Association between Oral Microbiome and Gastroesophageal Reflux Severity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Gastroesophageal Reflux Disorder
2.3. Microbiota Sampling and DNA Extraction
2.4. Statistical Analysis
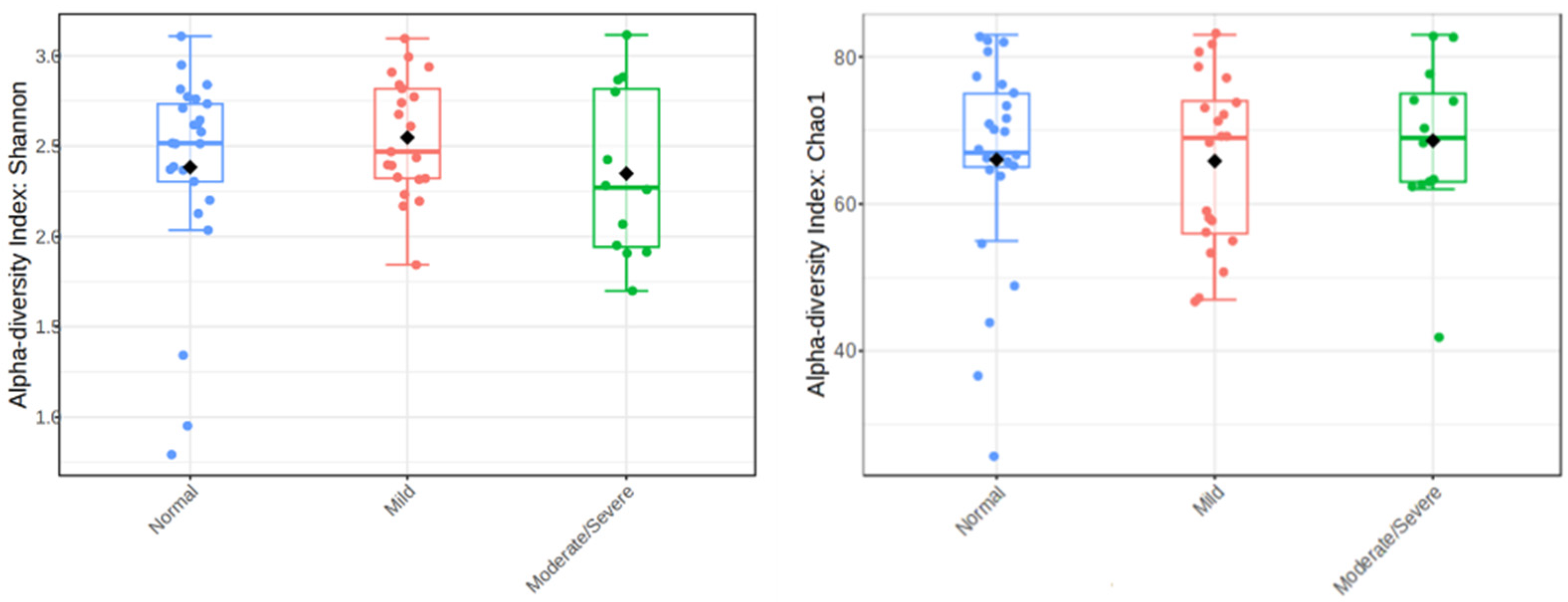
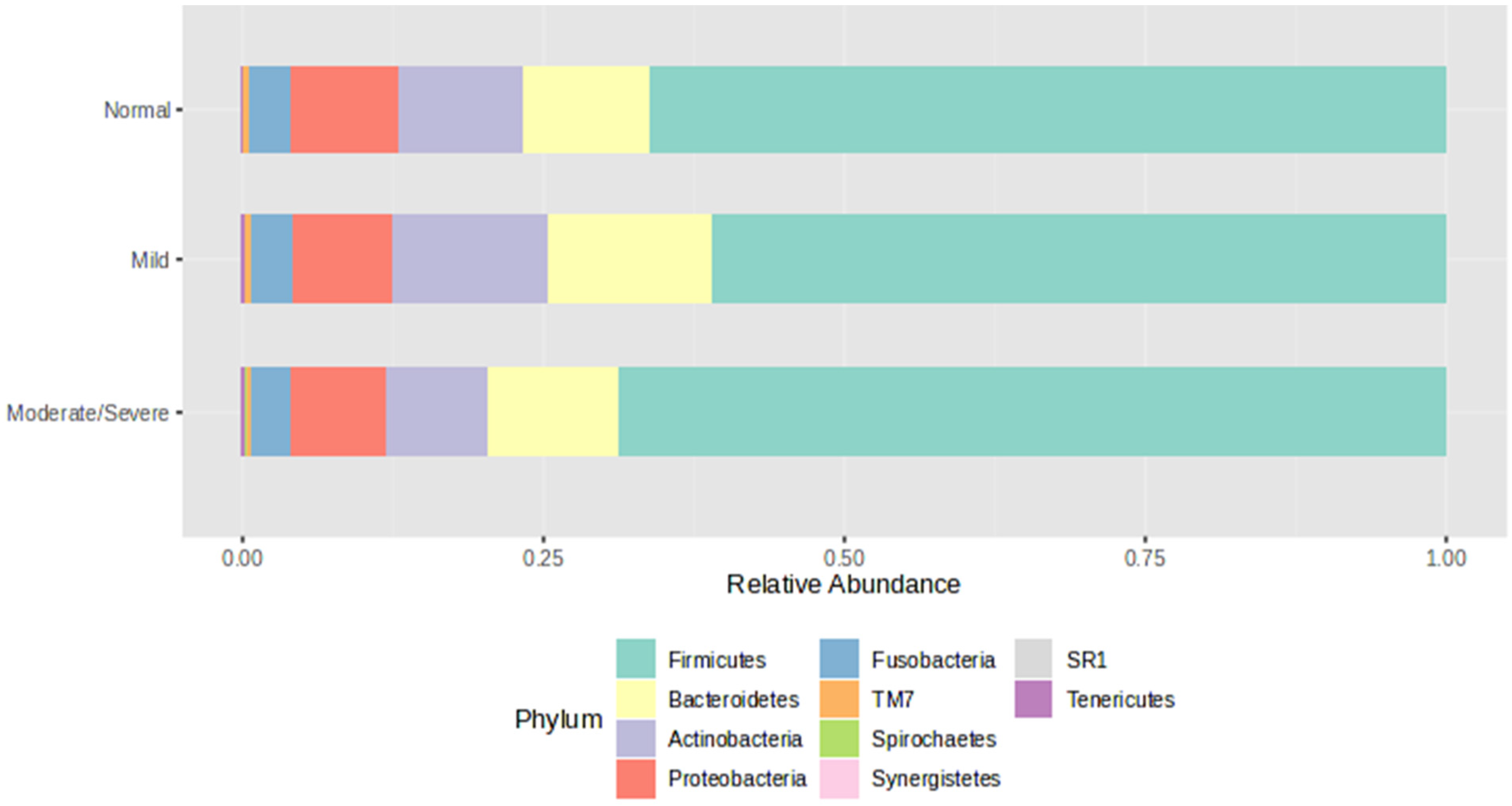
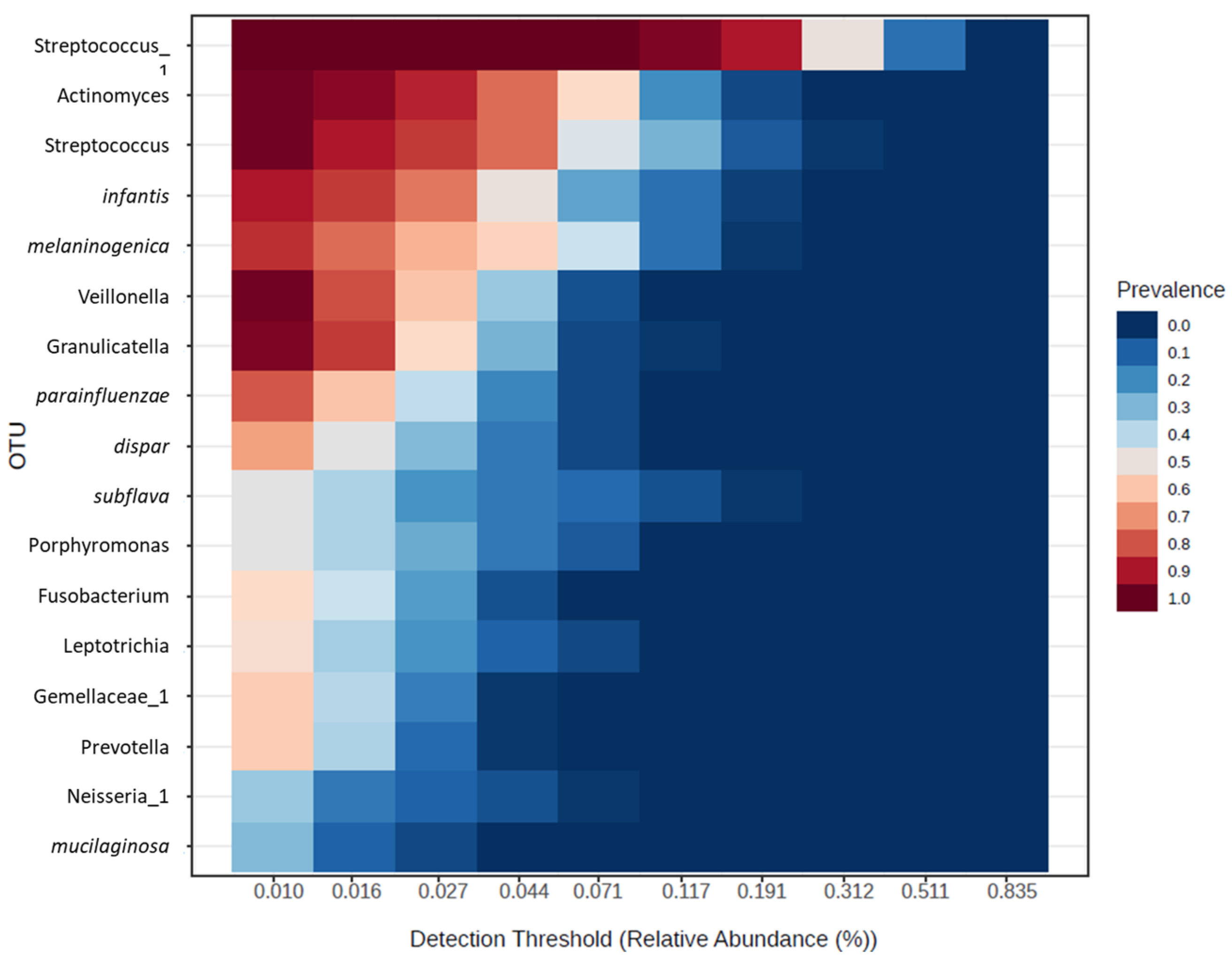
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eusebi, L.H.; Ratnakumaran, R.; Yuan, Y.; Solaymani-Dodaran, M.; Bazzoli, F.; Ford, A.C.J.G. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: A meta-analysis. Gut 2018, 67, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Nair, G.B. Homeostasis and dysbiosis of the gut microbiome in health and disease. J. Biosci. 2019, 44, 1–8. [Google Scholar] [CrossRef]
- Zhou, J.; Shrestha, P.; Qiu, Z.; Harman, D.G.; Teoh, W.-C.; Al-Sohaily, S.; Liem, H.; Turner, I.; Ho, V. Distinct microbiota dysbiosis in patients with non-erosive reflux disease and esophageal adenocarcinoma. J. Clin. Med. 2020, 9, 2162. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Chung, S.W.; Auh, Q.-S.; Hong, S.-J.; Lee, Y.-A.; Jung, J.; Lee, G.-J.; Park, H.J.; Shin, S.-I.; Hong, J.-Y.J.D. Progress in oral microbiome related to oral and systemic diseases: An update. Diagnostics 2021, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Yang, M.; Xu, D.; Zhang, G.; Cai, Y.; Yang, B.; Wang, X.; Yu, Y. Alterations of the salivary microbiota in gastroesophageal reflux disease. J. Oral Biosci. 2023, 65, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Y.; Zhao, Q.; Yan, Y.; Yang, T.; Xia, Y.; Chen, H. Patients with reflux esophagitis possess a possible different oral microbiota compared with healthy controls. Front. Pharmacol. 2020, 11, 548452. [Google Scholar] [CrossRef] [PubMed]
- Kawar, N.; Park, S.G.; Schwartz, J.L.; Callahan, N.; Obrez, A.; Yang, B.; Chen, Z.; Adami, G.R. Salivary microbiome with gastroesophageal reflux disease and treatment. Sci. Rep. 2021, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.F.; DeMeester, T.R. Development of the 24-hour intraesophageal pH monitoring composite scoring system. J. Clin. Gastroenterol. 1986, 8, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Padua, F.; Herbella, F.A.; Patti, M.G. Lyon consensus pH monitoring gray zone is more prone to be actual gastroesophageal reflux disease according to the demeester score. J. Gastrointest. Surg. 2021, 25, 2218–2220. [Google Scholar] [CrossRef]
- Liu, S.; Xu, M.; Yang, J.; Qi, H.; He, F.; Zhao, X.; Zhou, P.; Zhang, L.; Ming, D.J.C. Research on Gastroesophageal Reflux Disease Based on Dynamic Features of Ambulatory 24-Hour Esophageal pH Monitoring. Comput. Math. Methods Med. 2017, 2017, 9239074. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: Comprehensive statistical, functional and integrative analysis of microbiome data. Nucleic Acids Res. 2023, 51, W310–W318. [Google Scholar] [CrossRef]
- Vakil, N.; Van Zanten, S.V.; Kahrilas, P.; Dent, J.; Jones, R. The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am. J. Gastroenterol. 2006, 101, 1900–1920. [Google Scholar] [CrossRef]
- Azzam, R.S.J. Are the persistent symptoms to proton pump inhibitor therapy due to refractory gastroesophageal reflux disease or to other disorders? Arq. Gastroenterol. 2018, 55, 85–91. [Google Scholar] [CrossRef]
- Yang, L.; Lu, X.; Nossa, C.W.; Francois, F.; Peek, R.M.; Pei, Z.J.G. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology 2009, 137, 588–597. [Google Scholar] [CrossRef]
- Lundmark, A.; Hu, Y.O.; Huss, M.; Johannsen, G.; Andersson, A.F.; Yucel-Lindberg, T.J. Identification of salivary microbiota and its association with host inflammatory mediators in periodontitis. Front. Cell. Infect. Microbiol. 2019, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef]
- Visser, M.; Ellen, R.J. New insights into the emerging role of oral spirochaetes in periodontal disease. Clin. Microbiol. Infect. 2011, 17, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Bik, E.M.; Long, C.D.; Armitage, G.C.; Loomer, P.; Emerson, J.; Mongodin, E.F.; Nelson, K.E.; Gill, S.R.; Fraser-Liggett, C.M.; Relman, D.A. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J. 2010, 4, 962–974. [Google Scholar] [CrossRef]
- Kharitonova, M.; Vankov, P.; Abdrakhmanov, A.; Mamaeva, E.; Yakovleva, G.; Ilinskaya, O. The composition of microbial communities in inflammatory periodontal diseases in young adults Tatars. AIMS Microbiol. 2021, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jiang, X.; Zhu, F.; Yang, R.; Yu, X.; Zhou, X.; Tang, N. Characteristics of the oral and gastric microbiome in patients with early-stage intramucosal esophageal squamous cell carcinoma. BMC Microbiol. 2024, 24, 88. [Google Scholar] [CrossRef]
- Downes, J.; Munson, M.A.; Radford, D.R.; Spratt, D.A.; Wade, W.G. Shuttleworthia satelles gen. nov., sp. nov., isolated from the human oral cavity. Int. J. Syst. Evol. Microbiol. 2002, 52, 1469–1475. [Google Scholar] [PubMed]
- Sizova, M.V.; Muller, P.; Panikov, N.; Mandalakis, M.; Hohmann, T.; Hazen, A.; Fowle, W.; Prozorov, T.; Bazylinski, D.A.; Epstein, S.S.; et al. Stomatobaculum longum gen. nov., sp. nov., an obligately anaerobic bacterium from the human oral cavity. Int. J. Syst. Evol. Microbiol. 2013, 63, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Könönen, E.; Gursoy, U.K. Oral Prevotella species and their connection to events of clinical relevance in gastrointestinal and respiratory tracts. Front. Microbiol. 2022, 12, 798763. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Sagitov, I.I.; Akhmetova, R.F.; Saleeva, G.T.; Kiassov, A.P.; Gogoleva, N.E.; Shagimardanova, E.I.; Ziganshin, A.M. Comparison of the Microbiota and Inorganic Anion Content in the Saliva of Patients with Gastroesophageal Reflux Disease and Gastroesophageal Reflux Disease—Free Individuals. BioMed Res. Int. 2020, 2020, 2681791. [Google Scholar] [CrossRef] [PubMed]
- Hall, V. Actinomyces—Gathering evidence of human colonization and infection. Anaerobe 2008, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ranjitkar, S.; Kaidonis, J.A.; Smales, R.J. Gastroesophageal reflux disease and tooth erosion. Int. J. Dent. 2012, 2012, 479850. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.M.; Houston, K.; Keenan, L.; Yoo, B.S.; Parekh, P.J.; Johnson, D.A. Role of microbial dysbiosis in the pathogenesis of esophageal mucosal disease: A paradigm shift from acid to bacteria? World J. Gastroenterol. 2021, 27, 2054. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Winckler, B.; Lu, M.; Cheng, H.; Yuan, Z.; Yang, Y.; Jin, L.; Ye, W.J. Oral microbiota and risk for esophageal squamous cell carcinoma in a high-risk area of China. PLoS ONE 2015, 10, e0143603. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-Y.; Tang, C.Y.; Tan, T.-S.; Chen, K.-H.; Liao, K.-H.; Liou, M.-L. Subgingival microbiota in individuals with severe chronic periodontitis. J. Microbiol. Immunol. Infect. 2018, 51, 226–234. [Google Scholar] [CrossRef]
- Boutin, S.; Hagenfeld, D.; Zimmermann, H.; El Sayed, N.; Höpker, T.; Greiser, H.K.; Becher, H.; Kim, T.-S.; Dalpke, A.H. Clustering of subgingival microbiota reveals microbial disease ecotypes associated with clinical stages of periodontitis in a cross-sectional study. Front. Microbiol. 2017, 8, 340. [Google Scholar] [CrossRef]
- Colombo, A.P.V.; Bennet, S.; Cotton, S.L.; Goodson, J.M.; Kent, R.; Haffajee, A.D.; Socransky, S.S.; Hasturk, H.; Van Dyke, T.E.; Dewhirst, F.E. Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: Comparison between good responders and individuals with refractory periodontitis using the human oral microbe identification microarray. J. Periodontol. 2012, 83, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Mishiro, T.; Oka, K.; Kuroki, Y.; Takahashi, M.; Tatsumi, K.; Saitoh, T.; Tobita, H.; Ishimura, N.; Sato, S.; Ishihara, S.; et al. Oral microbiome alterations of healthy volunteers with proton pump inhibitor. J. Gastroenterol. Hepatol. 2018, 33, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Loke, C.; Lee, J.; Sander, S.; Mei, L.; Farella, M.J. Factors affecting intra-oral pH—A review. J. Oral Rehabil. 2016, 43, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Sujatha, S.; Jalihal, U.; Devi, Y.; Rakesh, N.; Chauhan, P.; Sharma, S.J. Oral pH in gastroesophageal reflux disease. Indian J. Gastroenterol. 2016, 35, 186–189. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Normal Group (n = 25) | Mild Group (n = 21) | Moderate/Severe Group (n = 12) | p Value |
|---|---|---|---|---|
| Gender | ||||
| Female, n (%) | 17 (68%) | 12 (57%) | 5 (42%) | 0.72 |
| Male, n (%) | 8 (32%) | 9 (43%) | 7 (58%) | 0.54 |
| Age, median (IQR) | 61 (47–68) | 61 (47–64) | 53 (40–64) | 0.34 |
| BMI median (IQR) | 23 (23–26) | 24 (23–25) | 23 (23–25) | 0.91 |
| DeMeester Score, median (IQR) | 4.4 (3.2–9.8) | 23.3 (18.2–27.2) | 96.2 (78.9–142.5) | 0.32×10−5 |
| Normal vs. Mild | |||||
| Phylum | Taxon Name | log2FC | logCPM | p Value | FDR |
| Actinobacteria | s_dentocariosa | 3.22 | 12.63 | 3.31 × 10−5 | 0.003 |
| Firmicutes | g_moryella | −1.70 | 11.50 | 8.64 × 10−4 | 0.025 |
| Firmicutes | o_Clostridiales_1 | −1.76 | 10.37 | 8.69 ×10−4 | 0.025 |
| Proteobacteria | g_lautropia | 2.15 | 11.94 | 0.0011 | 0.025 |
| Normal vs. Moderate/Severe | |||||
| Phylum | Feature | log2FC | logCPM | p Value | FDR |
| Firmicutes | g_schwartzia | −2.46 | 7.51 | 1.50 × 10−5 | 0.001 |
| TM7 | f_Rs_045 | −2.06 | 7.95 | 1.31 × 10−4 | 0.006 |
| Bacteroidetes | g_paludibacter | −2.23 | 7.98 | 2.13 × 10−4 | 0.006 |
| Firmicutes | s_satelles | −2.16 | 7.95 | 3.06 × 10−4 | 0.007 |
| Bacteroidetes | o_Bacteroidales_1 | −1.93 | 9.21 | 5.09 × 10−4 | 0.010 |
| Spirochaete | −1.69 | 9.41 | 9.03 × 10−4 | 0.010 | |
| Spirochaete | g_Treponema | −1.54 | 7.22 | 9.83 × 10−4 | 0.015 |
| Spirochaete | s_socranskii | −1.54 | 7.22 | 0.002 | 0.025 |
| Actinobacteria | s_aeria | 2.67 | 11.61 | 0.003 | 0.033 |
| Mild vs. Moderate/Severe | |||||
| Phylum | Feature | log2FC | logCPM | p Value | FDR |
| Bacteroidetes | s_pallens | 3.36 | 12.71 | 2.85 × 10−4 | 0.025 |
| Firmicutes | s_ satelles | −1.94 | 6.89 | 6.60 × 10−4 | 0.029 |
| Bacteroidetes | g_Paludibacter | −2.02 | 7.98 | 0.001 | 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Power, D.J.; Ho, V.; Zhou, J. Association between Oral Microbiome and Gastroesophageal Reflux Severity. J. Clin. Med. 2024, 13, 4479. https://doi.org/10.3390/jcm13154479
Power DJ, Ho V, Zhou J. Association between Oral Microbiome and Gastroesophageal Reflux Severity. Journal of Clinical Medicine. 2024; 13(15):4479. https://doi.org/10.3390/jcm13154479
Chicago/Turabian StylePower, Declan J., Vincent Ho, and Jerry Zhou. 2024. "Association between Oral Microbiome and Gastroesophageal Reflux Severity" Journal of Clinical Medicine 13, no. 15: 4479. https://doi.org/10.3390/jcm13154479







