MASLD-Related Hepatocarcinoma: Special Features and Challenges
Abstract
:1. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
1.1. Concept and Nomenclature
1.2. Epidemiology
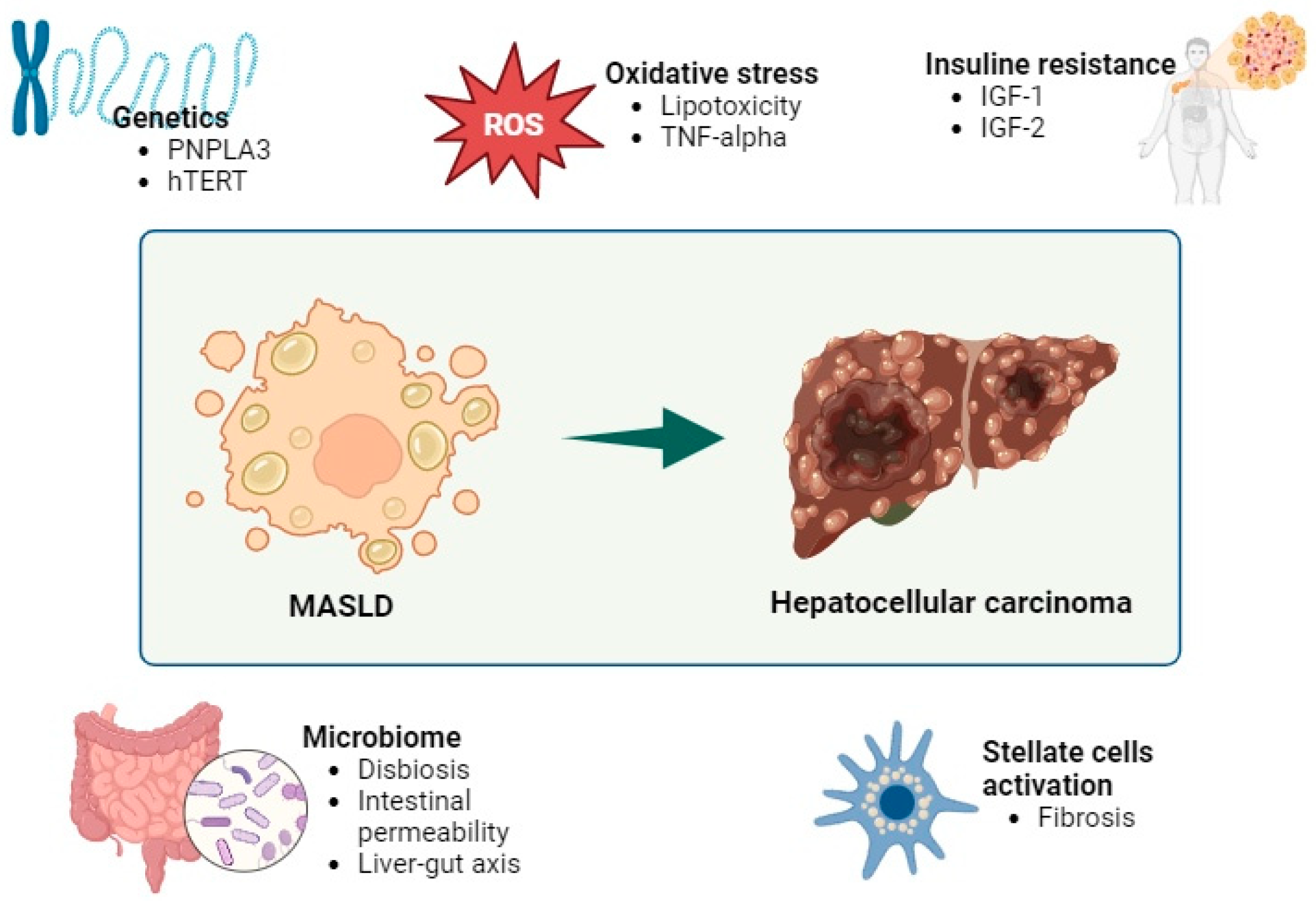
1.3. Etiopathogenesis
2. MASLD-Related Complications
2.1. Cardiovascular Complications
2.2. Extrahepatic Neoplasms
3. Hepatocellular Carcinoma and MASLD
3.1. Pathogenesis
3.2. Epidemiology
3.3. Clinical Presentation
3.4. Screening
3.5. Prevention
3.6. Treatment of HCC Related to MASLD
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ludwig, J.; Viggiano, T.R.; McGill, D.B.; Oh, B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980, 55, 434–438. [Google Scholar] [PubMed]
- Han, S.K.; Baik, S.K.; Kim, M.Y. Non-alcoholic fatty liver disease: Definition and subtypes. Clin. Mol. Hepatol. 2023, 29, S5–S16. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Paik, J.M.; Stepanova, M.; Ong, J.; Alqahtani, S.; Henry, L. Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and nonalcoholic fatty liver disease. J. Hepatol. 2024, 80, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Lai, J.C.; Wong, G.L.; Wong, V.W.; Yip, T.C. Can we use old NAFLD data under the new MASLD definition? J. Hepatol. 2023, 80, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Tinajero, M.G.; Malik, V.S. An update on the epidemiology of type 2 diabetes: A global perspective. Endocrinol. Metab. Clin. N. Am. 2021, 50, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Touros, A.; Kim, W.R. Nonalcoholic Fatty Liver Disease and Metabolic Syndrome. Clin. Liver Dis. 2018, 22, 133–140. [Google Scholar] [CrossRef]
- Qureshi, K.; Abrams, G.A. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World, J. Gastroenterol. 2007, 13, 3540–3553. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Wasilewska, N.; Lebensztejn, D.M. Non-alcoholic fatty liver disease and lipotoxicity. Clin. Exp. Hepatol. 2021, 7, 1–6. [Google Scholar] [CrossRef]
- Cannito, S.; Morello, E.; Bocca, C.; Foglia, B.; Benetti, E.; Novo, E.; Chiazza, F.; Rogazzo, M.; Fantozzi, R.; Povero, D.; et al. Microvesicules released from fat-laden cells promote activation of hepatocellular NLRP3 inflammasome: A pro-inflammatory link between lipotoxicity and non-alcoholic steatohepatitis. PLoS ONE 2017, 12, e0172575. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Alidadipour, A.; Saed, V.; Sayyadi, F.; Jadidi, Y.; Davoudi, M.; Amraee, F.; Jadidi, N.; Afrisham, R. Hepatokines: Unveiling the molecular and cellular mechanisms connecting hepatic tissue to insulin resistance and inflammation. Acta Diabetol. 2024; 1–23, epub ahead of print. [Google Scholar] [CrossRef]
- Zhou, X.-D.; Targher, G.; Byrne, C.D.; Somers, V.; Kim, S.U.; Chahal, C.A.A.; Wong, V.W.-S.; Cai, J.; Shapiro, M.D.; Eslam, M.; et al. An international multidisciplinary consensus statement on MAFLD and the risk of CVD. Hepatol. Int. 2023, 17, 773–791. [Google Scholar] [CrossRef]
- Yoneda, M.; Yamamoto, T.; Honda, Y.; Imajo, K.; Ogawa, Y.; Kessoku, T.; Kobayashi, T.; Nogami, A.; Higurashi, T.; Kato, S.; et al. Risk of cardiovascular disease in patients with fatty liver disease as defined from the metabolic dysfunction associated fatty liver disease or nonalcoholic fatty liver disease point of view: A retrospective nationwide claims database study in Japan. J. Gastroenterol. 2021, 56, 1022–1032. [Google Scholar] [CrossRef]
- Zhou, X.-D.; Cai, J.; Targher, G.; Byrne, C.D.; Shapiro, M.D.; Sung, K.-C.; Somers, V.K.; Chahal, C.A.A.; George, J.; Chen, L.-L.; et al. Metabolic dysfunction-associated fatty liver disease and implications for cardiovascular risk and disease prevention. Cardiovasc. Diabetol. 2022, 21, 270. [Google Scholar] [CrossRef]
- Gaiani, K.; Jornayvaz, F.R. Pathophysiology of NASH in endocrine diseases. Endocr. Connect. 2021, 2, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Sanna, C.; Rosso, C.; Marietti, M.; Bugianesi, E. Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers. Int. J. Mol. Sci. 2016, 17, 717. [Google Scholar] [CrossRef]
- Mitsala, A.; Tsalikidis, C.; Romanidis, K.; Pitiakoudis, M. Non-Alcoholic Fatty Liver Disease and Extrahepatic Cancers: A Wolf in Sheep’s Clothing? Curr. Oncol. 2022, 29, 4478–4510. [Google Scholar] [CrossRef] [PubMed]
- Streba, L.A.M.; Vere, C.C.; Rogoveanu, I.; Streba, C.T. Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: An open question. World J. Gastroenterol. 2015, 21, 4103–4110. [Google Scholar] [CrossRef]
- Vetrano, E.; Rinaldi, L.; Mormone, A.; Giorgione, C.; Galiero, R.; Caturano, A.; Nevola, R.; Marfella, R.; Sasso, F.C. Non-alcoholic fatty liver disease (NAFLD), Type 2 diabetes and non-viral hepatocarcinoma: Pathophysiological mechanisms and new therapeutic strategies. Biomedicines 2023, 11, 468. [Google Scholar] [CrossRef]
- Takakura, K.; Oikawa, T.; Nakano, M.; Saeki, C.; Torisu, Y.; Kajihara, M.; Saruta, M. Recent insights into the multiple pathways driving non-alcoholic steatohepatitis-derived hepatocellular carcinoma. Front. Oncol. 2019, 9, 762. [Google Scholar] [CrossRef]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2020, 18, 223–238. [Google Scholar] [CrossRef]
- Behary, J.; Amorim, N.; Jiang, X.-T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Goto, T.; Hirotsu, Y.; Masuzaki, R.; Moriyama, M.; Omata, M. Molecular mechanisms: Connections between nonal-coholic fatty liver disease, steatohepatitis and hepatocellular carcinoma. Int. J. Mol. Sci. 2020, 21, 1525. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.J.H.; Ng, C.H.; Lin, S.Y.; Pan, X.H.; Tay, P.; Lim, W.H.; Teng, M.; Syn, N.; Lim, G.; Yong, J.N.; et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: A systematic review and meta-analysis. Lancet Oncol. 2022, 23, 521–530. [Google Scholar] [CrossRef]
- Cernea, S.; Onișor, D. Screening and interventions to prevent nonalcoholic fatty liver disease/nonalcoholic steatohepatitis-associated hepatocellular carcinoma. World J. Gastroenterol. 2023, 29, 286–309. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, H.; Craig, D.; Barker, R.; Spiers, G.; Stow, D.; Anstee, Q.M.; Hanratty, B. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020, 17, e1003100. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Chen, T.-I.; Yang, T.-H.; Yin, S.-C.; Lu, S.-N.; Liu, X.-R.; Gao, Y.-Z.; Lin, C.-J.; Huang, C.-W.; Huang, J.-F.; et al. Long-term Risks of Cirrhosis and Hepatocellular Carcinoma Across Steatotic Liver Disease Subtypes. Am. J. Gastroenterol. 2024; advance online publication. [Google Scholar] [CrossRef]
- Yasui, K.; Hashimoto, E.; Komorizono, Y.; Koike, K.; Arii, S.; Imai, Y.; Shima, T.; Kanbara, Y.; Saibara, T.; Mori, T.; et al. Characteristics of Patients with Nonalcoholic Steatohepatitis Who Develop Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2011, 9, 428–433. [Google Scholar] [CrossRef]
- Vitale, A.; Svegliati-Baroni, G.; Ortolani, A.; Cucco, M.; Dalla Riva, G.V.; Giannini, E.G.; Piscaglia, F.; Rapaccini, G.; Di Marco, M.; Caturelli, E.; et al. Epidemiological trends and trajectories of MAFLD-associated hepatocellular carcinoma 2002–2033: The ITA.LI.CA database. Gut 2023, 72, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.G.; Wentworth, B.J.; Zimmet, A.; Rinella, M.E.; Loomba, R.; Caldwell, S.H.; Argo, C.K. Systematic review with meta-analysis: Risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment. Pharmacol. Ther. 2018, 48, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Lim, J.K.; Patton, H.; El-Serag, H.B. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2020, 158, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- EASL clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [CrossRef] [PubMed]
- Taru, M.-G.; Lupsor-Platon, M. Exploring Opportunities to Enhance the Screening and Surveillance of Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease (NAFLD) through Risk Stratification Algorithms Incorporating Ultrasound Elastography. Cancers 2023, 15, 4097. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V. The Growing Landscape of NAFLD-Associated Hepatocellular Carcinoma and Its Impact in Surveillance. GE-Port. J. Gastroenterol. 2023, 31, 14–23. [Google Scholar] [CrossRef]
- Alexander, M.; Loomis, A.K.; Van Der Lei, J.; Duarte-Salles, T.; Prieto-Alhambra, D.; Ansell, D.; Pasqua, A.; Lapi, F.; Rijnbeek, P.; Mosseveld, M.; et al. Risks and clinical predic-tors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: Real-world study of 18 million pa-tients in four European cohorts. BMC Med. 2019, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.A.; Patil, R.; Harrison, S.A. NAFLD-related hepatocellular carcinoma: The growing challenge. Hepatology 2023, 77, 323–338. [Google Scholar] [CrossRef]
- Cagnin, S.; Donghia, R.; Martini, A.; Pesole, P.L.; Coletta, S.; Shahini, E.; Boninsegna, G.; Biasiolo, A.; Pontisso, P.; Giannelli, G. Galad Score as a Prognostic Marker for Patients with Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 16485. [Google Scholar] [CrossRef]
- Guan, M.-C.; Zhang, S.-Y.; Ding, Q.; Li, N.; Fu, T.-T.; Zhang, G.-X.; He, Q.-Q.; Shen, F.; Yang, T.; Zhu, H. The Performance of GALAD Score for Diagnosing Hepatocellular Carcinoma in Patients with Chronic Liver Diseases: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 949. [Google Scholar] [CrossRef]
- Berhane, S.; Toyoda, H.; Tada, T.; Kumada, T.; Kagebayashi, C.; Satomura, S.; Schweitzer, N.; Vogel, A.; Manns, M.P.; Benckert, J.; et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin. Gastroenterol. Hepatol. 2016, 14, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.L.; Xu, D.; Wicha, M.S.; Lok, A.S.; Parikh, N.D. Uility of liquid biopsy analysis in detection of hepatocellular carcinoma, determination of prognosis, and disease monitoring: A systematic review. Clin. Gastroenterol. Hepatol. 2020, 18, 2879–2902. [Google Scholar] [CrossRef] [PubMed]
- Lange, N.F.; Radu, P.; Dufour, J.-F. Prevention of NAFLD-associated HCC: Role of lifestyle and chemoprevention. J. Hepatol. 2021, 75, 1217–1227. [Google Scholar] [CrossRef]
- Geh, D.; Anstee, Q.M.; Reeves, H.L. NAFLD-Associated HCC: Progress and Opportunities. J. Hepatocell. Carcinoma 2021, 8, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, S.E.; Schlesinger, S.; Aleksandrova, K.; Jochem, C.; Jenab, M.; Gunter, M.J.; Overvad, K.; Tjønneland, A.; Boutron-Ruault, M.C.; Carbonnel, F.; et al. Association between physical activity and risk of hepatobiliary cancers: A multinational cohort study. J. Hepatol. 2019, 70, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.I.; Khan, M.U.; Kodali, S.; Shetty, A.; Bell, S.M.; Victor, D. Hepatocellular Carcinoma Due to Nonalcoholic Fatty Liver Disease: Current Concepts and Future Challenges. J. Hepatocell. Carcinoma 2022, 9, 477–496. [Google Scholar] [CrossRef] [PubMed]
- Marengo, A.; Rosso, C.; Bugianesi, E. Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis. Annu. Rev. Med. 2016, 67, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Poly, T.N.; Walther, B.A.; Yang, H.-C.; Li, Y.-C.J. Statin Use and the Risk of Hepatocellular Carcinoma: A Meta-Analysis of Observational Studies. Cancers 2020, 12, 671. [Google Scholar] [CrossRef]
- Pinter, M.; Pinato, D.J.; Ramadori, P.; Heikenwalder, M. NASH and Hepatocellular carcinoma: Immunology and immunotheray. Clin. Cancer Res. 2023, 29, 513–520. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagüe-Caballero, C.; Casas-Deza, D.; Pascual-Oliver, A.; Espina-Cadena, S.; Arbones-Mainar, J.M.; Bernal-Monterde, V. MASLD-Related Hepatocarcinoma: Special Features and Challenges. J. Clin. Med. 2024, 13, 4657. https://doi.org/10.3390/jcm13164657
Yagüe-Caballero C, Casas-Deza D, Pascual-Oliver A, Espina-Cadena S, Arbones-Mainar JM, Bernal-Monterde V. MASLD-Related Hepatocarcinoma: Special Features and Challenges. Journal of Clinical Medicine. 2024; 13(16):4657. https://doi.org/10.3390/jcm13164657
Chicago/Turabian StyleYagüe-Caballero, Carmen, Diego Casas-Deza, Andrea Pascual-Oliver, Silvia Espina-Cadena, Jose M. Arbones-Mainar, and Vanesa Bernal-Monterde. 2024. "MASLD-Related Hepatocarcinoma: Special Features and Challenges" Journal of Clinical Medicine 13, no. 16: 4657. https://doi.org/10.3390/jcm13164657






