7) | 205 | (1.7) |
| - Group C (others) | 476 | (9.6) | 524 | (14) | 1508 | (13) | 1115 | (9.3) |
| Crescendo-TIA/Stroke-in-evolution | 135 | (2.7) | 348 | (9.6) | 664 | (5.9) | 495 | (4.1) |
| Simultaneous procedures # | 139 | (2.8) | 66 | (1.8) | 382 | (3.4) | 197 | (1.6) |
| Others ° | 202 | (4.1) | 110 | (3.0) | 462 | (4.1) | 423 | (3.5) |
| Certified Stroke-Unit | Certified Vascular Centre | |||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | |||||||
| Yes | No | Yes | No | |||||
| Specialists available at center | ||||||||
| Vascular surgeon | 4857 | (98) | 3615 | (100) | 10,557 | (94) | 11,427 | (96) |
| Neurologist | 4826 | (97) | 2882 | (80) | 10823 | (96) | 5109 | (43) |
| Heart surgery | 1843 | (37) | 3132 | (86) | 3811 | (34) | 4793 | (40) |
| Internal medicine/Angiology | 2575 | (52) | 2725 | (75) | 4656 | (41) | 5971 | (50) |
| Internal medicine/Cardiology | 4969 | (100) | 3489 | (96) | 10,866 | (97) | 10,524 | (88) |
| Neurosurgery | 4574 | (92) | 3058 | (84) | 8361 | (74) | 4799 | (40) |
| Neuroradiology | 3753 | (76) | 2794 | (77) | 5494 | (49) | 2932 | (25) |
| Certified Quality management system | ||||||||
| DIN ISO EN 9001 | 1136 | (23) | 518 | (14) | 1441 | (13) | 2229 | (19) |
| KTQ | 159 | (3.2) | 144 | (4.0) | 1277 | (11) | 552 | (4.6) |
| proCum Cert | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 526 | (4.4) |
| none of these | 3674 | (74) | 2963 | (82) | 8539 | (76) | 8635 | (72) |
| Centre annual caseload (median; Q1–Q3) | ||||||||
| - All CEA | 94 | (76–120) | 54 | (37–98) | 34 | (10–65) | 14 | (3–35) |
| CEA in Group A | 59 | (34–74) | 25 | (15–58) | 16 | (2–32) | 6.0 | (1–21) |
| CEA in Group B | 30 | (26–45) | 27 | (14–38) | 14 | (3–26) | 4.0 | (1–12) |
| CEA in Group C | 1 | (0–4) | 1 | (0–3) | 0 | (0–1) | 0 | (0–1) |
| - All CAS | 11 | (3–29) | 3 | (1–20) | 4 | (0–19) | 0 | (0–4) |
| CAS in Group A | 5.5 | (2–11) | 2.0 | (0–4) | 1.0 | (0–5) | 0.0 | (0–2) |
| CAS in Group B | 3.0 | (0–6) | 0.0 | (0–2) | 1.0 | (0–6) | 0.0 | (0–1) |
| CAS in Group C | 0 | (0–2) | 0 | (0–1) | 0 | (0–1) | 0 | (0–0) |
| Regional settlement structure | ||||||||
| Independent city | 2986 | (60) | 2836 | (78) | 5290 | (47) | 3849 | (32) |
| Urban district | 159 | (3.2) | 144 | (4.0) | 867 | (7.7) | 1251 | (10) |
| Rural district | 1170 | (24) | 463 | (13) | 1779 | (16) | 2557 | (21) |
| Sparsely populated region | 654 | (13) | 182 | (5.0) | 3321 | (30) | 4285 | (36) |
| Certified Stroke-Unit | Certified Vascular Centre | |||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | |||||||
| Yes | No | Yes | No | |||||
| Time interval * | ||||||||
| 0–2 days | 243 | (14) | 186 | (17) | 643 | (15) | 376 | (10) |
| 3–7 days | 627 | (37) | 437 | (41) | 1662 | (39) | 1398 | (38) |
| 8–14 days | 282 | (17) | 149 | (14) | 652 | (15) | 722 | (19) |
| 15–180 days | 460 | (27) | 228 | (21) | 889 | (21) | 1104 | (30) |
| Neurological assessment | ||||||||
| Pre-procedural | 3821 | (77) | 1941 | (54) | 8012 | (71) | 6903 | (58) |
| Post-procedural | 4151 | (84) | 1162 | (32) | 6918 | (61) | 5474 | (46) |
| Pre- and post-procedural | 3399 | (68) | 1107 | (31) | 6119 | (54) | 4732 | (40) |
| Perioperative antiplatelet medication | ||||||||
| Aspirin monotherapy | 3601 | (72) | 3225 | (89) | 8211 | (73) | 9431 | (79) |
| Clopidogrel mono | 128 | (2.6) | 44 | (1.2) | 244 | (2.2) | 329 | (2.8) |
| Other monotherapy | 9 | (0.2) | 13 | (0.4) | 76 | (0.7) | 31 | (0.3) |
| Dual antiplatelet medication | 715 | (14) | 250 | (6.9) | 1680 | (15) | 1524 | (13) |
| None | 516 | (10) | 93 | (2.6) | 1046 | (9.3) | 627 | (5.3) |
| Treatment by indication group | ||||||||
| - Group A (asymptomatic) | ||||||||
| CEA | 2427 | (86) | 1837 | (90) | 4592 | (84) | 6086 | (86) |
| CAS | 385 | (14) | 196 | (10) | 886 | (16) | 1029 | (14) |
| - Group B (symptomatic, elective) | ||||||||
| CEA | 1505 | (90) | 983 | (92) | 3367 | (79) | 3333 | (90) |
| CAS | 176 | (10) | 85 | (8.0) | 904 | (21) | 379 | (10) |
| - Group C (others) | ||||||||
| CEA | 296 | (62) | 413 | (79) | 723 | (48) | 932 | (84) |
| CAS | 180 | (38) | 111 | (21) | 785 | (52) | 183 | (16) |
| Type of anesthesia ° | ||||||||
| Local anesthesia | 796 | (26) | 292 | (15) | 1710 | (30) | 1950 | (23) |
| General anesthesia | 2279 | (73) | 1608 | (84) | 3903 | (68) | 6218 | (73) |
| Combined anesthesia | 41 | (1.3) | 8 | (0.4) | 119 | (2.1) | 357 | (4.2) |
| Intraprocedural monitoring § | ||||||||
| Electroencephalography | 46 | (2.3) | 135 | (28) | 150 | (3.5) | 207 | (4.0) |
| Transcranial Cerebral Oximetry | 631 | (31) | 53 | (11) | 538 | (13) | 824 | (16) |
| Somato-sensory evoked potentials | 831 | (41) | 118 | (24) | 2026 | (48) | 2122 | (41) |
| Other methods | 497 | (25) | 184 | (38) | 1518 | (36) | 2063 | (40) |
| Certified Stroke-Unit | Certified Vascular Centre | |||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | |||||||
| Yes | No | Yes | No | |||||
| Carotid endarterectomy (CEA) | ||||||||
| Any stroke or death | ||||||||
| - Group A: asymptomatic | 18/2427 | (0.7) | 27/1837 | (1.5) | 47/4592 | (1.0) | 74/6086 | (1.2) |
| - Group B: symptomatic, elective | 27/1505 | (1.8) | 24/983 | (2.4) | 92/3367 | (2.7) | 76/3333 | (2.3) |
| - Group C1: symptomatic, emergency | 5/64 | (7.8) | 27/299 | (9.0) | 16/299 | (5.4) | 40/430 | (9.3) |
| - Group C2: Simultaneous procedures | 6/72 | (8.3) | 5/35 | (14) | 14/159 | (8.8) | 11/124 | (8.9) |
| - Group C3: Others | 12/160 | (7.5) | 8/79 | (10) | 16/265 | (6.0) | 24/378 | (6.3) |
| Carotid artery stenting (CAS) | ||||||||
| Any stroke or death | ||||||||
| - Group A: asymptomatic | 3/385 | (0.8) | 1/196 | (0.5) | 33/886 | (3.7) | 22/1029 | (2.1) |
| - Group B: symptomatic, elective | 6/176 | (3.4) | 4/85 | (4.7) | 32/904 | (3.5) | 17/379 | (4.5) |
| - Group C1: symptomatic, emergency | 9/71 | (13) | 8/49 | (16) | 37/365 | (10) | 6/65 | (9.2) |
| - Group C2: Simultaneous procedures | 4/67 | (6.0) | 6/31 | (19) | 27/223 | (12) | 6/73 | (8.2) |
| - Group C3: Others | 8/42 | (19) | 2/31 | (6.5) | 21/197 | (11) | 3/45 | (6.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saicic, S.; Knappich, C.; Kallmayer, M.; Kirchhoff, F.; Bohmann, B.; Lohe, V.; Naher, S.; Böhm, J.; Lückerath, S.; Eckstein, H.-H.; et al. Association of Centre Quality Certification with Characteristics of Patients, Management, and Outcomes Following Carotid Endarterectomy or Carotid Artery Stenting. J. Clin. Med. 2024, 13, 4407. https://doi.org/10.3390/jcm13154407
Saicic S, Knappich C, Kallmayer M, Kirchhoff F, Bohmann B, Lohe V, Naher S, Böhm J, Lückerath S, Eckstein H-H, et al. Association of Centre Quality Certification with Characteristics of Patients, Management, and Outcomes Following Carotid Endarterectomy or Carotid Artery Stenting. Journal of Clinical Medicine. 2024; 13(15):4407. https://doi.org/10.3390/jcm13154407
Chicago/Turabian StyleSaicic, Stefan, Christoph Knappich, Michael Kallmayer, Felix Kirchhoff, Bianca Bohmann, Vanessa Lohe, Shamsun Naher, Julian Böhm, Sofie Lückerath, Hans-Henning Eckstein, and et al. 2024. "Association of Centre Quality Certification with Characteristics of Patients, Management, and Outcomes Following Carotid Endarterectomy or Carotid Artery Stenting" Journal of Clinical Medicine 13, no. 15: 4407. https://doi.org/10.3390/jcm13154407
APA StyleSaicic, S., Knappich, C., Kallmayer, M., Kirchhoff, F., Bohmann, B., Lohe, V., Naher, S., Böhm, J., Lückerath, S., Eckstein, H.-H., & Kuehnl, A. (2024). Association of Centre Quality Certification with Characteristics of Patients, Management, and Outcomes Following Carotid Endarterectomy or Carotid Artery Stenting. Journal of Clinical Medicine, 13(15), 4407. https://doi.org/10.3390/jcm13154407





