Sensory Profiles Predict Symptoms of Central Sensitization in Low Back Pain: A Predictive Model Research Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethics
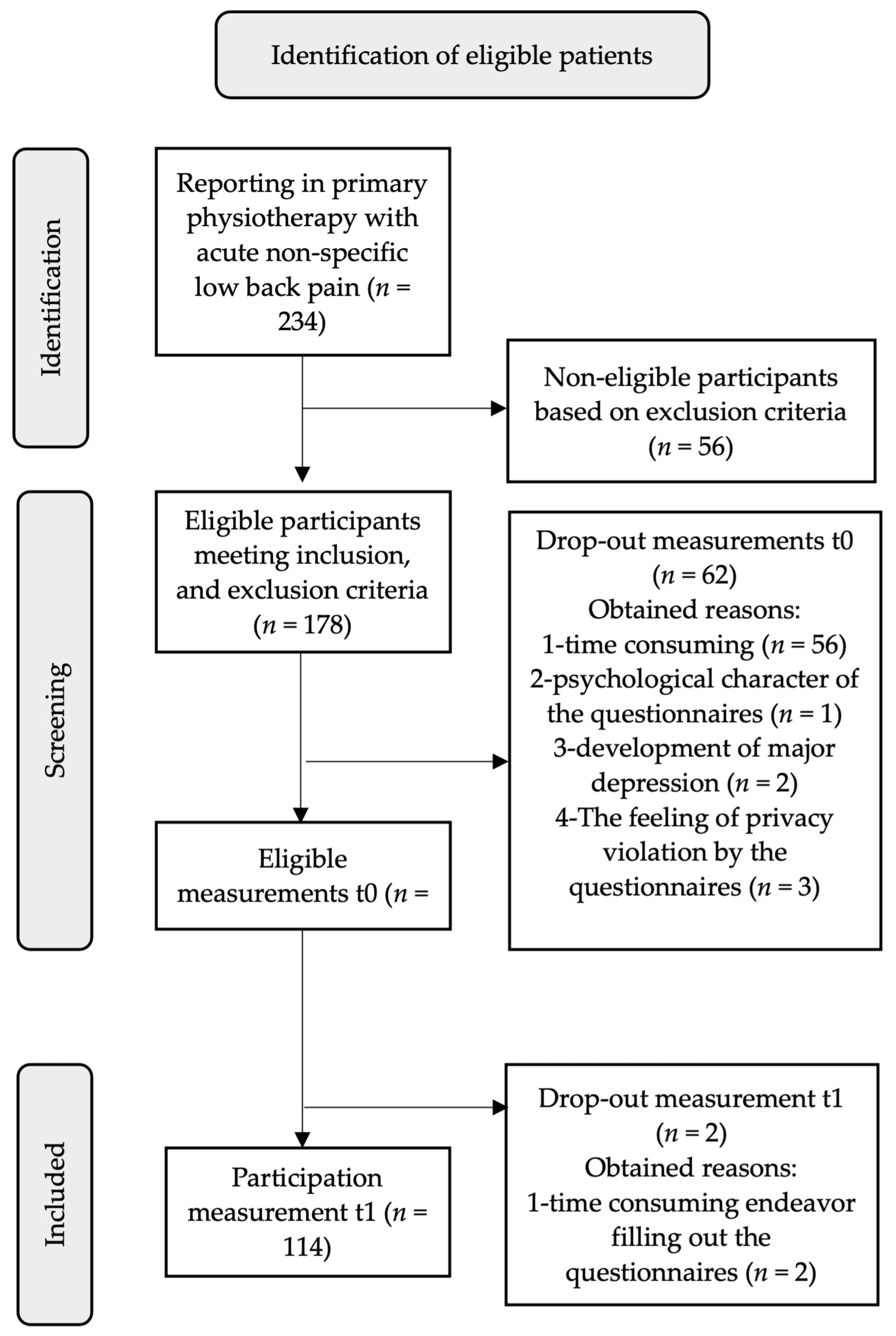
2.3. Setting and Participants
2.4. Data Collection and Measurement Procedures
2.5. Measurements
2.5.1. Adolescent/Adult Sensory Profile (AASP)
2.5.2. Known Factors
Numeric Pain Rating Scale (NPRS)
State-Trait Anxiety Inventory (STAI-DY1, STAI-DY2)
Becks Depression Inventory (BDI)
Pain Catastrophizing Scale (PCS)
2.5.3. Central Sensitization Inventory (CSI)
2.6. Data Analysis
3. Results
- Coefficient of determination R2 = 0.38; standard error = 4.38.
- Abbreviations: CSIt−1 = Central Sensitization Inventory score at 12 weeks; SSv.t0-score = Sensory Sensitive score at baseline; STAI.trait.t0-score = trait anxiety score at baseline.
| Linear Regression Analysis | Central Sensitization Inventory | |||||
| R2 | F-Ratio | B | Beta | p | S.E. | |
| Model | 0.38 | 31.15 (2; 103) | −5.84 | 0.19 | 4.38 | |
| SSv | 0.42 | 0.27 | 0.00 | 0.13 | ||
| STAI.trait | 0.53 | 0.45 | <0.001 | 0.10 | ||
| Logistic regression analysis | Central Sensitization Inventory | |||||
| Nagelkerke’s R2 | Wald (df), p | B | OR (95%CI) | S.E. | ||
| Model | 0.23 | 18.79 (1), <0.001 | −6.38 | 0.00 (1.01; 1.14) | 1.47 | |
| SSv | 3.07 (1), 0.04 | 0.06 | 1.07 (1.01; 1.14) | 0.04 | ||
| STAI.trait | 18.79 (1), <0.001 | 0.07 | 1.07 (1.01; 1.14) | 0.03 | ||
4. Discussion
4.1. Clinical Implications
4.2. Limitations
4.3. Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2021 Low Back Pain Collaborators. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: A systematic analysis of the global burden of disease study 2021. Lancet Rheumatol. 2023, 22, e316–e329. [Google Scholar] [CrossRef]
- Cayrol, T.; Draper-Rodi, J.; Fabre, L.; Pitance, L.; van den Broeke, E.N. Stuck in the Middle With You: Why a Broad-Brush Approach to Defining Central Sensitization Does Not Help Clinicians and Patients. J. Orthop. Sports Phys. Ther. 2021, 51, 204–206. [Google Scholar] [CrossRef]
- Nijs, J.; Malfliet, A.; Nishigami, T. Nociplastic pain and central sensitization in patients with chronic pain conditions: A terminology update for clinicians. Braz. J. Phys. Ther. 2023, 27, 100518. [Google Scholar] [CrossRef]
- Popkirov, S.; Enax-Krumova, E.K.; Mainka, T.; Hoheisel, M.; Hausteiner-Wiehle, C. Functional pain disorders-more than nociplastic pain. NeuroRehabilitation 2020, 47, 343–353. [Google Scholar] [CrossRef]
- Klyne, D.M.; Moseley, G.L.; Sterling, M.; Barbe, M.F.; Hodges, P.W. Are signs of central sensitization in acute low back pain a precursor to poor outcome? J. Pain 2019, 20, 994–1009. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef]
- Nijs, J.; Kosek, E.; Chiarotto, A.; Cook, C.; Danneels, L.A.; Fernández-de-Las-Peñas, C.; Hodges, P.W.; Koes, B.; Louw, A.; Ostelo, R.; et al. Nociceptive, neuropathic, or nociplastic low back pain? The low back pain phenotyping (BACPAP) consortium’s international and multidisciplinary consensus recommendations. Lancet Rheumatol. 2024, 6, e178–e188. [Google Scholar] [CrossRef]
- Dunn, W. The Impact of Sensory Processing Abilities on the Daily Lives of Young Children and Their Families: A Conceptual Model. Inf. Young Child. 1997, 9, 23–35. [Google Scholar] [CrossRef]
- Brown, C.; Tollefson, N.; Dunn, W.; Cromwell, R.; Filion, D. The adult sensory profile: Measuring patterns of sensory processing. Am. J. Occup. Ther. 2001, 55, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.R.; Nijs, J.; Smart, K.; Holmes, P.; Yeowell, G.; Goodwin, P.C. Prevalence of extreme trait sensory profiles and personality types in nonspecific chronic low back pain with predominant central sensitization: Secondary analysis of an international observational study. Pain Physician 2019, 22, 181–190. [Google Scholar] [CrossRef]
- Gräper, P.J.; Clark, J.R.; Thompson, B.L.; Hallegraeff, J.M. Evaluating sensory profiles in nociplastic chronic low back pain: A cross-sectional validation study. Physiother. Theory Pract. 2022, 29, 1508–1518. [Google Scholar] [CrossRef]
- Clark, J.; Nijs, J.; Yeowell, G.; Goodwin, P.C. What Are the Predictors of Altered Central Pain Modulation in Chronic Musculoskeletal Pain Populations? A Systematic Review. Pain Physician 2017, 20, 487–500. [Google Scholar] [CrossRef]
- Gräper, P.J.; Hartvigsen, J.; Scafoglieri, A.; Clark, J.R.; van Trijffel, E.; Hallegraeff, J.M. Sensory profiles and their role in the persistence of central sensitization symptoms in low back pain. A prospective cohort study. Physiother. Theory Pract. 2024, 11, 1–10. [Google Scholar] [CrossRef]
- Riley, R.D.; van der Windt, D.A.; Croft, P.; Moons, K.G.M. Prognosis Research in Health Care, Concepts, Methods, and Impact, 1st ed.; Oxford University Press: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef]
- Moons, K.G.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- WHO Guideline for Non-Surgical Management of Chronic Primary Low Back Pain in Adults in Primary and Community Care Settings. Available online: https://www.who.int/publications/i/item/9789240081789 (accessed on 13 June 2024).
- KNGF Guideline on Low Back Pain and Lumbosacral Radicular Syndrome. Available online: https://www.kngf.nl/binaries/content/assets/kennisplatform/onbeveiligd/guidelines/low-back-pain-2021/kngf_guide_on_low_back_pain_practice_guideline.pdf (accessed on 13 June 2014).
- Brown, C.; Dunn, W. Adolescent/Adult Sensory Profile; Pearson Education: San Antonio, TX, USA, 2002. [Google Scholar]
- Gándara-Gafo, B.; Santos-Del Riego, S.; Muñiz, J. Reference values for the adolescent/adult sensory profile in Spain. Am. J. Occup. Ther. 2019, 73, 7305205040p1–7305205040p8. [Google Scholar] [CrossRef]
- Nieminen, L.K.; Pyysalo, L.M.; Kankaanpää, M.J. Prognostic factors for pain chronicity in low back pain: A systematic review. Pain Rep. 2021, 6, e919. [Google Scholar] [CrossRef]
- Childs, J.D.; Piva, S.R.; Fritz, J.M. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine 2005, 30, 1331–1334. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Thombs, B.D.; Magyar-Russell, G.; Bass, E.B.; Stewart, K.J.; Tsilidis, K.K.; Bush, D.E.; Fauerbach, J.A.; McCann, U.D.; Ziegelstein, R.C. Performance characteristics of depression screening instruments in survivors of acute myocardial infarction: Review of the evidence. Psychosmatics 2007, 48, 185–194. [Google Scholar] [CrossRef]
- Smarr, K.L.; Keefer, A.L. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Care Res. 2011, 63 (Suppl. S11), S454–S466. [Google Scholar] [CrossRef]
- Arnau, R.C.; Meagher, M.W.; Norris, M.P.; Bramson, R. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychol. 2001, 20, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.A.; D’Eon, J.L. Psychometric properties of the Beck Depression Inventory--second edition (BDI-II) in individuals with chronic pain. Pain 2008, 137, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Schulte-van Maaren, Y.W.; Carlier, I.V.; Zitman, F.G.; van Hemert, A.M.; de Waal, M.W.; van der Does, A.J.; van Noorden, M.S.; Giltay, E.J. Reference values for major depression questionnaires: The Leiden Routine Outcome Monitoring Study. J. Affect. Disord. 2013, 149, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.; Thibault, P.; Andrikonyte, J.; Butler, H.; Catchlove, R.; Larivière, C. Psychological influences on repetition-induced summation of activity-related pain in patients with chronic low back pain. Pain 2009, 141, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Lamé, I.E.; Peters, M.L.; Kessels, A.G.; Van Kleef, M.; Patijn, J. Test-retest stability of the Pain Catastrophizing Scale and the Tampa Scale for Kinesiophobia in chronic pain over a longer period of time. J. Health Psychol. 2008, 13, 820–826. [Google Scholar] [CrossRef]
- Kregel, J.; Vuijk, P.J.; Descheemaeker, F.; Keizer, D.; van der Noord, R.; Nijs, J.; Cagnie, B.; Meeus, M.; van Wilgen, P. The Dutch Central Sensitization Inventory (CSI): Factor analysis, discriminative power, and test-retest reliability. Clin. J. Pain 2016, 32, 624–630. [Google Scholar] [CrossRef]
- Neblett, R.; Cohen, H.; Choi, Y.; Hartzell, M.M.; Williams, M.; Mayer, T.G.; Gatchel, R.J. The Central Sensitization Inventory (CSI): Establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J. Pain 2013, 14, 438–445. [Google Scholar] [CrossRef]
- Mayer, T.G.; Neblett, R.; Cohen, H.; Howard, K.J.; Choi, Y.H.; Williams, M.J.; Perez, Y.; Gatchel, R.J. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012, 12, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Neblett, R.; Hartzell, M.M.; Mayer, T.G.; Cohen, H.; Gatchel, R.J. Establishing clinically relevant severity levels for the central sensitization inventory. Pain Pract. 2017, 17, 166–175. [Google Scholar] [CrossRef]
- Scerbo, T.; Colasurdo, J.; Dunn, S.; Unger, J.; Nijs, J.; Cook, C. Measurement properties of the central sensitization inventory: A systematic review. Pain Pract. 2018, 18, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Masconi, K.L.; Matsha, T.E.; Echouffo-Tcheugui, J.B.; Erasmus, R.T.; Kengne, A.P. Reporting and handling of missing data in predictive research for prevalent undiagnosed type 2 diabetes mellitus: A systematic review. EPMA J. 2015, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Field, A. Discovering Statistics Using SPSS, 3rd ed.; SAGE Publications: London, UK, 2018. [Google Scholar]
- Hamilton, D.F.; Ghert, M.; Simpson, A.H. Interpreting regression models in clinical outcome studies. Bone Jt. Res. 2015, 4, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Nijs, J.; George, S.Z.; Clauw, D.J.; Fernández-de-las-Peñas, C.; Kosek, E.; Ickmans, K.; Fernández-Canero, J.; Polli, A.; Kapreli, E.; Huysmans, E.; et al. Central sensitization in chronic pain conditions: Latest discoveries and their potential for precision medicine. Lancet 2021, 3, e383–e392. [Google Scholar] [CrossRef]
- Schuttert, I.; Wolff, A.P.; Schiphorst Preuper, R.H.R.; Malmberg, A.G.G.A.; Reneman, M.F.; Timmerman, H. Validity of the Central Sensitization Inventory to Address Human Assumed Central Sensitization: Newly Proposed Clinically Relevant Values and Associations. J. Clin. Med. 2023, 23, 4849. [Google Scholar] [CrossRef]
- Nijs, J.; Torres-Cueco, R.; van Wilgen, C.P.; Girbes, E.L.; Struyf, F.; Roussel, N.; van Oosterwijck, J.; Daenenm, L.; Kuppens, K.; Vanwerweeen, L.; et al. Applying modern pain neuroscience in clinical practice: Criteria for the classification of central sensitization pain. Pain Physician 2014, 17, 447–457. [Google Scholar] [CrossRef]
- Glymour, M.M.; Weuve, J.; Berkman, L.F.; Kawachi, I.; Robins, J.M. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am. J. Epidemiol. 2005, 162, 267–278. [Google Scholar] [CrossRef]
- Vander Weele, T.J. Confounding and effect modification: Distribution and measure. Epidemiol. Methods 2012, 1, 55–82. [Google Scholar] [CrossRef] [PubMed]
- Bettini, L.; Moore, K. Central Sensitization in Functional Chronic Pain Syndromes: Overview and Clinical Application. Pain Manag. Nurs. 2016, 17, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Royston, P.; Altman, D.G.; Sauerbrei, W. Dichotomizing continuous predictors in multiple regression: A bad idea. Stat. Med. 2006, 25, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Australian Commission on Safety and Quality in Health Care 2022 Low Back Pain Clinical Care Standard. Clinical Care Standards, Australia. Available online: https://www.safetyandquality.gov.au/standards/clinical-care-standards/low-back-pain-clinical-care-standard/information-clinicians#what-clinicians-need-to-know (accessed on 13 June 2024).
- George, S.Z.; Fritz, J.M.; Silfies, S.P.; Schneider, M.J.; Beneciuk, J.M.; Lentz, T.A.; Gilliam, J.R.; Hendren, S.; Norman, K.S. Interventions for the management of acute and chronic low back pain: Revision 2021. J. Orthop. Sports Phys. Ther. 2021, 51, CPG1–CPG60. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Loggia, M.L.; Polli, A.; Moens, M.; Huysmans, E.; Goudman, L.; Meeus, M.; Vanderweeën, L.; Ickmans, K.; Clauw, D. Sleep disturbances and severe stress as glial activators: Key targets for treating central sensitization in chronic pain patients? Expert Opin. Ther. Targets 2017, 21, 817–826. [Google Scholar] [CrossRef]
- Caumo, W.; Antunes, L.C.; Elkfury, J.L.; Herbstrith, E.G.; Busanello Sipmann, R.; Souza, A.; Torres, I.L.; Souza Dos Santos, V.; Neblett, R. The central sensitization inventory validated and adapted for a Brazilian population: Psychometric properties and its relationship with brain-derived neurotrophic factor. J. Pain Res. 2017, 10, 2109–2122. [Google Scholar] [CrossRef]
- Bittencourt, J.V.; de Melo Magalhães Amaral, A.C.; Rodrigues, P.V.; Corrêa, L.A.; Silva, B.M.; Reis, F.J.J.; Nogueira, L.A.C. Diagnostic accuracy of the clinical indicators to identify central sensitization pain in patients with musculoskeletal pain. Arch. Physiother. 2021, 11, 2. [Google Scholar] [CrossRef]
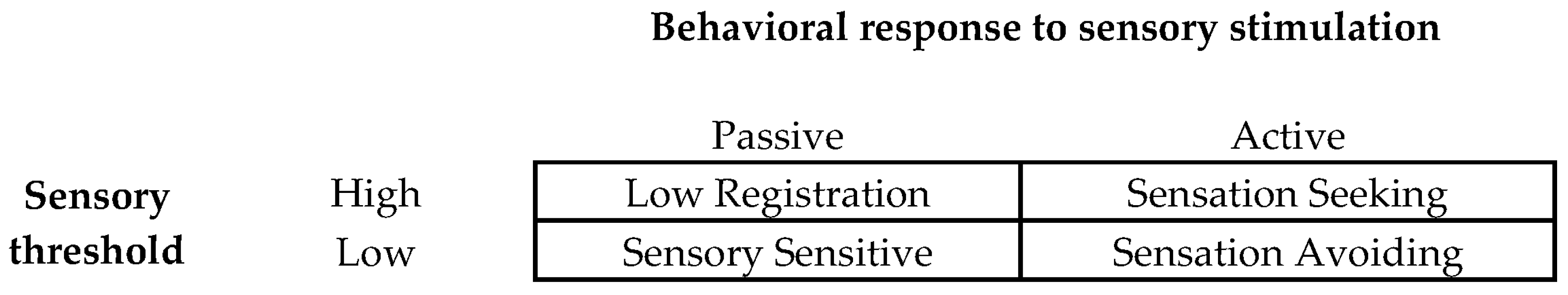

| Baseline | |
|---|---|
| Male (n) (%) | 64 (56.1) |
| Age (yrs.) (SD) | 45 (11.1) |
| Widespread pain (n) (%) | 41 (36.0) |
| Duration of LBP (wks.) (SD) | 3.0 (1.5) |
| Severity of LBP (NPRS) (SD) | 6.1 (1.9) |
| Severity of leg pain (NPRS) (SD) | 1.6 (2.3) |
| Recurrent episodes (n) (%) | 81 (71.1) |
| Variable | Baseline | 12 Weeks | Paired Sample t-Test | ||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean Difference | 95%CI | Two-Sided (p) | |
| CSI-A | 30.33 (12.40) | 27.33 (12.28) | −3.00 | 1.09; 4.41 | <0.001 |
| Sensory Profiles | |||||
| Low Registration | 27.63 (7.18) | 27.09 (7.13) | −0.54 | −0.65; 0.95 | 0.71 |
| Sensation Seeking | 45.18 (7.40) | 45.19 (8.40) | 0.01 | −0.80; 0.97 | 0.85 |
| Sensory Sensitive | 31.48 (8.07) | 31.29 (8.80) | −0.19 | −0.96; 0.85 | 0.90 |
| Sensation Avoiding | 31.81 (8.97) | 31.08 (8.36) | −0.73 | −0.40; 1.40 | 0.27 |
| NPRS | 6.06 (1.95) | 1.96 (1.95) | −4.10 | 3.91; 4.91 | <0.001 |
| STAI state | 35.67 (11.30) | 34.82 (11.50) | −0.85 | −0.72; 2.90 | 0.24 |
| STAI trait | 37.96 (10.37) | 36.86 (10.84) | −1.07 | −0.65; 2.46 | 0.25 |
| BDI | 8.94 (8.47) | 6.78 (6.94) | −2.16 | 1.35; 4.15 | <0.001 |
| PCS cat. | 10.25 (9.29) | 7.52 (9.29) | −2.73 | 1.17; 4.45 | <0.001 |
| PCS hel. | 4.05 (4.29) | 2.84 (3.99) | −1.21 | 0.43; 1.91 | 0.002 |
| PCS rum. | 4.33 (3.84) | 3.19 (3.79) | −1.14 | 0.61; 1.98 | <0.001 |
| PCS mag. | 1.80 (2.11) | 1.30 (2.02) | −0.50 | 3.91; 4.91 | <0.001 |
| R2 | F-Ratio | B | p | S.E. | |
|---|---|---|---|---|---|
| LR | 0.11 | 13.22 (1; 104) | 0.60 | <0.001 | 0.17 |
| SSk | 0.00 | 0.01 (1; 104) | −0.01 | 0.93 | 0.16 |
| SSv | 0.22 | 28.60 (1; 104) | 0.71 | <0.001 | 0.13 |
| SA | 0.24 | 31.90 (1; 104) | 0.71 | <0.001 | 0.13 |
| Age | 0.00 | 0.09 (1; 104) | 0.03 | 0.76 | 0.11 |
| Duration | 0.00 | 0.46 (1; 103) | −0.57 | 0.50 | 0.83 |
| NPRS | 0.00 | 0.30 (1; 103) | 0.34 | 0.59 | 0.62 |
| STAI-state | 0.26 | 35.34 (1; 103) | 0.55 | <0.001 | 0.09 |
| STAI-trait | 0.32 | 48.08 (1; 104) | 0.67 | <0.001 | 0.10 |
| BDI | 0.09 | 10.17 (1; 99) | 0.44 | 0.00 | 0.14 |
| PCS-cat | 0.01 | 0.80 (1; 101) | 0.12 | 0.37 | 0.13 |
| PCS-help | 0.00 | 0.04 (1; 102) | 0.06 | 0.84 | 0.29 |
| PCS-rum | 0.00 | 0.35 (1; 102) | 0.18 | 0.56 | 0.31 |
| PCS-mag | 0.06 | 6.66 (1; 101) | 1.45 | <0.001 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gräper, P.J.; Scafoglieri, A.; Clark, J.R.; Hallegraeff, J.M. Sensory Profiles Predict Symptoms of Central Sensitization in Low Back Pain: A Predictive Model Research Study. J. Clin. Med. 2024, 13, 4677. https://doi.org/10.3390/jcm13164677
Gräper PJ, Scafoglieri A, Clark JR, Hallegraeff JM. Sensory Profiles Predict Symptoms of Central Sensitization in Low Back Pain: A Predictive Model Research Study. Journal of Clinical Medicine. 2024; 13(16):4677. https://doi.org/10.3390/jcm13164677
Chicago/Turabian StyleGräper, Pieter J., Aldo Scafoglieri, Jacqueline R. Clark, and Joannes M. Hallegraeff. 2024. "Sensory Profiles Predict Symptoms of Central Sensitization in Low Back Pain: A Predictive Model Research Study" Journal of Clinical Medicine 13, no. 16: 4677. https://doi.org/10.3390/jcm13164677






