Retinitis Pigmentosa and Therapeutic Approaches: A Systematic Review
Abstract
:1. Introduction
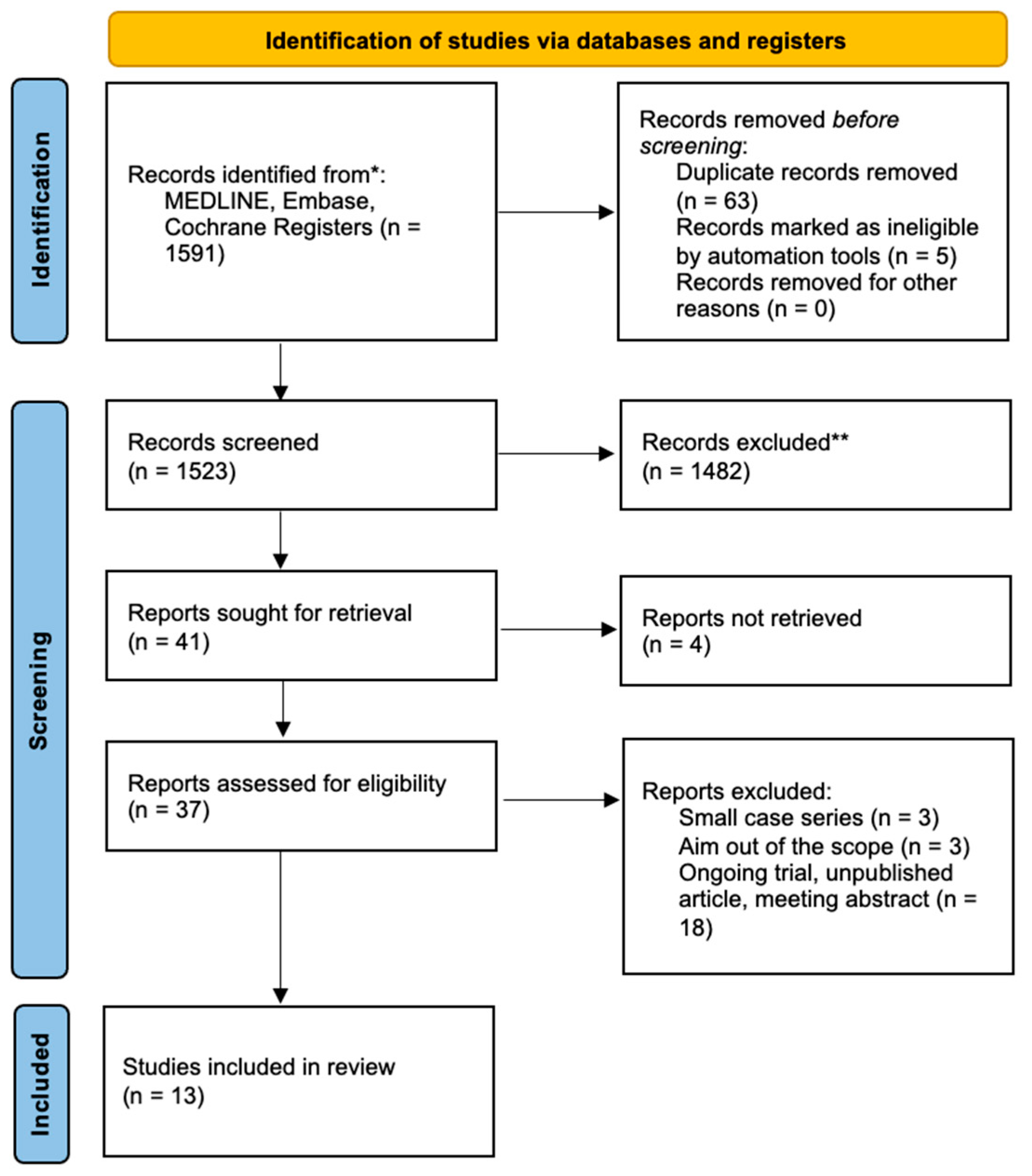
2. Materials and Methods
3. Results
3.1. Study Characteristics
3.2. Mesenchymal Stem Cells
3.3. Gene Therapy
3.4. Docoexhanoic Acid
3.5. Oral Valproic Acid
3.6. Oral QLT091001
3.7. Retina Implant Alpha IMS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Database | Number of Retrieved Records |
| MEDLINE (Ovid) | 855 |
| Embase (Ovid) | 1339 |
| Cochrane Systematic Reviews, Cochrane Register of Controlled Trials | 85 |
| Number of records before deduplication | 2279 |
| Number of records after deduplication | 1612 |
- retinitis pigmentosa/or alstrom syndrome/or bardet-biedl syndrome/or (retinitis pigmentosa or alstrom* or (bardet adj1 biedl) or biedl syndrome* or ((retina* pigment* or tapetoretina* or tapeto retina*) adj3 (dystroph* or degenerat*))).tw,kf.
- genetic therapy/or rnai therapeutics/or targeted gene repair/or ((gene* adj3 (therap* or treatment*)) or (gene* adj3 repair*) or ((antisense or anti-sense) adj3 (therap* or treatment*)) or ((germ line or germline) adj3 (therap* or treatment*)) or ((oligonucleotide* or oligo-nucleotide*) adj3 (therap* or treatment*)) or (ribozyme* adj3 (therap* or treatment*))).tw,kf.
- ((gene* or DNA or RNA) adj2 (immuni?ation* or vaccination*)).tw,kf.
- ((gene* replacement* or mitochondrial replacement* or RNA interference* or RNAi or viral based or virus based) and (therap* or treatment*)).tw,kf.
- Stem Cell Transplantation/or Induced Pluripotent Stem Cells/or stem cell*.tw,kf.
- stem cells/or pluripotent stem cells/or embryonic stem cells/or human embryonic stem cells/or induced pluripotent stem cells/or (IPSC or IPS cell*).tw,kf.
- Optogenetics/or (optogenetic* or opto-genetic*).tw,kf.
- or/2–7
- 1 and 8
- exp models, animal/or ((Animal Experimentation/or exp Animals/) not Humans/) or (veterinar* or animal or animals or swine or rabbit or rabbits or rodent or rodents or rat or rats or mouse or mice or hamster or hamsters or pig or pigs or piglet or piglets or porcine or pigeon* or horse* or equine or cow or cows or bovine or goat or goats or sheep or lamb or lambs or monkey or monkeys or murine or ovine or dog or dogs or canine or cat or cats or feline or dolphin* or fish or zebrafish).ti.
- 9 not 10
- limit 11 to english language
- retina pigment degeneration/or retinitis pigmentosa/or Alstrom syndrome/or Bardet Biedl syndrome/or (retinitis pigmentosa or alstrom* or (bardet adj1 biedl) or biedl syndrome* or ((retina* pigment* or tapetoretina* or tapeto retina*) adj3 (dystroph* or degenerat*))).tw,kf.
- gene therapy/or antisense therapy/or cell based gene therapy/or exp gene replacement therapy/or exp genetic immunization/or germ line gene therapy/or nonviral gene therapy/or oligonucleotide therapy/or ribozyme therapy/or rnai therapeutics/or somatic gene therapy/or stem cell gene therapy/or viral gene therapy/
- ((gene* adj3 (therap* or treatment*)) or (gene* adj3 repair*) or ((antisense or anti-sense) adj3 (therap* or treatment*)) or ((germ line or germline) adj3 (therap* or treatment*)) or ((oligonucleotide* or oligo-nucleotide*) adj3 (therap* or treatment*)) or (ribozyme* adj3 (therap* or treatment*))).tw,kf.
- ((gene* or DNA or RNA) adj2 (immuni?ation* or vaccination*)).tw,kf.
- ((gene* replacement* or mitochondrial replacement* or RNA interference* or RNAi or viral based or virus based) and (therap* or treatment*)).tw,kf.
- stem cell transplantation/or induced pluripotent stem cell/or pluripotent stem cell/or embryonic stem cell/or human embryonic stem cell/or (stem cell* or IPSC or IPS cell*).tw,kf.
- optogenetics/or (optogenetic* or opto-genetic*).tw,kf.
- or/2–7
- 1 and 8
- exp animal model/or ((exp animal/or nonhuman/) not exp human/) or (veterinar* or animal or animals or swine or rabbit or rabbits or rodent or rodents or rat or rats or mouse or mice or hamster or hamsters or pig or pigs or piglet or piglets or porcine or pigeon* or horse* or equine or cow or cows or bovine or goat or goats or sheep or lamb or lambs or monkey or monkeys or murine or ovine or dog or dogs or canine or cat or cats or feline or dolphin* or zebrafish* or fish).ti.
- 9 not 10
- limit 11 to (conference abstracts or “preprints (unpublished, non-peer reviewed)”)
- 11 not 12
- limit 13 to english language
- #1.
- MeSH descriptor: [Retinitis Pigmentosa] explode all trees
- #2.
- (“retinitis pigmentosa” OR alstrom* OR (bardet NEAR/1 biedl) OR (biedl NEXT syndrome*) OR ((retina* pigment* OR tapetoretina* OR “tapeto retina” OR “tapeto retinal”) NEAR/3 (dystroph* OR degenerat*))):ti,ab,kw
- #3.
- #1 OR #2
- #4.
- MeSH descriptor: [Genetic Therapy] explode all trees
- #5.
- ((gene* NEAR/3 (therap* OR treatment*)) OR (gene* NEAR/3 repair*) OR ((antisense OR “anti-sense”) NEAR/3 (therap* OR treatment*)) OR ((“germ line” OR germline) NEAR/3 (therap* OR treatment*)) OR ((oligonucleotide* OR (oligo NEXT nucleotide*)) NEAR/3 (therap* OR treatment*)) OR (ribozyme* NEAR/3 (therap* OR treatment*))):ti,ab,kw
- #6.
- (((gene* OR DNA OR RNA) NEXT/2 (immuni?ation* OR vaccination*))):ti,ab,kw
- #7.
- ((gene* NEXT replacement*) OR (mitochondrial NEXT replacement*) OR (RNA NEXT interference*) OR RNAi OR “viral based” OR “virus based”):ti,ab,kw
- #8.
- (therap* OR treatment*)
- #9.
- #7 AND #8
- #10.
- MeSH descriptor: [Stem Cell Transplantation] this term only
- #11.
- MeSH descriptor: [Induced Pluripotent Stem Cells] this term only
- #12.
- MeSH descriptor: [Stem Cells] this term only
- #13.
- MeSH descriptor: [Pluripotent Stem Cells] explode all trees
- #14.
- ((IPSC OR IPS NEXT cell* OR stem NEXT cell*)):ti,ab,kw
- #15.
- MeSH descriptor: [Optogenetics] this term only 2
- #16.
- (optogenetic* OR opto NEXT genetic*):ti,ab,kw
- #17.
- #4 OR #5 OR #6 OR #7 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16
- #18.
- #3 AND #17
Appendix B
| Title | Include (N° of Eyes) | Exclude | Explanation for Exclusion | |
| 1 | Clinical trial of intravitreal injection of autologous bone marrow stem cells in patients with retinitis pigmentosa. ClinicalTrials.gov, 0(0). Retrieved from https://clinicaltrials.gov/show/NCT02280135 (2014), Access on 1 January 2020. | Excluded | Unpublished article | |
| 2 | Zhao, T., Lie, H., Wang, F., Liu, Y., Meng, X., Yin, Z., & Li, S. (2021). Comparative study of a modified sub-Tenon’s capsule injection of triamcinolone acetonide and the intravenous infusion of umbilical cord mesenchymal stem cells in retinitis pigmentosa combined with macular edema. Frontiers in Pharmacology, 12. [9] | [1] 40 eyes (20 patients) | ||
| 3 | Hoffman, D. R., Hughbanks-Wheaton, D. K., Spencer, R., Fish, G. E., Pearson, N. S., Wang, Y. Z., Klein, M., Takacs, A., Locke, K. G., & Birch, D. G. (2015). Docosahexaenoic acid slows visual field progression in X-linked retinitis pigmentosa: Ancillary outcomes of the DHAX trial. Investigative Ophthalmology & Visual Science, 56(11), 6646–6653. [16] | [2] 102 eyes (51 patients) | ||
| 4 | Birch, D. G., Bernstein, P. S., Iannacone, A., Pennesi, M. E., Lam, B. L., Heckenlively, J., Csaky, K., Hartnett, M. E., Winthrop, K. L., Jayasundera, T., et al. (2018). Effect of oral valproic acid vs placebo for vision loss in patients with autosomal dominant retinitis pigmentosa: A randomized phase 2 multicenter placebo-controlled clinical trial. JAMA Ophthalmology, 136(8), 849–856. [18] | [3] 180 eyes (90 patiens) | ||
| 5 | Russell, S., Bennett, J., Wellman, J. A., Chung, D. C., Yu, Z. F., Tillman, A., Wittes, J., Pappas, J., Elci, O., McCague, S., et al. (2017). Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. The Lancet, 390(10097), 849–860. | Excluded | Preliminary results of an included article/aim out of scope | |
| 6 | Maguire, A. M., Russell, S., Wellman, J. A., Chung, D. C., Yu, Z. F., Tillman, A., Wittes, J., Pappas, J., Elci, O., Marshall, K. A., et al. (2019). Efficacy, safety, and durability of voretigene neparvovec-rzyl in RPE65 mutation-associated inherited retinal dystrophy: Results of phase 1 and 3 trials. Ophthalmology, 126(9), 1273–1285. | Excluded | Preliminary results of an included article/aim out of scope | |
| 7 | Euctr, F. R. (2014). Study of SAR421869 in Patients With Retinitis Pigmentosa associated with Usher Syndrome Type 1B. [Journal Article]. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2012-002574-31-FR Access on 20 February 2024 | Excluded | Unpublished article | |
| 8 | Maguire, A. M., Russell, S., Chung, D. C., Yu, Z. F., Tillman, A., Drack, A. V., Simonelli, F., Leroy, B. P., Reape, K. Z., High, K. A., et al. (2021). Durability of voretigene neparvovec for biallelic RPE65-mediated inherited retinal disease: Phase 3 results at 3 and 4 years. Ophthalmology, 128(10), 1460–1468. [15] | [4] 58 eyes (29 patients) | ||
| 9 | Cehajic-Kapetanovic, J., Xue, K., Martinez-Fernandez de la Camara, C., Nanda, A., Davies, A., Wood, L. J., Salvetti, A. P., Fischer, M. D., Aylward, J. W., Barnard, A. R., Jolly, J. K., Luo, E., Lujan, B. J., Ong, T., Girach, A., Black, G. C. M., Gregori, N. Z., Davis, J. L., Rosa, P. R., Lotery, A. J., Lam, B. L., Stanga, P. E., & MacLaren, R. E. (2020). Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nature Medicine, 26(3), 354–359. [14] | [5] (18 patients) | ||
| 10 | Zhao, T., Liang, Q., Meng, X., Duan, P., Wang, F., Li, S., Liu, Y., & Yin, Z. Q. (2020). Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells Maintains and Partially Improves Visual Function in Patients with Advanced Retinitis Pigmentosa. Stem Cells & Development, 29(16), 1029–1037. [8] | [6] 64 eyes (32 patients) | ||
| 11 | Tuekprakhon, A., Sangkitporn, S., Trinavarat, A., Pawestri, A. R., Vamvanij, V., Ruangchainikom, M., Luksanapruksa, P., Pongpaksupasin, P., Khorchai, A., Dambua, A., Boonchu, P., Yodtup, C., Uiprasertkul, M., Sangkitporn, S., & Atchaneeyasakul, L. O. (2021). Intravitreal autologous mesenchymal stem cell transplantation: a non-randomized phase I clinical trial in patients with retinitis pigmentosa. Stem Cell Research & Therapy, 12(1), 52. [12] | [7] 14 eyes (14 patients) | ||
| 12 | Liao, D., Boyer, D. S., Kaiser, P., Kuppermann, B. D., Heier, J., Mehta, M., Joseph, A., Kammer, R., Mills, B., Yang, J., et al. (2021). Intravitreal injection of allogeneic human retinal progenitor cells (hRPC) for treatment of retinitis pigmentosa: a prospective randomized controlled phase 2b trial. Investigative Ophthalmology & Visual Science, 62(8). | Excluded | Meeting abstract/unpublished article | |
| 13 | Ozmert, E., & Arslan, U. (2023). Management of retinitis pigmentosa via Wharton’s jelly-derived mesenchymal stem cells or combination with Magnovision: 3-year prospective results. Stem Cells Translational Medicine, 12(10), 631–650. [11] | [8] 130 eyes (80 patients) | ||
| 14 | Limoli, P. G., Limoli, C. S. S., Morales, M. U., & Vingolo, E. M. (2020). Mesenchymal stem cell surgery, rescue and regeneration in retinitis pigmentosa: Clinical and rehabilitative prognostic aspects. Restorative Neurology & Neuroscience, 38(3), 223–237. [13] | [9] 34 eyes (25 patients) | ||
| 15 | Hu, Y., Du, Y., Jin, Y., Feng, K., Chen, H., Han, L., Qu, H., & Ma, Z. (2023). A Novel Surgical Approach for Big Sheet Allogenic Retinal Pigment Epithelium-Bruch Membrane Complex Transplantation Into the Subretinal Space. Retina, 43(10), 1816–1819. | Excluded | Small case series | |
| 16 | Hoffman, D. R., Locke, K. G., Wheaton, D. H., Fish, G. E., Spencer, R., & Birch, D. G. (2004). A randomized, placebo-controlled clinical trial of docosahexaenoic acid supplementation for X-linked retinitis pigmentosa. American Journal of Ophthalmology, 137(4), 704–718. [17] | [10] 88 eyes (44 patients) | ||
| 17 | Scholl, H. P., Moore, A. T., Koenekoop, R. K., Wen, Y., Fishman, G. A., van den Born, L. I., … & Bowles, K. (2015). Safety and proof-of-concept study of oral QLT091001 in retinitis pigmentosa due to inherited deficiencies of retinal pigment epithelial 65 protein (RPE65) or lecithin: retinol acyltransferase (LRAT). PloS one, 10(12), e0143846. [19] | [11] 36 eyes (18 patients) | ||
| 18 | Sobaci, G., Sevinc, K., Ovali, E., Ozmert, E., Ozdek, S., Yilmaz, G., … & Akkoyun, I. (2015). Submacular allogeneic ectomesenchymal stem cell transplantation in retinitis pigmentosa: one-year results. Investigative ophthalmology & visual science, 56(7), 2276. | Excluded | Small case series | |
| 19 | Stingl, K., Bartz-Schmidt, K. U., Besch, D., Chee, C. K., Gekeler, F., Groppe, M., Jackson, T. L., MacLaren, R. E., Koitschev, A., & Kusnyerik, A. (2015). Subretinal Visual Implant Alpha IMS–Clinical trial interim report. Vision research, 111, 149–160. [20] | [12] 29 eyes (29 patients) | ||
| 20 | Kahraman, N. S., & Oner, A. (2020). Umbilical cord derived mesenchymal stem cell implantation in retinitis pigmentosa: a 6-month follow-up results of a phase 3 trial. International Journal of Ophthalmology, 13(9), 1423–1429. [10] | [13] 124 eyes (82 patients) | ||
| 21 | Russell, S., Bennett, J., Maguire, A. M., & High, K. A. (2018). Voretigene neparvovec-rzyl for the treatment of biallelic RPE65 mutation-associated retinal dystrophy. Expert Opinion on Orphan Drugs, 6(8), 457–464. | Excluded | Expert opinion | |
| 22 | Euctr, G. B. (2016). A Clinical Trial of Retinal Gene Therapy for X-linked Retinitis Pigmentosa using AAV8. [Protocol]. Retrieved from https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2016-003852-60-GB Accesed on 20 February 2024 | Excluded | Unpublished article | |
| 23 | A clinical trial to evaluate the effect of bone marrow-derived stem cells in diseases like dry age-related macular degeneration and retinitis pigmentosa. [Protocol]. Retrieved from https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2010/091/000639 (2010) Accesed on 20 February 2024 | Excluded | Unpublished article | |
| 24 | Mohanty, S., Batabyal, S., Kim, S., Carlson, M., Ayyagari, A., Rittimann, J., Tchedre, K., & Chavala, S. H. (2022). Double-masked, Randomized, sham-controlled, Multicenter Phase 2b study of Multi-Characteristic Opsin enabled vision restoration in patients with advanced retinitis pigmentosa: design and Development of novel endpoints. Investigative ophthalmology & visual science, 63(7), 1722-F0040. | Excluded | Meeting abstract/unpublished article | |
| 25 | Boyer, D. S., Bergstrom, L., Emanuelli, A., Gonzalez, V. H., Wykoff, C. C., Gupta, S., Liao, D. S., Zak, V., Chavala, S. H., Mohanty, S., et al. (2023). Efficacy and safety of MCO-010 optogenetic therapy for vision restoration in patients with severe vision loss due to retinitis pigmentosa: a phase 2b randomized, sham-controlled, multi-center, multi-dose, double-masked clinical trial (RESTORE). Investigative ophthalmology & visual science, 64(8), 5443. | Excluded | Meeting abstract/unpublished article | |
| 26 | Nct. (2021). Efficacy and Safety of MCO-010 Optogenetic Therapy in Adults With Retinitis Pigmentosa. Journal of Clinical Trials, 0(0). Retrieved from https://clinicaltrials.gov/show/NCT04945772 Accesed on 20 February 2024 | Excluded | Unpublished article | |
| 27 | Euctr, D. K. (2021). Gene Therapy Trial for Patients with Retinitis Pigmentosa Due to a Gene Defect on Chromosome X. Journal of Clinical Trials, 0(0). Retrieved from https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-002873-88-DK Accesed on 20 February 2024 | Excluded | Unpublished article | |
| 28 | Yusupov, A. Y. (1956). Implantation of catgut in the treatment of some eye diseases. Sborn, 0(0), 303-307. Retrieved from https://ezproxy.uio.no/login?url=http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=emcl1&AN=281398832 Accesed on 20 February 2024 | Excluded | ||
| 29 | Satarian, L., Nourinia, R., Safi, S., Kanavi, M. R., Jarughi, N., Daftarian, N., Arab, L., Aghdami, N., Ahmadieh, H., & Baharvand, H. (2017). Intravitreal Injection of Bone Marrow Mesenchymal Stem Cells in Patients with Advanced Retinitis Pigmentosa; a Safety Study. Journal of Ophthalmic & Vision Research, 12(1), 58–64. | Excluded | Aim out of scope | |
| 30 | Liao, D., Gonzalez, V., Emanuelli, A., Gupta, S., Wykoff, C., Zak, V., Ayyagari, A., Bataybal, S., Chavala, S., Koester, J., et al. (2023). Optogenetic Therapy with MCO-010 for Vision Restoration in Patients with Severe Sight Loss Due to Retinitis Pigmentosa: the Phase 2b RESTORE Study. Molecular Therapy, 31(4), 399. | Excluded | Abstract/unpublished article | |
| 31 | Euctr, N. O. (2021). A Phase 2/3 study to evaluate efficacy, safety, and tolerability of QR-421a in subjects with advanced vision loss. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2021-002729-74-NO Accesed on 20 February 2024 | Excluded | Unpublished article | |
| 32 | Euctr, N. O. (2021). A Phase 2/3 study to evaluate efficacy, safety, and tolerability of QR-421a in subjects with advanced vision loss. [Journal Article]. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2021-002729-74-NO Accesed on 20 February 2024 | Excluded | Unpublished article | |
| 33 | Nct. (2023). Role of UC-MSC and CM to Inhibit Vision Loss in Retinitis Pigmentosa Phase I/II. [Journal Article]. https://clinicaltrials.gov/show/NCT05909488 Accesed on 20 February 2024 | Excluded | Unpublished article | |
| 34 | Euctr, D. K. (2016). A study in subjects with rare inherited eye conditions caused by gene mutations to see if treatment with QLT091001 is safe and works to improve subjects’ vision. | Excluded | Unpublished article | |
| 35 | Euctr, F. R. (2018). Study to evaluate QR-421a in subjects with retinitis pigmentosa (RP) due to mutations in exon 13 of the USH2A Gene | Excluded | Unpublished article | |
| 36 | Euctr, G. B. (2008). An open-label dose escalation study of an adeno-associated virus vector (AAV2/2-hRPE65p-hRPE65) for gene therapy of severe early-onset retinal degeneration—RPE65 gene therapy. | Excluded | Unpublished article | |
| 37 | Oner, A., Gonen, Z. B., Sinim, N., Cetin, M., & Ozkul, Y. (2016). Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: a phase I clinical safety study. Stem Cell Research and Therapy, 7(1), 1–12. | Excluded | Small case series |
References
- Hamel, C. Retinitis pigmentosa. Orphanet J. Rare Dis. 2006, 1, 40. [Google Scholar] [CrossRef]
- Cross, N.; Van Steen, C.; Zegaoui, Y.; Satherley, A.; Angelillo, L. Retinitis Pigmentosa: Burden of Disease and Current Unmet Needs. Clin. Ophthalmol. 2022, 16, 1993–2010. [Google Scholar] [CrossRef]
- Menghini, M.; Cehajic-Kapetanovic, J.; MacLaren, R.E. Monitoring progression of retinitis pigmentosa: Current recommendations and recent advances. Expert Opin. Orphan Drugs 2020, 8, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.K.; Tsai, Y.T.; Tsang, S.H. Emerging Treatments for Retinitis Pigmentosa: Genes and stem cells, as well as new electronic and medical therapies, are gaining ground. Retin. Physician 2015, 12, 52. [Google Scholar] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Howick, J.; Chalmers, I.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H. The 2011 Oxford CEBM Evidence Levels of Evidence (Introductory Document). Oxford Centre for Evidence-Based Medicine. Available online: http://www.cebm.net/index.aspx?o=5653 (accessed on 5 May 2024).
- GRADE Home. GRADE Home. Available online: http://www.gradeworkinggroup.org (accessed on 5 May 2024).
- Zhao, T.; Liang, Q.; Meng, X.; Duan, P.; Wang, F.; Li, S.; Liu, Y.; Yin, Z.Q. Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells Maintains and Partially Improves Visual Function in Patients with Advanced Retinitis Pigmentosa. Stem Cells Dev. 2020, 29, 1029–1037. [Google Scholar] [CrossRef]
- Zhao, T.; Lie, H.; Wang, F.; Liu, Y.; Meng, X.; Yin, Z.; Li, S. Comparative Study of a Modified Sub-Tenon’s Capsule Injection of Triamcinolone Acetonide and the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Retinitis Pigmentosa Combined with Macular Edema. Front. Pharmacol. 2021, 12, 694225. [Google Scholar] [CrossRef]
- Kahraman, N.S.; Oner, A. Umbilical cord derived mesenchymal stem cell implantation in retinitis pigmentosa: A 6-month follow-up results of a phase 3 trial. Int. J. Ophthalmol. 2020, 13, 1423–1429. [Google Scholar] [CrossRef]
- Ozmert, E.; Arslan, U. Management of Retinitis Pigmentosa Via Wharton’s Jelly-Derived Mesenchymal Stem Cells or Combination with Magnovision: 3-Year Prospective Results. Stem Cells Transl. Med. 2023, 12, 631–650. [Google Scholar] [CrossRef] [PubMed]
- Tuekprakhon, A.; Sangkitporn, S.; Trinavarat, A.; Pawestri, A.R.; Vamvanij, V.; Ruangchainikom, M.; Luksanapruksa, P.; Pongpaksupasin, P.; Khorchai, A.; Dambua, A.; et al. Intravitreal autologous mesenchymal stem cell transplantation: A non-randomized phase I clinical trial in patients with retinitis pigmentosa. Stem Cell Res. Ther. 2021, 12, 52. [Google Scholar] [CrossRef]
- Limoli, P.G.; Limoli, C.S.S.; Morales, M.U.; Vingolo, E.M. Mesenchymal stem cell surgery, rescue and regeneration in retinitis pigmentosa: Clinical and rehabilitative prognostic aspects. Restor. Neurol. Neurosci. 2020, 38, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Cehajic-Kapetanovic, J.; Xue, K.; De la Camara, C.M.-F.; Nanda, A.; Davies, A.; Wood, L.J.; Salvetti, A.P.; Fischer, M.D.; Aylward, J.W.; Barnard, A.R.; et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat. Med. 2020, 26, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A.M.; Russell, S.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Drack, A.V.; Simonelli, F.; Leroy, B.P.; Reape, K.Z.; High, K.A.; et al. Durability of Voretigene Neparvovec for Biallelic RPE65-Mediated Inherited Retinal Disease. Ophthalmology 2021, 128, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.R.; Hughbanks-Wheaton, D.K.; Spencer, R.; Fish, G.E.; Pearson, N.S.; Wang, Y.-Z.; Klein, M.; Takacs, A.; Locke, K.G.; Birch, D.G. Docosahexaenoic Acid Slows Visual Field Progression in X-Linked Retinitis Pigmentosa: Ancillary Outcomes of the DHAX Trial. Investig. Opthalmol. Vis. Sci. 2015, 56, 6646–6653. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.R.; Locke, K.G.; Wheaton, D.H.; Fish, G.E.; Spencer, R.; Birch, D.G. A randomized, placebo-controlled clinical trial of docosahexaenoic acid supplementation for X-linked retinitis pigmentosa. Arch. Ophthalmol. 2004, 137, 704–718. [Google Scholar] [CrossRef]
- Birch, D.G.; Bernstein, P.S.; Iannacone, A.; Pennesi, M.E.; Lam, B.L.; Heckenlively, J.; Csaky, K.; Hartnett, M.E.; Winthrop, K.L.; Jayasundera, T.; et al. Effect of Oral Valproic Acid vs Placebo for Vision Loss in Patients with Autosomal Dominant Retinitis Pigmentosa. JAMA Ophthalmol. 2018, 136, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Scholl, H.P.N.; Moore, A.T.; Koenekoop, R.K.; Wen, Y.; Fishman, G.A.; Born, L.I.v.D.; Bittner, A.; Bowles, K.; Fletcher, E.C.; Collison, F.T.; et al. Safety and Proof-of-Concept Study of Oral QLT091001 in Retinitis Pigmentosa Due to Inherited Deficiencies of Retinal Pigment Epithelial 65 Protein (RPE65) or Lecithin: Retinol Acyltransferase (LRAT). PLoS ONE 2015, 10, e0143846. [Google Scholar] [CrossRef] [PubMed]
- Stingl, K.; Bartz-Schmidt, K.U.; Besch, D.; Chee, C.K.; Cottriall, C.L.; Gekeler, F.; Groppe, M.; Jackson, T.L.; MacLaren, R.E.; Koitschev, A.; et al. Subretinal Visual Implant Alpha IMS—Clinical trial interim report. Vis. Res. 2015, 111, 149–160. [Google Scholar] [CrossRef]
- Qian, T.W.; Xu, X.; Qian, T. Research progress of treatment strategies for retinitis pigmentosa. Zhonghua Yan Ke Za Zhi 2017, 53, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F. Current Pharmacological Concepts in the Treatment of the Retinitis Pigmentosa. Adv. Exp. Med. Biol. 2018, 1074, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Kamde, S.P.; Anjankar, A. Retinitis Pigmentosa: Pathogenesis, Diagnostic Findings, and Treatment. Cureus 2023, 15, e48006. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, S.; Li, P.; Yao, K. Retinitis Pigmentosa: Progress in Molecular Pathology and Biotherapeutical Strategies. Int. J. Mol. Sci. 2022, 23, 4883. [Google Scholar] [CrossRef] [PubMed]
- Diakatou, M.; Dubois, G.; Erkilic, N.; Sanjurjo-Soriano, C.; Meunier, I.; Kalatzis, V. Allele-Specific Knockout by CRISPR/Cas to Treat Autosomal Dominant Retinitis Pigmentosa Caused by the G56R Mutation in NR2E3. Int. J. Mol. Sci. 2021, 22, 2607. [Google Scholar] [CrossRef] [PubMed]
- Benati, D.; Patrizi, C.; Recchia, A. Gene editing prospects for treating inherited retinal diseases. J. Med. Genet. 2020, 57, 437–444. [Google Scholar] [CrossRef]
- Botto, C.; Rucli, M.; Tekinsoy, M.D.; Pulman, J.; Sahel, J.-A.; Dalkara, D. Early and late stage gene therapy interventions for inherited retinal degenerations. Prog. Retin. Eye Res. 2022, 86, 100975. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Edwards, T.L.; O’hare, F.; Hickey, D.G.; Wang, J.-H.; Liu, Z.; Ayton, L.N. Gene therapy for inherited retinal diseases: Progress and possibilities. Clin. Exp. Optom. 2021, 104, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Tzameret, A.; Sher, I.; Belkin, M.; Treves, A.J.; Meir, A.; Nagler, A.; Levkovitch-Verbin, H.; Rotenstreich, Y.; Solomon, A.S. Epiretinal transplantation of human bone marrow mesenchymal stem cells rescues retinal and vision function in a rat model of retinal degeneration. Stem Cell Res. 2015, 15, 387–394. [Google Scholar] [CrossRef]
- Ahmadieh, H.; Baharvand, H.; Satarian, L.; Nourinia, R.; Safi, S.; Kanavi, M.R.; Jarughi, N.; Daftarian, N.; Arab, L.; Aghdami, N. Intravitreal injection of bone marrow mesenchymal stem cells in patients with advanced retinitis pigmentosa; a safety study. J. Ophthalmic Vis. Res. 2017, 12, 58–64. [Google Scholar] [CrossRef]
- Mead, B.; Tomarev, S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl. Med. 2017, 6, 1273–1285. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, Y.; Wang, Y.; Zhang, D.; Shen, B.; Luo, M.; Gu, P. Progress of stem/progenitor cell-based therapy for retinal degeneration. J. Transl. Med. 2017, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jaganathan, B.G. Stem Cell Therapy for Retinal Degeneration: The Evidence to Date. Biol. Targets Ther. 2021, 15, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, P.E.; Freeman, S.C.; Janot, A.C. Novel stem cell and gene therapy in diabetic retinopathy, age related macular degeneration, and retinitis pigmentosa. Int. J. Retin. Vitr. 2019, 5, 7. [Google Scholar] [CrossRef]
- Holan, V.; Palacka, K.; Hermankova, B. Mesenchymal Stem Cell-Based Therapy for Retinal Degenerative Diseases: Experimental Models and Clinical Trials. Cells 2021, 10, 588. [Google Scholar] [CrossRef]
- Jin, Z.-B.; Gao, M.-L.; Deng, W.-L.; Wu, K.-C.; Sugita, S.; Mandai, M.; Takahashi, M. Stemming retinal regeneration with pluripotent stem cells. Prog. Retin. Eye Res. 2019, 69, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Shabanan, S.H.; Seyedmirzaei, H.; Barnea, A.; Hanaei, S.; Rezaei, N. Stem cell transplantation as a progressing treatment for retinitis pigmentosa. Cell Tissue Res. 2022, 387, 177–205. [Google Scholar] [CrossRef]
- Hu, D.; Hou, X.; Pan, F.; Sun, L.-J.; Bai, Q.; Wang, Y.-S. Valproate reduces retinal ganglion cell apoptosis in rats after optic nerve crush. Neural Regen. Res. 2023, 18, 1607–1612. [Google Scholar] [CrossRef]
- Gao, S.; Qin, T.; Liu, Z.; Caceres, M.A.; Ronchi, C.F.; Chen, C.-Y.O.; Yeum, K.-J.; Taylor, A.; Blumberg, J.B.; Liu, Y.; et al. Lutein and zeaxanthin supplementation reduces H2O2-induced oxidative damage in human lens epithelial cells. Mol. Vis. 2011, 17, 3180. [Google Scholar]
- Zeviani, M.; Carelli, V. Mitochondrial Retinopathies. Int. J. Mol. Sci. 2022, 23, 210. [Google Scholar] [CrossRef] [PubMed]
- Gallenga, C.E.; Lonardi, M.; Pacetti, S.; Violanti, S.S.; Tassinari, P.; Di Virgilio, F.; Tognon, M.; Perri, P. Molecular Mechanisms Related to Oxidative Stress in Retinitis Pigmentosa. Antioxidants 2021, 10, 848. [Google Scholar] [CrossRef]
- Song, D.-J.; Bao, X.-L.; Fan, B.; Li, G.-Y. Mechanism of Cone Degeneration in Retinitis Pigmentosa. Cell. Mol. Neurobiol. 2022, 43, 1037–1048. [Google Scholar] [CrossRef]
- Murakami, Y.; Nakabeppu, Y.; Sonoda, K.-H. Oxidative Stress and Microglial Response in Retinitis Pigmentosa. Int. J. Mol. Sci. 2020, 21, 7170. [Google Scholar] [CrossRef]
- McClements, M.E.; Staurenghi, F.; MacLaren, R.E.; Cehajic-Kapetanovic, J. Optogenetic Gene Therapy for the Degenerate Retina: Recent Advances. Front. Neurosci. 2020, 14, 570909. [Google Scholar] [CrossRef]
- Prosseda, P.P.; Tran, M.; Kowal, T.; Wang, B.; Sun, Y. Advances in Ophthalmic Optogenetics: Approaches and Applications. Biomolecules 2022, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Sugano, E. Optogenetics-Mediated Gene Therapy for Retinal Diseases. Adv. Exp. Med. Biol. 2021, 1293, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.R.; Gilbert, F. Restoring vision using optogenetics without being blind to the risks. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 260, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Bellapianta, A.; Cetkovic, A.; Bolz, M.; Salti, A. Retinal Organoids and Retinal Prostheses: An Overview. Int. J. Mol. Sci. 2022, 23, 2922. [Google Scholar] [CrossRef]
- Özmert, E.; Arslan, U. Retinal Prostheses and Artificial Vision. Turk. J. Ophthalmol. 2019, 49, 213–219. [Google Scholar] [CrossRef]
- Palanker, D. Electronic Retinal Prostheses. Cold Spring Harb. Perspect. Med. 2023, 13, a041525. [Google Scholar] [CrossRef]
- Berger, A.; Devenyi, R.; Héon, E. Retinal Prosthesis System for Advanced Retinitis Pigmentosa: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2016, 16, 1. [Google Scholar]
- Rachitskaya, A.V.; Yuan, A. Argus II retinal prosthesis system: An update. Ophthalmic Genet. 2016, 37, 260–266. [Google Scholar] [CrossRef]
- Finn, A.P.; Grewal, D.S.; Vajzovic, L. Argus II retinal prosthesis system: A review of patient selection criteria, surgical considerations, and post-operative outcomes. Clin. Ophthalmol. 2018, 12, 1089. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Peng, X.; Ren, Q. Retinitis pigmentosa patients’ attitudes toward participation in retinal prosthesis trials. Contemp. Clin. Trials 2012, 33, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Borgonovi, E.; Taylor, R.S.; Sahel, J.-A.; Rizzo, S.; Stanga, P.E.; Kukreja, A.; Walter, P. The cost-effectiveness of the Argus II retinal prosthesis in Retinitis Pigmentosa patients. BMC Ophthalmol. 2014, 14, 49. [Google Scholar] [CrossRef]
- Prévot, P.-H.; Gehere, K.; Arcizet, F.; Akolkar, H.; Khoei, M.A.; Blaize, K.; Oubari, O.; Daye, P.; Lanoë, M.; Valet, M.; et al. Behavioural responses to a photovoltaic subretinal prosthesis implanted in non-human primates. Nat. Biomed. Eng. 2020, 4, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.; Kuriyan, A.E. Optoelectronic Devices for Vision Restoration. Curr. Ophthalmol. Rep. 2020, 8, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ostad-Ahmadi, Z.; Daemi, A.; Modabberi, M.-R.; Mostafaie, A. Safety, effectiveness, and cost-effectiveness of Argus II in patients with retinitis pigmentosa: A systematic review. Int. J. Ophthalmol. 2021, 14, 310–316. [Google Scholar] [CrossRef] [PubMed]
| N° | Title | Author (Year) | Study Design | Study Sample | Investigated Treatment | Methods | Outcomes | Main Findings | GRADE |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells Maintains and Partially Improves Visual Function in Patients with Advanced Retinitis Pigmentosa | Zhao T. et al. (2020) [8] | Prospective, open label, single-arm, phase I/II clinical trial | 32 adult patients (64 eyes), male and female | Intravenous infusion of human umbilical cord mesenchymal stem cells (UCMSCs) | Single infusion of 108 UCMSCs (250 mL); 12-month follow-up; evaluated safety, CMT, visual field sensitivity, BCVA, FVEP, and NEI-VFQ-25 scores at set intervals | No adverse effects Stable CMT Non-significant BCVA increase No significant change in visual field sensitivity and FVEP Improved NEI-VFQ-25 at 3 months | UCMSC infusion well-tolerated; short-term improvement in BCVA and quality of life; significant NEI-VFQ-25 improvement at 3 months; no significant long-term effects on visual field sensitivity or FVEP. Short-term benefits likely due to diminishing functional properties over time | Moderate |
| 2 | Comparative Study of a Modified Sub-Tenon’s Capsule Injection of Triamcinolone Acetonide and the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Retinitis Pigmentosa Combined With Macular Edema | Zhao T. et al. (2021) [9] | Prospective, open label, randomized, phase I/II clinical trial | 20 adult patients (40 eyes), male and female | Comparison of sub-Tenon’s injection of triamcinolone acetonide (TA) and intravenous infusion of umbilical cord mesenchymal stem cells (UCMSCs) in RP patients with macular edema | TA: 20 mg injection; UCMSCs: 3 × 106 (250 mL) infusion; 6-month follow-up; evaluated safety, CMT, visual field sensitivity, BCVA, and FVEP | No severe adverse effects in both groups TA reduced CMT significantly at 1 week, 1 month, and 2 months; UCMSCs at 1 month and had greater reduction at 6 months TA increased FVEP P2 at 2 months; UCMSCs at 6 months No significant visual acuity or field differences | Both treatments were safe. TA reduced macular edema quickly; UCMSCs had longer-lasting effects and better visual function improvement over time | Moderate |
| 3 | Umbilical cord derived mesenchymal stem cell implantation in retinitis pigmentosa: a 6-month follow-up results of a phase 3 trial | Neslihan Sinim Kahraman (2020) [10] | Prospective, single-center, phase III clinical study | 82 patients (124 eyes) | 5 million UCMSCs injected into the suprachoroidal area via surgery | Injection by experienced surgeon; evaluations at presurgery, 1 day, 1 week, 1-, 3-, and 6-months postsurgery | No serious systemic or ocular complications Significant improvements in BCVA and VF (p < 0.05) Significant improvements in mfERG P1 wave amplitudes and implicit times in central areas | Safe procedure with significant improvements in visual acuity, visual field, and retinal function | Moderate |
| 4 | Management of Retinitis Pigmentosa Via Wharton’s Jelly-Derived Mesenchymal Stem Cells or Combination With Magnovision: 3-Year Prospective Results | Ozmert (2023) [11] | Prospective, sequential, open-label clinical study | 80 patients (130 eyes) | Comparison of sub-Tenon WJ-MSC-only, Magnovision-only, combined WJ-MSC and Magnovision, and control groups in RP patients | Group 1: Sub-Tenon WJ-MSC (34 eyes)—Group 2: Magnovision (32 eyes)—Group 3: Combined WJ-MSC and Magnovision (32 eyes)—Group 4: Control (no treatment, 32 eyes) | Primary: Fundus autofluorescence surface area (FAF-field) Secondary: ETDRS visual acuity (BCVA), ellipsoid zone widths (EZWs), fundus perimetry deviation index (FPDI), full-field multiluminance ERG | FAF-field changes: Group 1: 0.39 mm2, Group 2: 1.50 mm2, Group 3: 0.07 mm2, Group 4: 12.04 mm2 EZW, BCVA, FPDI changes: Group 4 > Groups 1, 2 > Group 3—ERG-m changes: Group 3 > Groups 1, 2, 4 | High |
| 5 | Intravitreal autologous mesenchymal stem cell transplantation: a non-randomized phase I clinical trial in patients with retinitis pigmentosa | Tuekprakhon et al. (2021) [12] | Prospective, open-label, non-randomized phase I clinical trial | 14 adult patients (14 eyes treated, fellow eye control), male and female | Intravitreal injection of autologous BM-MSCs from posterior iliac crest | Single injection in right eye; left eye as control; 3 groups based on MSC quantity (1 × 106 cells, 5 × 106 cells, and 1 × 107 cells) | Primary: Safety assessed through various measures Secondary: BCVA, visual fields, central subfield thickness, ERG | No serious adverse events Stable IOP Transient increase in anterior chamber cells and flare Slight BCVA improvement Subjective quality of life improvements reported | Low |
| 6 | Mesenchymal stem cell surgery, rescue and regeneration in retinitis pigmentosa: clinical and rehabilitative prognostic aspects | Limoli et al. (2020) [13] | Retrospective clinical study | 25 patients, 11 women and 14 men, with an average age of 45.9 ± 18.36 years (34 eyes) | Autograft of mesenchymal cells of fat cells and PRP using LRRT (between the choroid and sclera) | All eyes were divided into two groups based on central retinal thickness (FT recorded by SD-OCT): Group A (≤190 μm) and Group B (>190 μm) | Mean BCVA, mean close-up visus, average threshold sensitivity, average threshold of close-up visus with magnifying system, percentage of change | BCVA changes: Group A varied from 0.89 to 0.85 logMAR (+4.16%, p = 0.9701); Group B varied from 0.45 to 0.37 logMAR (+16.31%, p = 0.9083) | Moderate |
| 7 | Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR | Kapetanovic (2020) [14] | Gene therapy trial | 18 patients | Increasing concentrations of codon-optimized AAV2 serotype 8 vector (AAV8.coRPGR) | Vector delivery into subretinal space via two-step injection | Primary safety endpoint: Incidence of dose-limiting toxicities and treatment-emergent adverse events over 24 months. Secondary endpoints: Changes in retinal sensitivity, BCVA, SD-OCT, and autofluorescence over 24 months | Dose–response effects observed, with mid-dose patients showing gains in retinal sensitivity and visual field improvement. Visual acuity returned to baseline levels by 3 months postsurgery. Subjective improvement in visual clarity and field of vision reported by all patients at 1-month follow-up. Functional assessment showed similar visual acuity to baseline | Moderate |
| 8 | Durability of Voretigene Neparvovec for Biallelic RPE65-Mediated Inherited Retinal Disease Phase 3 Results at 3 and 4 Years | Albert M Maguire et al. (2021) [15] | Open-label, randomized, controlled phase III trial | 29 male and female patients with RPE65-mutated IRD | Gene augmentation therapy with recombinant AAV vector voretigene neparvovec-rzyl (VN) | Randomization between original intervention (n = 19) and delayed intervention control (n = 10). Treatment: Intervention group received 1.5 × 1011 vg of VN in each eye. Controls switched to intervention after 1 year | Long-term efficacy and safety assessment over 5 years: multiluminance mobility test, full-field light sensitivity threshold, visual field, and visual acuity | Safety: No product-related serious adverse events Both groups showed consistent but not significant improvements in ambulatory navigation, light sensitivity, and visual field over 3 to 4 years compared to baseline One delayed intervention group patient experienced foveal loss attributed to the administration procedure | High |
| 9 | Docosahexaenoic Acid Slows Visual Field Progression in X-Linked Retinitis Pigmentosa: Ancillary Outcomes of the DHAX Trial | Dennis R. Hoffman et al. (2015) [16] | Single-site, placebo-controlled, randomized clinical trial | 51 patients (29 treated and 22 placebo) | Oral DHA supplementation | XLRP patients (age: 7–31) received 30 mg/kg/d or placebo for 4 years. Follow-up: Visual outcomes annually; RBC-DHA every 6 months | RBC-DHA levels increased 4-fold over placebo (p < 0.0001) No significant differences in visual acuity, shape discrimination, or fundus appearance Reduced progression in dark-adapted thresholds and visual field sensitivity with DHA supplementation (p < 0.05) | No significant changes in ERG function between groups DHA supplementation showed less progression in dark-adapted thresholds compared to placebo over 4 years Significant reductions in disease progression rates for various visual field measures with DHA supplementation | High |
| 10 | A Randomized, Placebo-controlled Clinical Trial of Docosahexaenoic Acid Supplementation for X-linked Retinitis Pigmentosa | Dennis R. Hoffman et al. (2003) [17] | 4-year prospective randomized clinical trial | 44 male patients with XLRP (mean age = 16 years; range = 4–38 years) received DHA (400 mg/d; n = 23; +DHA group) or placebo (n = 21) | Oral supplementation of docosahexaenoic acid | Male patients with XLRP (mean age = 16 years; range = 4–38 years) received DHA (400 mg/d; n = 23; +DHA group) or placebo (n = 21). Follow-up: RBC-DHA concentrations assessed every 6 months. Full-field cone ERGs, visual acuity, dark adaptation, visual fields, rod ERGs, and fundus photos recorded annually | RBC-DHA increased 2.5-fold in +DHA group No significant difference in cone ERG function between groups Preservation of cone ERG function correlated with RBC-DHA Less change in fundus appearance in +DHA group Subset analysis showed DHA supplementation reduced rod ERG loss in patients aged < 12 years and preserved cone ERG function in patients ≥ 12 years | Although DHA-supplemented patients had significantly higher RBC-DHA levels, cone ERG functional loss rate was not significantly different between groups | High |
| 11 | Effect of Oral Valproic Acid vs. Placebo for Vision Loss in Patients With Autosomal Dominant Retinitis Pigmentosa A Randomized Phase 2 Multicenter Placebo-Controlled Clinical Trial | David G. Birch et al. (2018) [18] | Multicenter, phase II, prospective, interventional, placebo-controlled, double-masked randomized clinical trial | 90 male and female patients with autosomal dominant RP | Oral VPA 500–1000 mg daily | Participants randomized to receive VPA (n= 46) or placebo (n = 44) for 12 months. Dose selection based on proof-of-concept studies. Follow-up visits at 8, 26, 39, 52, and 65 weeks | Primary outcome: Change in kinetic perimetry (KP) visual field area (VFA) assessed by the III4e isopter between baseline and week 52. Secondary outcomes: Visual function measures | The study did not meet its primary endpoint at 12 months, showing no change in visual field area between groups No significant improvement in any secondary outcomes observed between the two groups The study does not support the use of valproic acid to enhance visual function in AD-RP patients | Very high |
| 12 | Safety and Proof-of-Concept Study of Oral QLT091001 in Retinitis Pigmentosa Due to Inherited Deficiencies of Retinal Pigment Epithelial 65 Protein (RPE65) or Lecithin: Retinol Acyltransferase (LRAT) | Hendrik P. n. Scholl et al. (2015) [19] | International, multicenter, open-label, proof-of-concept study | 18 patients with RPE65- or LRAT-related retinitis pigmentosa with autosomal recessive RP due to biallelic mutations in either the RPE65 or LRAT gene confirmed in an accredited molecular genetic laboratory and between 5 and 65 years of age | Oral QLT091001 | Patients received 40 mg/m2/day QLT091001 for 7 days | Within 2 months, 44% showed a 20% increase in retinal area 67% showed a 5-letter ETDRS score increase | Baseline outer segment OS layer value was significantly lower in non-responders QLT091001 improved visual field and/or acuity in RP patients | High |
| 13 | Subretinal Visual Implant Alpha IMS—Clinical trial interim report | Katarina Stingl et al. (2015) [20] | International multicenter, single-arm, clinical trial | 29 male and female patients with hereditary retinal degeneration (retinitis pigmentosa n = 25; cone–rod dystrophy n = 4). Patients had either light perception without projection (20 participants) or no light perception | Retina Implant Alpha IMS | Surgical implantation of microchip subretinally in one eye. Participants compared vision with implant’s power on or off. Follow-up for 1 year with visual function tests and monitoring | Primary: Improvements in daily activities and mobility. Secondary: Enhanced visual acuity and object recognition | 72% showed better daily living and mobility 86% demonstrated improved visual acuity and recognition - Better detection and recognition of shapes and objects with the implant on - Improved gray level perception and light localization 86% experienced improved light perception and localization with the implant Few SAEs reported, mostly resolved successfully | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Confalonieri, F.; La Rosa, A.; Ottonelli, G.; Barone, G.; Ferraro, V.; Di Maria, A.; Romano, M.; Randazzo, A.; Vallejo-Garcia, J.L.; Vinciguerra, P.; et al. Retinitis Pigmentosa and Therapeutic Approaches: A Systematic Review. J. Clin. Med. 2024, 13, 4680. https://doi.org/10.3390/jcm13164680
Confalonieri F, La Rosa A, Ottonelli G, Barone G, Ferraro V, Di Maria A, Romano M, Randazzo A, Vallejo-Garcia JL, Vinciguerra P, et al. Retinitis Pigmentosa and Therapeutic Approaches: A Systematic Review. Journal of Clinical Medicine. 2024; 13(16):4680. https://doi.org/10.3390/jcm13164680
Chicago/Turabian StyleConfalonieri, Filippo, Antonio La Rosa, Giovanni Ottonelli, Gianmaria Barone, Vanessa Ferraro, Alessandra Di Maria, Mary Romano, Alessandro Randazzo, Josè Luis Vallejo-Garcia, Paolo Vinciguerra, and et al. 2024. "Retinitis Pigmentosa and Therapeutic Approaches: A Systematic Review" Journal of Clinical Medicine 13, no. 16: 4680. https://doi.org/10.3390/jcm13164680
APA StyleConfalonieri, F., La Rosa, A., Ottonelli, G., Barone, G., Ferraro, V., Di Maria, A., Romano, M., Randazzo, A., Vallejo-Garcia, J. L., Vinciguerra, P., & Petrovski, G. (2024). Retinitis Pigmentosa and Therapeutic Approaches: A Systematic Review. Journal of Clinical Medicine, 13(16), 4680. https://doi.org/10.3390/jcm13164680








