Novel and Previously Known Mutations of the KCNV2 Gene Cause Various Variants of the Clinical Course of Cone Dystrophy with Supernormal Rod Response in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Assessment
2.2. Electrophysiological Assessment
2.3. Molecular Genetics
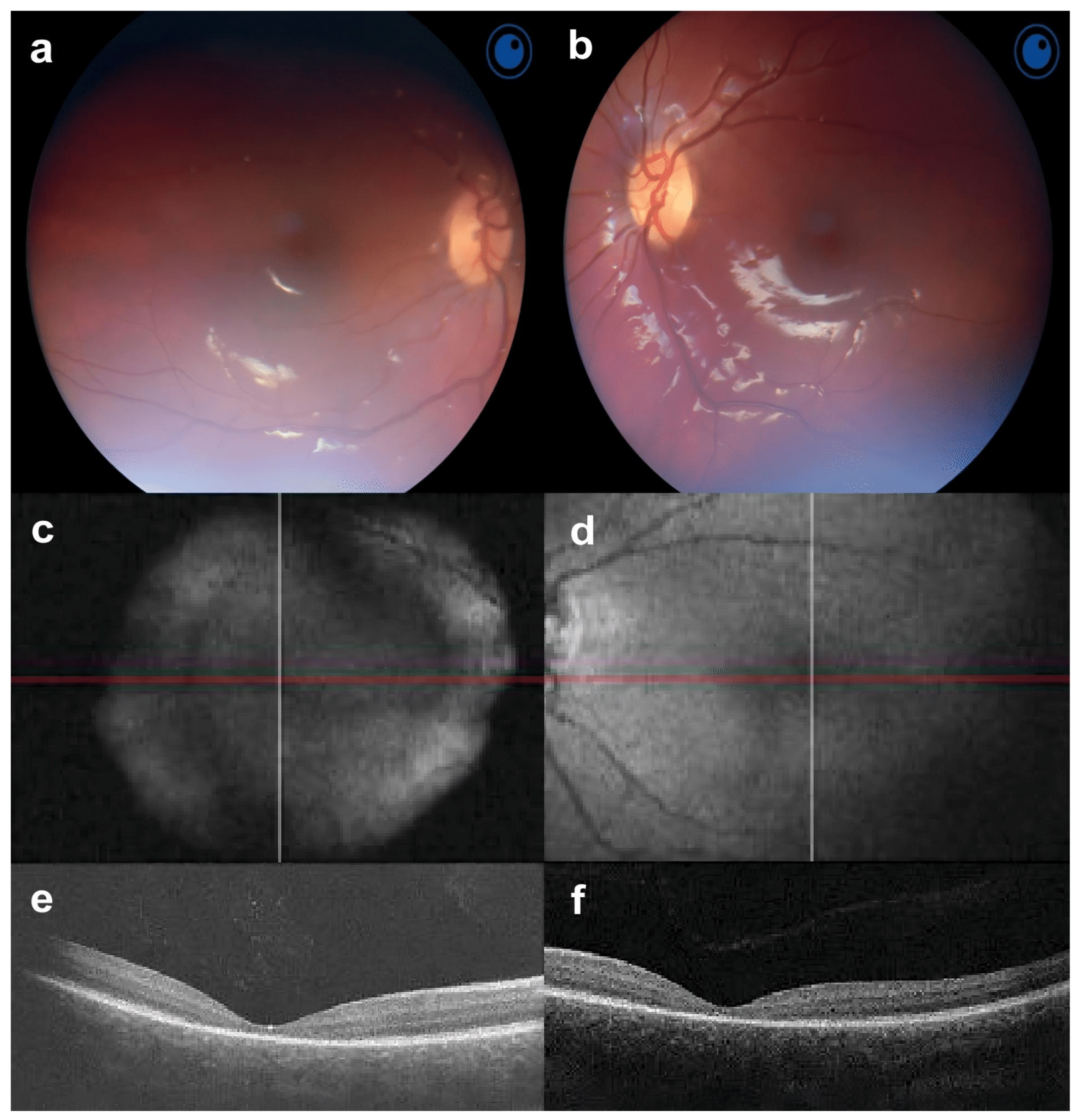
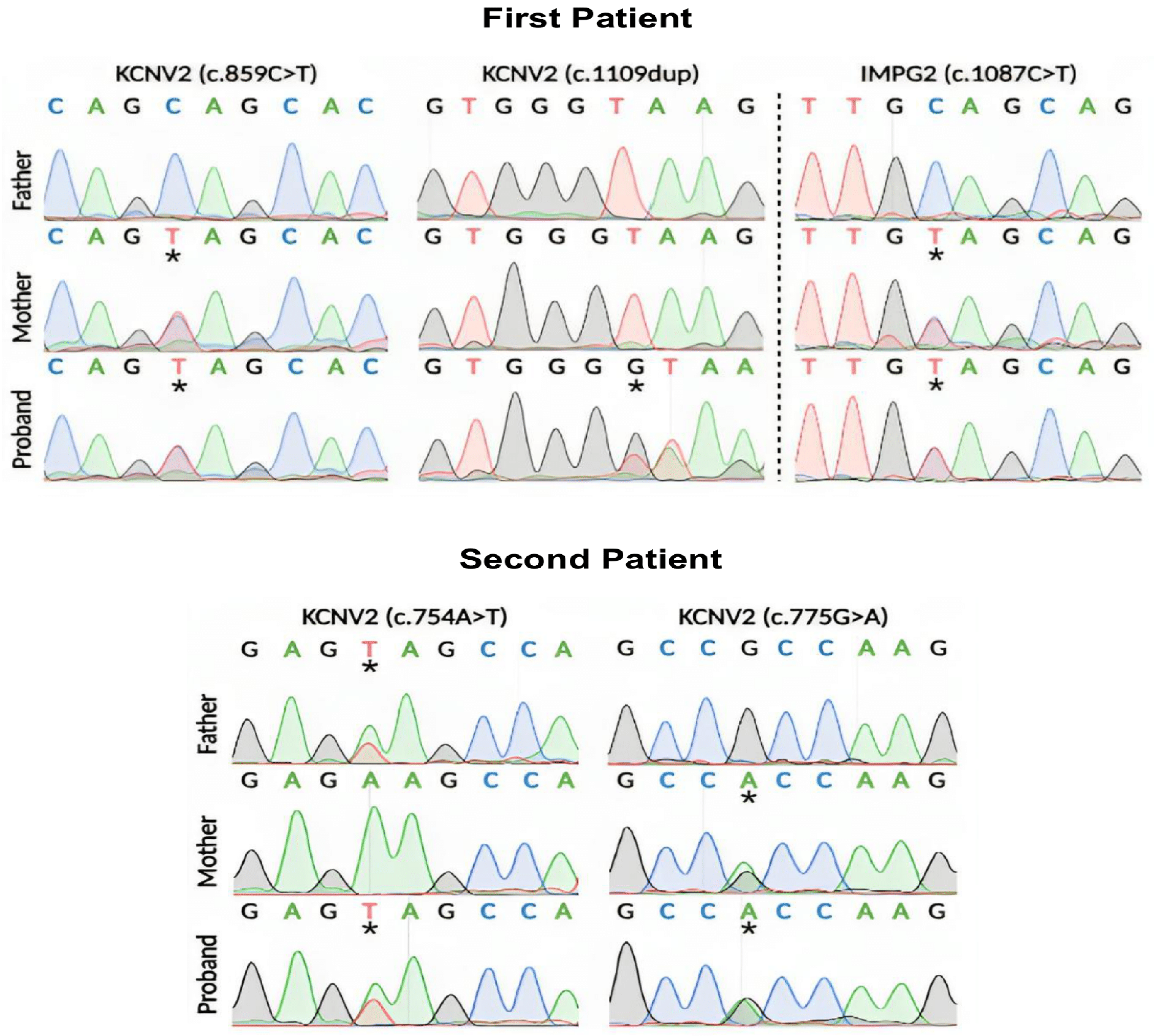
3. Results
3.1. Subjects and Clinical Findings
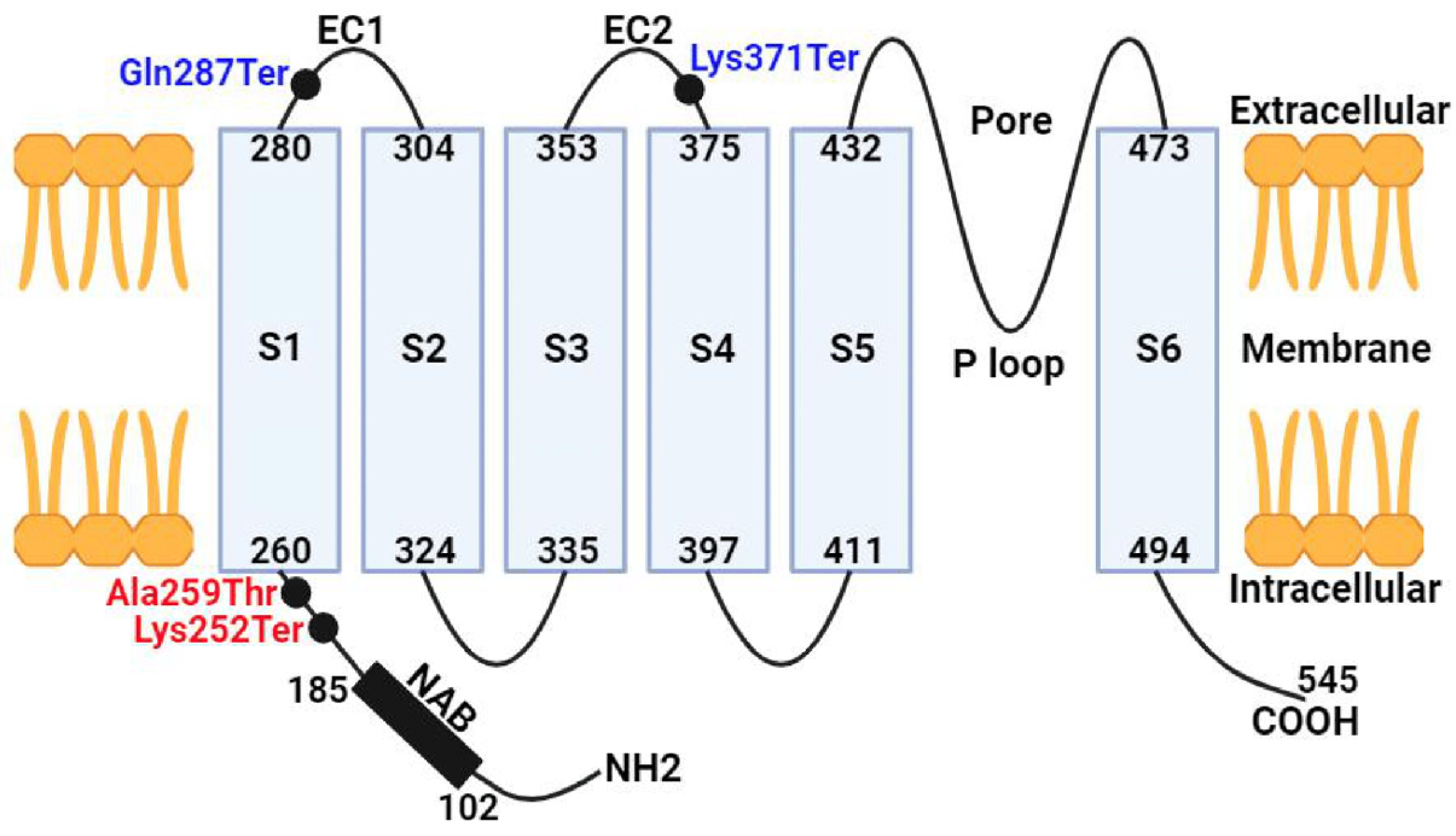
3.2. Molecular Genetics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, H.; Cowing, J.A.; Michaelides, M.; Wilkie, S.E.; Jeffery, G.; Jenkins, S.A.; Mester, V.; Bird, A.C.; Robson, A.G.; Holder, G.E.; et al. Mutations in the gene KCNV2 encoding a voltage-gated potassium channel subunit cause “cone dystrophy with supernormal rod electroretinogram” in humans. Am. J. Hum. Genet. 2006, 79, 574–579. [Google Scholar] [CrossRef]
- Wissinger, B.; Dangel, S.; Jagle, H.; Hansen, L.; Baumann, B.; Rudolph, G.; Wolf, C.; Bonin, M.; Koeppen, K.; Ladewig, T.; et al. Cone dystrophy with supernormal rod response is strictly associated with mutations in KCNV2. Investig. Ophthalmol. Vis. Sci. 2008, 49, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Robson, A.G.; Fujinami, K.; Leo, S.M.; Vincent, A.; Nasser, F.; Cabral De Guimarães, T.A.; Khateb, S.; Pontikos, N.; Fujinami-Yokokawa, Y.; et al. KCNV2 associated retinopathy: Genetics, electrophysiology, and clinical course-KCNV2 study group report 1. Am. J. Ophthalmol. 2021, 225, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, M.; Holder, G.E.; Webster, A.R.; Hunt, D.M.; Bird, A.C.; Fitzke, F.W.; Mollon, J.D.; Moore, A.T. A detailed phenotypic study of “cone dystrophy with supernormal rod ERG”. Br. J. Ophthalmol. 2005, 89, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.G.; Webster, A.R.; Michaelides, M.; Downes, S.M.; Cowing, J.A.; Hunt, D.M.; Moore, A.T.; Holder, G.E. “Cone dystrophy with supernormal rod electroretinogram”: A comprehensive genotype/phenotype study including fundus autofluorescence and extensive electrophysiology. Retina 2010, 30, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, E.; Yasir, Z.H.; Khan, A.M.; Raddadi, O.; Khandekar, R.; Alateeq, N.; Nowilaty, S.; AlShahrani, N.; Schatz, P. Analysis of retinal structure and function in cone dystrophy with supernormal rod response. Doc. Ophthalmol. 2020, 141, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, L.H.; Bell, P.; Maguire, A.M.; Xiao, R.; Hopkins, T.B.; Grant, R.; Bennett, J.; Wilson, J.M. AAV9 targets cone photoreceptors in the nonhuman primate retina. PLoS ONE 2013, 8, e53463. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, Y.; Huang, L.; Jiang, W.; Wu, N.; Song, Y.; Lu, Y.; Ma, Y. Compound heterozygous KCNV2 variants contribute to cone dystrophy with supernormal rod responses in a Chinese family. Mol. Genet. Genom. Med. 2021, 9, e1795. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, T.A.C.; Georgiou, M.; Robson, A.G.; Michaelides, M. KCNV2 retinopathy: Clinical features, molecular genetics and directions for future therapy. Ophthalmic Genet. 2020, 41, 208–215. [Google Scholar] [CrossRef]
- Thiagalingam, S.; McGee, T.L.; Weleber, R.G.; Sandberg, M.A.; Trzupek, K.M.; Berson, E.L.; Dryja, T.P. Novel mutations in the KCNV2 gene in patients with cone dystrophy and a supernormal rod electroretinogram. Ophthalmic Genet. 2007, 28, 135–142. [Google Scholar] [CrossRef]
- Alsalloum, A.; Mityaeva, O.; Kegeles, E.; Khavina, E.; Volchkov, P. Generation of two human induced pluripotent stem cell lines (ABi001-A and ABi002-A) from cone dystrophy with supernormal rod response patients caused by KCNV2 mutation. Stem Cell Res. 2023, 69, 103099. [Google Scholar] [CrossRef] [PubMed]
- Bach, M.; Brigell, M.G.; Hawlina, M.; Holder, G.E.; Johnson, M.A.; McCulloch, D.L.; Meigen, T.; Viswanathan, S. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc. Ophthalmol. 2013, 126, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Czirjak, G.; Toth, Z.E.; Enyedi, P. Characterization of the heteromericpotassium channel formed by kv2.1 and the retinal subunit kv8.2 in Xenopusoocytes. J. Neurophysiol. 2007, 98, 1213–1222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vincent, A.; Wright, T.; Garcia-Sanchez, Y.; Kisilak, M.; Campbell, M.; Westall, C.; Héon, E. Phenotypic characteristics including in vivo cone photoreceptor mosaic in KCNV2-related “cone dystrophy with supernormal rod electroretinogram”. Investig. Ophthalmol. Vis. Sci. 2013, 54, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, K.; Tsunoda, K.; Nakamura, N.; Kato, Y.; Noda, T.; Shinoda, K.; Tomita, K.; Hatase, T.; Usui, T.; Akahori, M.; et al. Molecular characteristics of four Japanese cases with KCNV2 retinopathy: Report of novel disease-causing variants. Mol. Vis. 2013, 19, 1580–1590. [Google Scholar] [PubMed] [PubMed Central]
- Wabbels, B.; Demmler, A.; Paunescu, K.; Wegscheider, E.; Preising, M.N.; Lorenz, B. Fundus autofluorescence in children and teenagers with hereditary retinal diseases. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 36–45. [Google Scholar] [CrossRef]
- Robson, A.G.; Michaelides, M.; Saihan, Z.; Bird, A.C.; Webster, A.R.; Moore, A.T.; Fitzke, F.W.; Holder, G.E. Functional characteristics of patients with retinal dystrophy that manifest abnormal parafoveal annuli of high density fundus autofluorescence; a review and update. Doc. Ophthalmol. 2008, 116, 79–89. [Google Scholar] [CrossRef]
- Robson, A.G.; Michaelides, M.; Luong, V.A.; Holder, G.E.; Bird, A.C.; Webster, A.R.; Moore, A.T.; Fitzke, F.W. Functional correlates of fundus autofluorescence abnormalities in patients with RPGR or RIMS1 mutations causing cone or cone rod dystrophy. Br. J. Ophthalmol. 2008, 92, 95–102. [Google Scholar] [CrossRef]
- Sergouniotis, P.I.; Holder, G.E.; Robson, A.G.; Michaelides, M.; Webster, A.R.; Moore, A.T. High-resolution optical coherence tomography imaging in KCNV2 retinopathy. Br. J. Ophthalmol. 2012, 96, 213–217. [Google Scholar] [CrossRef]
- Zobor, D.; Kohl, S.; Wissinger, B.; Zrenner, E.; Jägle, H. Rod and cone function in patients with KCNV2 retinopathy. PLoS ONE. 2012, 7, e46762. [Google Scholar] [CrossRef]
- Vázquez-Domínguez, I.; Li, C.H.Z.; Fadaie, Z.; Haer-Wigman, L.; Cremers, F.P.M.; Garanto, A.; Hoyng, C.B.; Roosing, S. Identification of a Complex Allele in IMPG2 as a Cause of Adult-Onset Vitelliform Macular Dystrophy. Investig. Ophthalmol. Vis. Sci. 2022, 63, 27. [Google Scholar] [CrossRef] [PubMed]
- van Huet, R.A.; Collin, R.W.; Smiatkowska, A.M.; Klaver, C.C.; Hoyng, C.B.; Simonelli, F.; Khan, M.I.; Qamar, R.; Banin, E.; Cremers, F.P.; et al. IMPG2-associated retinitis pigmentosa displays relatively early macular involvement. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3939–3953. [Google Scholar] [CrossRef] [PubMed]
- Esteves-Leandro, J.; Torres-Costa, S.; Estrela-Silva, S.; Santos-Silva, R.; Brandão, E.; Grangeia, A.; Fernandes, S.; Oliveira, R.; Falcão-Reis, F.; Rocha-Sousa, A. Cone dystrophy with supernormal rod responses: A rare KCNV2 gene variant. Eur. J. Ophthalmol. 2022, 32, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Wissinger, B.; Schaich, S.; Baumann, B.; Bonin, M.; Jägle, H.; Friedburg, C.; Varsányi, B.; Hoyng, C.B.; Dollfus, H.; Heckenlively, J.R.; et al. Large deletions of the KCNV2 gene are common in patients with cone dystrophy with supernormal rod response. Hum. Mutat. 2011, 32, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Sakti, D.H.; Cornish, E.E.; Ali, H.; Retsas, S.; Raza, M.; Saakova, N.; Carvalho, L.S.; Nash, B.M.; Jamieson, R.V.; Grigg, J.R. Natural history and biomarkers of KCNV2-associated retinopathy. Clin. Exp. Ophthalmol. 2024, 52, 528–544. [Google Scholar] [CrossRef] [PubMed]
- Collison, F.T.; Park, J.C.; Fishman, G.A.; Stone, E.M.; McAnany, J.J. Two-color pupillometry in KCNV2 retinopathy. Doc. Ophthalmol. 2019, 139, 11–20. [Google Scholar] [CrossRef]
- Jiang, X.; Rashwan, R.; Voigt, V.; Nerbonne, J.; Hunt, D.M.; Carvalho, L.S. Molecular, Cellular and Functional Changes in the Retinas of Young Adult Mice Lacking the Voltage-Gated K+ Channel Subunits Kv8.2 and K2.1. Int. J. Mol. Sci. 2021, 22, 4877. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsalloum, A.; Mosin, I.; Shefer, K.; Mingaleva, N.; Kim, A.; Feoktistova, S.; Malyugin, B.; Boiko, E.; Sultanov, S.; Mityaeva, O.; et al. Novel and Previously Known Mutations of the KCNV2 Gene Cause Various Variants of the Clinical Course of Cone Dystrophy with Supernormal Rod Response in Children. J. Clin. Med. 2024, 13, 4592. https://doi.org/10.3390/jcm13164592
Alsalloum A, Mosin I, Shefer K, Mingaleva N, Kim A, Feoktistova S, Malyugin B, Boiko E, Sultanov S, Mityaeva O, et al. Novel and Previously Known Mutations of the KCNV2 Gene Cause Various Variants of the Clinical Course of Cone Dystrophy with Supernormal Rod Response in Children. Journal of Clinical Medicine. 2024; 13(16):4592. https://doi.org/10.3390/jcm13164592
Chicago/Turabian StyleAlsalloum, Almaqdad, Ilya Mosin, Kristina Shefer, Natalia Mingaleva, Alexander Kim, Sofya Feoktistova, Boris Malyugin, Ernest Boiko, Shamil Sultanov, Olga Mityaeva, and et al. 2024. "Novel and Previously Known Mutations of the KCNV2 Gene Cause Various Variants of the Clinical Course of Cone Dystrophy with Supernormal Rod Response in Children" Journal of Clinical Medicine 13, no. 16: 4592. https://doi.org/10.3390/jcm13164592
APA StyleAlsalloum, A., Mosin, I., Shefer, K., Mingaleva, N., Kim, A., Feoktistova, S., Malyugin, B., Boiko, E., Sultanov, S., Mityaeva, O., & Volchkov, P. (2024). Novel and Previously Known Mutations of the KCNV2 Gene Cause Various Variants of the Clinical Course of Cone Dystrophy with Supernormal Rod Response in Children. Journal of Clinical Medicine, 13(16), 4592. https://doi.org/10.3390/jcm13164592







