Advancements in Early Detection and Screening Strategies for Pancreatic Cancer: From Genetic Susceptibility to Novel Biomarkers
Abstract
:1. Introduction
2. Risk Factors for Pancreatic Cancer
2.1. Genetic and Familial Risk Factors
2.1.1. Peutz–Jeghers Syndrome
2.1.2. Lynch Syndrome
2.1.3. Hereditary Breast and Ovarian Cancer
2.1.4. Familial Atypical Multiple Mole Melanoma (FAMMM)
2.1.5. Li–Fraumeni Syndrome (LFS)
2.1.6. Familial Adenomatous Polyposis (FAP)
2.1.7. Ataxia–Telangiectasia Mutated (ATM)
2.1.8. Cystic Fibrosis
2.1.9. Hereditary Pancreatitis
2.2. Non-Genetic and Environmental Risk Factors
3. Age-Appropriate Screening for Pancreatic Cancer in High-Risk Groups
4. How Often Should Patients Be Screened?
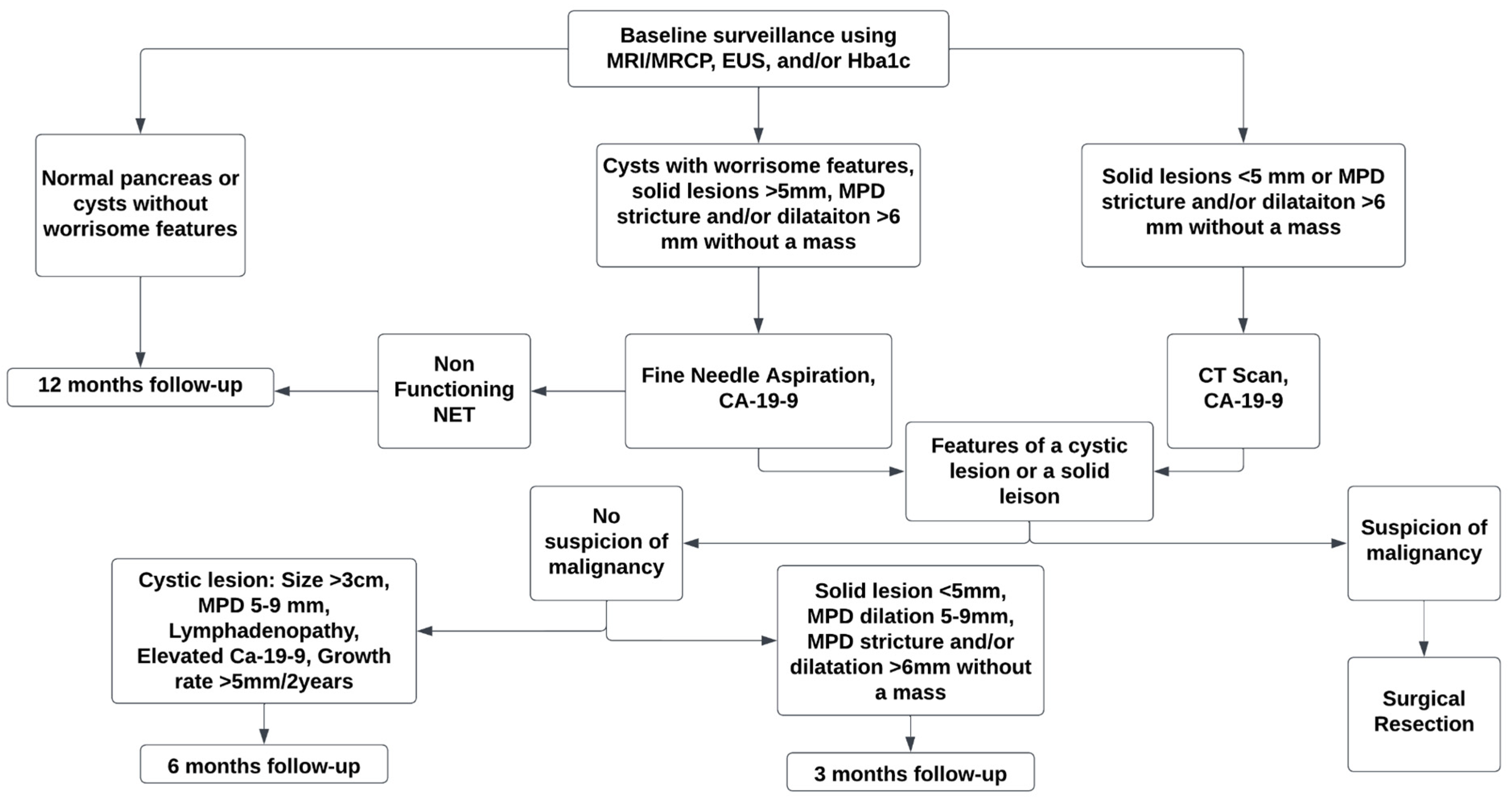
5. Modalities for Screening of Pancreatic Cancer
Snapshotting Pancreatic Cancer: Exploring Early Detection with Imaging Techniques
6. Efficacy and Limitations of Current Screening Strategies for Pancreatic Cancer
6.1. Challenges in Sensitivity and Specificity
6.2. False-Positive Rates
6.3. Long-Term Outcomes
6.4. Psychological Impact
7. Promoter Methylation of ADAMTS1, BNC1, and Novel cfDNA Methylation Markers
8. Exploring Novel Biomarkers for Pancreatic Cancer Detection
9. Unlocking the Potential of Circulating Exosomes: A Promising Avenue for Pancreatic Cancer Screening
10. Advancing Pancreatic Cancer Screening: Future Directions and Recommendations
10.1. Integration of Multi-Modal Approaches
10.2. Advancements in Technology
10.3. Personalized Risk Assessment
10.4. Interdisciplinary Collaboration
10.5. Prospective Studies
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pancreatic Cancer Facts. Hirshberg Found. Pancreat. Cancer Res. Available online: https://pancreatic.org/pancreatic-cancer/pancreatic-cancer-facts/ (accessed on 5 March 2024).
- Cancer Facts & Figures 2024. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2024-cancer-facts-figures.html (accessed on 5 March 2024).
- Cancer of the Pancreas—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 5 March 2024).
- Vasen, H.; Ibrahim, I.; Ponce, C.G.; Slater, E.P.; Matthäi, E.; Carrato, A.; Earl, J.; Robbers, K.; van Mil, A.M.; Potjer, T.; et al. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J. Clin. Oncol. 2016, 34, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Bilici, A. Prognostic Factors Related with Survival in Patients with Pancreatic Adenocarcinoma. World J. Gastroenterol. WJG 2014, 20, 10802–10812. [Google Scholar] [CrossRef]
- Survival Rates for Pancreatic Cancer. Available online: https://www.cancer.org/cancer/types/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 24 March 2024).
- Hruban, R.H.; Canto, M.; Goggins, M.; Schulick, R.; Klein, A.P. Update on Familial Pancreatic Cancer. Adv. Surg. 2010, 44, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Chari, S.T.; Kelly, K.; Hollingsworth, M.A.; Thayer, S.P.; Ahlquist, D.A.; Andersen, D.K.; Batra, S.K.; Brentnall, T.A.; Canto, M.; Cleeter, D.F.; et al. Early Detection of Sporadic Pancreatic Cancer. Pancreas 2015, 44, 693–712. [Google Scholar] [CrossRef] [PubMed]
- Waleleng, B.J.; Adiwinata, R.; Wenas, N.T.; Haroen, H.; Rotty, L.; Gosal, F.; Rotty, L.; Winarta, J.; Waleleng, A.; Simadibrata, M. Screening of Pancreatic Cancer: Target Population, Optimal Timing and How? Ann. Med. Surg. 2022, 84, 104814. [Google Scholar] [CrossRef] [PubMed]
- Pandol, S.J.; Apte, M.V.; Wilson, J.S.; Gukovskaya, A.S.; Edderkaoui, M. The Burning Question: Why Is Smoking a Risk Factor for Pancreatic Cancer? Pancreatol. Off. J. Int. Assoc. Pancreatol. IAP Al 2012, 12, 344–349. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Van Hoogstraten, L.; Gomez-Rubio, P.; Löhr, M.; Sharp, L.; Molero, X.; Márquez, M.; Michalski, C.W.; Farré, A.; Perea, J.; et al. Pancreatic Cancer Risk in Relation to Lifetime Smoking Patterns, Tobacco Type, and Dose-Response Relationships. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2020, 29, 1009–1018. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Gou, Y.-W.; Jin, W.-W.; Xiao, M.; Fang, H.-Y. Association between Alcohol Intake and the Risk of Pancreatic Cancer: A Dose–Response Meta-Analysis of Cohort Studies. BMC Cancer 2016, 16, 212. [Google Scholar] [CrossRef]
- Li, D. Diabetes and Pancreatic Cancer. Mol. Carcinog. 2012, 51, 64–74. [Google Scholar] [CrossRef]
- Kirkegård, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef]
- Klein, A.P. Genetic Susceptibility to Pancreatic Cancer. Mol. Carcinog. 2012, 51, 14–24. [Google Scholar] [CrossRef]
- Aslanian, H.R.; Lee, J.H.; Canto, M.I. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology 2020, 159, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Tajima, K.; Takezaki, T.; Hamajima, N.; Hirose, K.; Ito, H.; Tominaga, S. Epidemiology of Pancreatic Cancer in Japan: A Nested Case-Control Study from the Hospital-Based Epidemiologic Research Program at Aichi Cancer Center (HERPACC). Int. J. Epidemiol. 2003, 32, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Falk, R.T.; Pickle, L.W.; Fontham, E.T.; Correa, P.; Fraumeni, J.F. Life-Style Risk Factors for Pancreatic Cancer in Louisiana: A Case-Control Study. Am. J. Epidemiol. 1988, 128, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.P.; Brune, K.A.; Petersen, G.M.; Goggins, M.; Tersmette, A.C.; Offerhaus, G.J.A.; Griffin, C.; Cameron, J.L.; Yeo, C.J.; Kern, S.; et al. Prospective Risk of Pancreatic Cancer in Familial Pancreatic Cancer Kindreds. Cancer Res. 2004, 64, 2634–2638. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, S.; Brune, K.A.; Hruban, R.H.; Parmigiani, G.; Klein, A.P. PancPRO: Risk Assessment for Individuals with a Family History of Pancreatic Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Latchford, A.; Greenhalf, W.; Vitone, L.J.; Neoptolemos, J.P.; Lancaster, G.A.; Phillips, R.K.S. Peutz–Jeghers Syndrome and Screening for Pancreatic Cancer. Br. J. Surg. 2006, 93, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Korsse, S.E.; Harinck, F.; van Lier, M.G.F.; Biermann, K.; Offerhaus, G.J.A.; Krak, N.; Looman, C.W.N.; van Veelen, W.; Kuipers, E.J.; Wagner, A.; et al. Pancreatic Cancer Risk in Peutz-Jeghers Syndrome Patients: A Large Cohort Study and Implications for Surveillance. J. Med. Genet. 2013, 50, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Giardiello, F.M.; Brensinger, J.D.; Tersmette, A.C.; Goodman, S.N.; Petersen, G.M.; Booker, S.V.; Cruz–Correa, M.; Offerhaus, J.A. Very High Risk of Cancer in Familial Peutz–Jeghers Syndrome. Gastroenterology 2000, 119, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Resta, N.; Pierannunzio, D.; Lenato, G.M.; Stella, A.; Capocaccia, R.; Bagnulo, R.; Lastella, P.; Susca, F.C.; Bozzao, C.; Loconte, D.C.; et al. Cancer Risk Associated with STK11/LKB1 Germline Mutations in Peutz-Jeghers Syndrome Patients: Results of an Italian Multicenter Study. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2013, 45, 606–611. [Google Scholar] [CrossRef]
- Kastrinos, F.; Mukherjee, B.; Tayob, N.; Wang, F.; Sparr, J.; Raymond, V.M.; Bandipalliam, P.; Stoffel, E.M.; Gruber, S.B.; Syngal, S. The Risk of Pancreatic Cancer in Families with Lynch Syndrome. JAMA J. Am. Med. Assoc. 2009, 302, 1790–1795. [Google Scholar] [CrossRef]
- Borelli, I.; Casalis Cavalchini, G.C.; Del Peschio, S.; Micheletti, M.; Venesio, T.; Sarotto, I.; Allavena, A.; Delsedime, L.; Barberis, M.A.; Mandrile, G.; et al. A Founder MLH1 Mutation in Lynch Syndrome Families from Piedmont, Italy, Is Associated with an Increased Risk of Pancreatic Tumours and Diverse Immunohistochemical Patterns. Fam. Cancer 2014, 13, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Petrucelli, N.; Daly, M.B.; Pal, T. BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Choi, E.; Mun, G.; Lee, J.; Lee, H.; Cho, J.; Lee, Y.-S. BRCA1 Deficiency in Triple-Negative Breast Cancer: Protein Stability as a Basis for Therapy. Biomed. Pharmacother. 2023, 158, 114090. [Google Scholar] [CrossRef]
- Casaubon, J.T.; Kashyap, S.; Regan, J.-P. BRCA1 and BRCA2 Mutations. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Yoshida, R. Hereditary Breast and Ovarian Cancer (HBOC): Review of Its Molecular Characteristics, Screening, Treatment, and Prognosis. Breast Cancer Tokyo Jpn. 2021, 28, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Olakowski, M.; Bułdak, Ł. Current Status of Inherited Pancreatic Cancer. Hered. Cancer Clin. Pract. 2022, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Easton, D.F.; The Breast Cancer Linkage Consortium. Cancer Incidence in BRCA1 Mutation Carriers. JNCI J. Natl. Cancer Inst. 2002, 94, 1358–1365. [Google Scholar] [CrossRef]
- Breast Cancer Linkage Consortium. Cancer Risks in BRCA2 Mutation Carriers. J. Natl. Cancer Inst. 1999, 91, 1310–1316. [Google Scholar] [CrossRef]
- Hofstatter, E.W.; Domchek, S.M.; Miron, A.; Garber, J.; Wang, M.; Componeschi, K.; Boghossian, L.; Miron, P.L.; Nathanson, K.L.; Tung, N. PALB2 Mutations in Familial Breast and Pancreatic Cancer. Fam. Cancer 2011, 10. [Google Scholar] [CrossRef]
- Jones, S.; Hruban, R.H.; Kamiyama, M.; Borges, M.; Zhang, X.; Parsons, D.W.; Cheng, J.; Lin, H.; Palmisano, E.; Brune, K.; et al. Exomic Sequencing Identifies PALB2 as a Pancreatic Cancer Susceptibility Gene. Science 2009, 324, 217. [Google Scholar] [CrossRef]
- Slater, E.; Langer, P.; Niemczyk, E.; Strauch, K.; Butler, J.; Habbe, N.; Neoptolemos, J.; Greenhalf, W.; Bartsch, D. PALB2 Mutations in European Familial Pancreatic Cancer Families. Clin. Genet. 2010, 78, 490–494. [Google Scholar] [CrossRef]
- Goldstein, A.M.; Chan, M.; Harland, M.; Gillanders, E.M.; Hayward, N.K.; Avril, M.-F.; Azizi, E.; Bianchi-Scarra, G.; Bishop, D.T.; Bressac-de Paillerets, B.; et al. High-Risk Melanoma Susceptibility Genes and Pancreatic Cancer, Neural System Tumors, and Uveal Melanoma across GenoMEL. Cancer Res. 2006, 66, 9818–9828. [Google Scholar] [CrossRef] [PubMed]
- Vasen, H.f.a.; Gruis, N.a.; Frants, R.r.; van der Velden, P.a.; Hille, E.t.m.; Bergman, W. Risk of Developing Pancreatic Cancer in Families with Familial Atypical Multiple Mole Melanoma Associated with a Specific 19 Deletion of P16 (P16-Leiden). Int. J. Cancer 2000, 87, 809–811. [Google Scholar] [CrossRef]
- Li, F.P.; Fraumeni, J.F. Soft-Tissue Sarcomas, Breast Cancer, and Other Neoplasms. A Familial Syndrome? Ann. Intern. Med. 1969, 71, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Malkin, D.; Li, F.P.; Strong, L.C.; Fraumeni, J.F.; Nelson, C.E.; Kim, D.H.; Kassel, J.; Gryka, M.A.; Bischoff, F.Z.; Tainsky, M.A. Germ Line P53 Mutations in a Familial Syndrome of Breast Cancer, Sarcomas, and Other Neoplasms. Science 1990, 250, 1233–1238. [Google Scholar] [CrossRef]
- Srivastava, S.; Zou, Z.Q.; Pirollo, K.; Blattner, W.; Chang, E.H. Germ-Line Transmission of a Mutated P53 Gene in a Cancer-Prone Family with Li-Fraumeni Syndrome. Nature 1990, 348, 747–749. [Google Scholar] [CrossRef]
- Li, F.P.; Fraumeni, J.F.; Mulvihill, J.J.; Blattner, W.A.; Dreyfus, M.G.; Tucker, M.A.; Miller, R.W. A Cancer Family Syndrome in Twenty-Four Kindreds. Cancer Res. 1988, 48, 5358–5362. [Google Scholar]
- Amadou, A.; Achatz, M.I.W.; Hainaut, P. Revisiting Tumor Patterns and Penetrance in Germline TP53 Mutation Carriers: Temporal Phases of Li-Fraumeni Syndrome. Curr. Opin. Oncol. 2018, 30, 23–29. [Google Scholar] [CrossRef] [PubMed]
- DaVee, T.; Coronel, E.; Papafragkakis, C.; Thaiudom, S.; Lanke, G.; Chakinala, R.C.; Nogueras González, G.M.; Bhutani, M.S.; Ross, W.A.; Weston, B.R.; et al. Pancreatic Cancer Screening in High-Risk Individuals with Germline Genetic Mutations. Gastrointest. Endosc. 2018, 87, 1443–1450. [Google Scholar] [CrossRef]
- Ruijs, M.W.G.; Verhoef, S.; Rookus, M.A.; Pruntel, R.; van der Hout, A.H.; Hogervorst, F.B.L.; Kluijt, I.; Sijmons, R.H.; Aalfs, C.M.; Wagner, A.; et al. TP53 Germline Mutation Testing in 180 Families Suspected of Li-Fraumeni Syndrome: Mutation Detection Rate and Relative Frequency of Cancers in Different Familial Phenotypes. J. Med. Genet. 2010, 47, 421–428. [Google Scholar] [CrossRef]
- Pardillos Tomé, A.; Bajador Andreu, E.; Comín Orce, A.; Marcilla Córdoba, F. Familial Adenomatous Polyposis Associated with Pancreatic Neuroendocrine Tumour. Gastroenterol. Hepatol. Engl. Ed. 2021, 44, 130–131. [Google Scholar] [CrossRef]
- Maire, F.; Hammel, P.; Terris, B.; Olschwang, S.; O’Toole, D.; Sauvanet, A.; Palazzo, L.; Ponsot, P.; Laplane, B.; Lévy, P.; et al. Intraductal Papillary and Mucinous Pancreatic Tumour: A New Extracolonic Tumour in Familial Adenomatous Polyposis. Gut 2002, 51, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, P.; Davaro, E.P.; Doan, J.V.; Ising, M.E.; Evans, N.R.; Phillips, N.J.; Lai, J.; Guzman, M.A. Familial Adenomatous Polyposis Syndrome: An Update and Review of Extraintestinal Manifestations. Arch. Pathol. Lab. Med. 2019, 143, 1382–1398. [Google Scholar] [CrossRef] [PubMed]
- Karstensen, J.G.; Bülow, S.; Højen, H.; Jelsig, A.M.; Jespersen, N.; Andersen, K.K.; Wewer, M.D.; Burisch, J.; Pommergaard, H.C. Cancer in Patients With Familial Adenomatous Polyposis: A Nationwide Danish Cohort Study with Matched Controls. Gastroenterology 2023, 165, 573–581.e3. [Google Scholar] [CrossRef]
- Giardiello, F.M.; Offerhaus, G.J.; Lee, D.H.; Krush, A.J.; Tersmette, A.C.; Booker, S.V.; Kelley, N.C.; Hamilton, S.R. Increased Risk of Thyroid and Pancreatic Carcinoma in Familial Adenomatous Polyposis. Gut 1993, 34, 1394–1396. [Google Scholar] [CrossRef]
- Moussata, D.; Senouci, L.; Berger, F.; Scoazec, J.-Y.; Pinson, S.; Walter, T.; Lombard-Bohas, C.; Saurin, J.-C. Familial Adenomatous Polyposis and Pancreatic Cancer. Pancreas 2015, 44, 512–513. [Google Scholar] [CrossRef] [PubMed]
- Pitter, K.L.; Casey, D.L.; Lu, Y.C.; Hannum, M.; Zhang, Z.; Song, X.; Pecorari, I.; McMillan, B.; Ma, J.; Samstein, R.M.; et al. Pathogenic ATM Mutations in Cancer and a Genetic Basis for Radiotherapeutic Efficacy. J. Natl. Cancer Inst. 2021, 113, 266–273. [Google Scholar] [CrossRef]
- Lavin, M.F. Ataxia-Telangiectasia: From a Rare Disorder to a Paradigm for Cell Signalling and Cancer. Nat. Rev. Mol. Cell Biol. 2008, 9, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.-C.; Roberts, N.J.; Childs, E.; Porter, N.; Rabe, K.G.; Borgida, A.; Ukaegbu, C.; Goggins, M.G.; Hruban, R.H.; Zogopoulos, G.; et al. Risk of Pancreatic Cancer Among Individuals With Pathogenic Variants in the ATM Gene. JAMA Oncol. 2021, 7, 1664–1668. [Google Scholar] [CrossRef]
- Hall, M.J.; Bernhisel, R.; Hughes, E.; Larson, K.; Rosenthal, E.T.; Singh, N.A.; Lancaster, J.M.; Kurian, A.W. Germline Pathogenic Variants in the Ataxia Telangiectasia Mutated (ATM) Gene Are Associated with High and Moderate Risks for Multiple Cancers. Cancer Prev. Res. 2021, 14, 433–440. [Google Scholar] [CrossRef]
- Geoffroy-Perez, B.; Janin, N.; Ossian, K.; Laugé, A.; Croquette, M.F.; Griscelli, C.; Debré, M.; Bressac-de-Paillerets, B.; Aurias, A.; Stoppa-Lyonnet, D.; et al. Cancer Risk in Heterozygotes for Ataxia-Telangiectasia. Int. J. Cancer 2001, 93, 288–293. [Google Scholar] [CrossRef]
- Hannan, Z.; Yu, S.; Domchek, S.; Mamtani, R.; Reiss, K.A. Clinical Characteristics of Patients with Pancreatic Cancer and Pathogenic ATM Alterations. JNCI Cancer Spectr. 2021, 5, pkaa121. [Google Scholar] [CrossRef]
- Lopes-Pacheco, M. CFTR Modulators: The Changing Face of Cystic Fibrosis in the Era of Precision Medicine. Front. Pharmacol. 2019, 10, 1662. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Blankenheim, Z.; Scott, P.M.; Cormier, R.T. CFTR and Gastrointestinal Cancers: An Update. J. Pers. Med. 2022, 12, 868. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P.; Marshall, B.C.; Lowenfels, A.B. Risk of Pancreatic Cancer in Patients with Cystic Fibrosis. Gut 2007, 56, 1327–1328. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, R.R.; Petersen, G.M.; Rabe, K.G.; Holtegaard, L.M.; Lynch, P.J.; Bishop, M.D.; Highsmith, W.E. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Gene Mutations and Risk for Pancreatic Adenocarcinoma. Cancer 2010, 116, 203–209. [Google Scholar] [CrossRef]
- McWilliams, R.; Highsmith, W.E.; Rabe, K.G.; de Andrade, M.; Tordsen, L.A.; Holtegaard, L.M.; Petersen, G.M. Cystic Fibrosis Transmembrane Regulator Gene Carrier Status Is a Risk Factor for Young Onset Pancreatic Adenocarcinoma. Gut 2005, 54, 1661–1662. [Google Scholar] [CrossRef]
- Whitcomb, D.C.; Gorry, M.C.; Preston, R.A.; Furey, W.; Sossenheimer, M.J.; Ulrich, C.D.; Martin, S.P.; Gates, L.K.; Amann, S.T.; Toskes, P.P.; et al. Hereditary Pancreatitis Is Caused by a Mutation in the Cationic Trypsinogen Gene. Nat. Genet. 1996, 14, 141–145. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-Related Inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Lowenfels, A.B.; Maisonneuve, P.; DiMagno, E.P.; Elitsur, Y.; Gates, L.K., Jr.; Perrault, J.; Whitcomb, D.C.; International Hereditary Pancreatitis Study Group. Hereditary Pancreatitis and the Risk of Pancreatic Cancer. JNCI J. Natl. Cancer Inst. 1997, 89, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Mario, C.; Marilisa, F.; Kryssia, I.R.-C.; Pellegrino, C.; Ginevra, C.; Chiara, M.; Alberto, B.; Antonio, N.; Gioacchino, L.; Tiziana, M.; et al. Epidemiology and Risk Factors of Pancreatic Cancer. Acta Bio Medica Atenei Parm. 2018, 89, 141–146. [Google Scholar] [CrossRef]
- Iodice, S.; Gandini, S.; Maisonneuve, P.; Lowenfels, A.B. Tobacco and the Risk of Pancreatic Cancer: A Review and Meta-Analysis. Langenbecks Arch. Surg. 2008, 393, 535–545. [Google Scholar] [CrossRef]
- Tramacere, I.; Scotti, L.; Jenab, M.; Bagnardi, V.; Bellocco, R.; Rota, M.; Corrao, G.; Bravi, F.; Boffetta, P.; La Vecchia, C. Alcohol Drinking and Pancreatic Cancer Risk: A Meta-Analysis of the Dose-Risk Relation. Int. J. Cancer 2010, 126, 1474–1486. [Google Scholar] [CrossRef]
- Cavestro, G.M.; Comparato, G.; Nouvenne, A.; Sianesi, M.; Di Mario, F. The Race from Chronic Pancreatitis to Pancreatic Cancer. JOP J. Pancreas 2003, 4, 165–168. [Google Scholar]
- Raimondi, S.; Lowenfels, A.B.; Morselli-Labate, A.M.; Maisonneuve, P.; Pezzilli, R. Pancreatic Cancer in Chronic Pancreatitis; Aetiology, Incidence, and Early Detection. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N.; Wolk, A. Body Mass Index and Pancreatic Cancer Risk: A Meta-Analysis of Prospective Studies. Int. J. Cancer 2007, 120, 1993–1998. [Google Scholar] [CrossRef]
- Stolzenberg-Solomon, R.Z.; Newton, C.C.; Silverman, D.T.; Pollak, M.; Nogueira, L.M.; Weinstein, S.J.; Albanes, D.; Männistö, S.; Jacobs, E.J. Circulating Leptin and Risk of Pancreatic Cancer: A Pooled Analysis From 3 Cohorts. Am. J. Epidemiol. 2015, 182, 187–197. [Google Scholar] [CrossRef]
- Gullo, L.; Pezzilli, R.; Morselli-Labate, A.M.; Italian Pancreatic Cancer Study Group. Diabetes and the Risk of Pancreatic Cancer. N. Engl. J. Med. 1994, 331, 81–84. [Google Scholar] [CrossRef]
- Yang, J.; Waldron, R.T.; Su, H.-Y.; Moro, A.; Chang, H.-H.; Eibl, G.; Ferreri, K.; Kandeel, F.R.; Lugea, A.; Li, L.; et al. Insulin Promotes Proliferation and Fibrosing Responses in Activated Pancreatic Stellate Cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 311, G675–G687. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.V.; Wilson, J.S.; Lugea, A.; Pandol, S.J. A Starring Role for Stellate Cells in the Pancreatic Cancer Microenvironment. Gastroenterology 2013, 144, 1210–1219. [Google Scholar] [CrossRef]
- Paiella, S.; Salvia, R.; De Pastena, M.; Pollini, T.; Casetti, L.; Landoni, L.; Esposito, A.; Marchegiani, G.; Malleo, G.; De Marchi, G.; et al. Screening/Surveillance Programs for Pancreatic Cancer in Familial High-Risk Individuals: A Systematic Review and Proportion Meta-Analysis of Screening Results. Pancreatol. Off. J. Int. Assoc. Pancreatol. IAP Al 2018, 18, 420–428. [Google Scholar] [CrossRef]
- Goggins, M.; Overbeek, K.A.; Brand, R.; Syngal, S.; Del Chiaro, M.; Bartsch, D.K.; Bassi, C.; Carrato, A.; Farrell, J.; Fishman, E.K.; et al. Management of Patients with Increased Risk for Familial Pancreatic Cancer: Updated Recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Sawhney, M.S.; Calderwood, A.H.; Thosani, N.C.; Rebbeck, T.R.; Wani, S.; Canto, M.I.; Fishman, D.S.; Golan, T.; Hidalgo, M.; Kwon, R.S.; et al. ASGE Guideline on Screening for Pancreatic Cancer in Individuals with Genetic Susceptibility: Summary and Recommendations. Gastrointest. Endosc. 2022, 95, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Cancer Risk Management for People with an Inherited TP53 Mutation. Available online: https://www.facingourrisk.org/info/hereditary-cancer-and-genetic-testing/hereditary-cancer-genes-and-risk/genes-by-name/tp53/risk-management (accessed on 4 April 2024).
- Yu, J.; Blackford, A.L.; Dal Molin, M.; Wolfgang, C.L.; Goggins, M. Time to Progression of Pancreatic Ductal Adenocarcinoma from Low-to-High Tumour Stages. Gut 2015, 64, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Gangi, S.; Fletcher, J.G.; Nathan, M.A.; Christensen, J.A.; Harmsen, W.S.; Crownhart, B.S.; Chari, S.T. Time Interval Between Abnormalities Seen on CT and the Clinical Diagnosis of Pancreatic Cancer: Retrospective Review of CT Scans Obtained Before Diagnosis. Am. J. Roentgenol. 2004, 182, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Brune, K.A.; Lau, B.; Palmisano, E.; Canto, M.; Goggins, M.G.; Hruban, R.H.; Klein, A.P. Importance of Age of Onset in Pancreatic Cancer Kindreds. JNCI J. Natl. Cancer Inst. 2010, 102, 119–126. [Google Scholar] [CrossRef]
- Del Chiaro, M.; Zerbi, A.; Falconi, M.; Bertacca, L.; Polese, M.; Sartori, N.; Boggi, U.; Casari, G.; Longoni, B.M.; Salvia, R.; et al. Cancer Risk among the Relatives of Patients with Pancreatic Ductal Adenocarcinoma. Pancreatol. Off. J. Int. Assoc. Pancreatol. IAP Al 2007, 7, 459–469. [Google Scholar] [CrossRef]
- Lorenzo, D.; Rebours, V.; Maire, F.; Palazzo, M.; Gonzalez, J.-M.; Vullierme, M.-P.; Aubert, A.; Hammel, P.; Lévy, P.; de Mestier, L. Role of Endoscopic Ultrasound in the Screening and Follow-up of High-Risk Individuals for Familial Pancreatic Cancer. World J. Gastroenterol. 2019, 25, 5082–5096. [Google Scholar] [CrossRef] [PubMed]
- Harinck, F.; Konings, I.C.a.W.; Kluijt, I.; Poley, J.W.; van Hooft, J.E.; van Dullemen, H.M.; Nio, C.Y.; Krak, N.C.; Hermans, J.J.; Aalfs, C.M.; et al. A Multicentre Comparative Prospective Blinded Analysis of EUS and MRI for Screening of Pancreatic Cancer in High-Risk Individuals. Gut 2016, 65, 1505–1513. [Google Scholar] [CrossRef]
- Canto, M.I.; Hruban, R.H.; Fishman, E.K.; Kamel, I.R.; Schulick, R.; Zhang, Z.; Topazian, M.; Takahashi, N.; Fletcher, J.; Petersen, G.; et al. Frequent Detection of Pancreatic Lesions in Asymptomatic High-Risk Individuals. Gastroenterology 2012, 142, 796–804; quiz e14-15. [Google Scholar] [CrossRef]
- Yousaf, M.N.; Chaudhary, F.S.; Ehsan, A.; Suarez, A.L.; Muniraj, T.; Jamidar, P.; Aslanian, H.R.; Farrell, J.J. Endoscopic Ultrasound (EUS) and the Management of Pancreatic Cancer. BMJ Open Gastroenterol. 2020, 7, e000408. [Google Scholar] [CrossRef]
- Hewitt, M.J.; McPhail, M.J.W.; Possamai, L.; Dhar, A.; Vlavianos, P.; Monahan, K.J. EUS-Guided FNA for Diagnosis of Solid Pancreatic Neoplasms: A Meta-Analysis. Gastrointest. Endosc. 2012, 75, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, R.; Lu, Y.; Xia, Y.; Zhou, H. Diagnostic Accuracy of Endoscopic Ultrasound-Guided Fine-Needle Aspiration for Solid Pancreatic Lesion: A Systematic Review. J. Cancer Res. Clin. Oncol. 2012, 138, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Puli, S.R.; Kalva, N.; Bechtold, M.L.; Pamulaparthy, S.R.; Cashman, M.D.; Estes, N.C.; Pearl, R.H.; Volmar, F.-H.; Dillon, S.; Shekleton, M.F.; et al. Diagnostic Accuracy of Endoscopic Ultrasound in Pancreatic Neuroendocrine Tumors: A Systematic Review and Meta Analysis. World J. Gastroenterol. 2013, 19, 3678–3684. [Google Scholar] [CrossRef] [PubMed]
- Banafea, O.; Mghanga, F.P.; Zhao, J.; Zhao, R.; Zhu, L. Endoscopic Ultrasonography with Fine-Needle Aspiration for Histological Diagnosis of Solid Pancreatic Masses: A Meta-Analysis of Diagnostic Accuracy Studies. BMC Gastroenterol. 2016, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Topazian, M.; Goggins, M.G.; Syngal, S.; Saltzman, J.R.; Lee, J.H.; Farrell, J.J.; Canto, M.I. Linear-Array EUS Improves Detection of Pancreatic Lesions in High-Risk Individuals: A Randomized Tandem Study. Gastrointest. Endosc. 2015, 82, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Casà, C.; Piras, A.; D’Aviero, A.; Preziosi, F.; Mariani, S.; Cusumano, D.; Romano, A.; Boskoski, I.; Lenkowicz, J.; Dinapoli, N.; et al. The Impact of Radiomics in Diagnosis and Staging of Pancreatic Cancer. Ther. Adv. Gastrointest. Endosc. 2022, 15, 26317745221081596. [Google Scholar] [CrossRef] [PubMed]
- Keihanian, T.; Barkin, J.A.; Souto, E.O. Early Detection of Pancreatic Cancer: Risk Factors and the Current State of Screening Modalities. Gastroenterol. Hepatol. 2021, 17, 254–262. [Google Scholar]
- Screening for Pancreatic Cancer | Cancer Screening, Prevention, Control | JAMA | JAMA Network. Available online: https://jamanetwork.com/journals/jama/fullarticle/2740696 (accessed on 16 April 2024).
- Post, E. Routine Pancreatic Cancer Screening Improves Long-Term Survival for High-Risk Individuals, New Research Shows. Available online: https://pancan.org/stories/routine-pancreatic-cancer-screening-improves-long-term-survival-for-high-risk-individuals-new-research-shows/ (accessed on 16 April 2024).
- Lowe, T.; DeLuca, J.; Abenavoli, L.; Boccuto, L. Familial Pancreatic Cancer: A Case Study and Review of the Psychosocial Effects of Diagnoses on Families. Hered. Cancer Clin. Pract. 2023, 21, 17. [Google Scholar] [CrossRef]
- CancerConnect False-Positive Results Are Common with Cancer Screening. Available online: https://news.cancerconnect.com/ovarian-cancer/false-positive-results-are-common-with-cancer-screening (accessed on 16 April 2024).
- How to Improve the Efficacy of Gastric Cancer Screening? | Current Treatment Options in Gastroenterology. Available online: https://link.springer.com/article/10.1007/s11938-023-00430-4 (accessed on 16 April 2024).
- Karampini, E.; McCaughan, F. Circulating DNA in Solid Organ Cancers-Analysis and Clinical Application. QJM Mon. J. Assoc. Physicians 2016, 109, 223–227. [Google Scholar] [CrossRef]
- Cohen, J.D.; Javed, A.A.; Thoburn, C.; Wong, F.; Tie, J.; Gibbs, P.; Schmidt, C.M.; Yip-Schneider, M.T.; Allen, P.J.; Schattner, M.; et al. Combined Circulating Tumor DNA and Protein Biomarker-Based Liquid Biopsy for the Earlier Detection of Pancreatic Cancers. Proc. Natl. Acad. Sci. USA 2017, 114, 10202–10207. [Google Scholar] [CrossRef] [PubMed]
- Eissa, M.A.L.; Lerner, L.; Abdelfatah, E.; Shankar, N.; Canner, J.K.; Hasan, N.M.; Yaghoobi, V.; Huang, B.; Kerner, Z.; Takaesu, F.; et al. Promoter Methylation of ADAMTS1 and BNC1 as Potential Biomarkers for Early Detection of Pancreatic Cancer in Blood. Clin. Epigenetics 2019, 11, 59. [Google Scholar] [CrossRef]
- Singh, N.; Rashid, S.; Rashid, S.; Dash, N.R.; Gupta, S.; Saraya, A. Clinical Significance of Promoter Methylation Status of Tumor Suppressor Genes in Circulating DNA of Pancreatic Cancer Patients. J. Cancer Res. Clin. Oncol. 2020, 146, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Zhao, Q.; Wang, Z.; Lu, S.; Kang, Y.; Jin, G.; Tian, J. Genome-Wide Analysis of Cell-Free DNA Methylation Profiling for the Early Diagnosis of Pancreatic Cancer. Front. Genet. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T. Earlier Diagnosis of Pancreatic Cancer: Is It Possible? Cancers 2023, 15, 4430. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-J.; Li, H.-Y.; Gu, F. Diagnostic Significance of Serum Osteopontin Level for Pancreatic Cancer: A Meta-Analysis. Genet. Test. Mol. Biomark. 2014, 18, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, W. The Clinical Utility of the CA 19-9 Tumor-Associated Antigen. Am. J. Gastroenterol. 1990, 85, 350–355. [Google Scholar] [PubMed]
- Koprowski, H.; Steplewski, Z.; Mitchell, K.; Herlyn, M.; Herlyn, D.; Fuhrer, P. Colorectal Carcinoma Antigens Detected by Hybridoma Antibodies. Somatic Cell Genet. 1979, 5, 957–971. [Google Scholar] [CrossRef]
- Koprowski, H.; Herlyn, M.; Steplewski, Z.; Sears, H.F. Specific Antigen in Serum of Patients with Colon Carcinoma. Science 1981, 212, 53–55. [Google Scholar] [CrossRef]
- Homma, T.; Tsuchiya, R. The Study of the Mass Screening of Persons without Symptoms and of the Screening of Outpatients with Gastrointestinal Complaints or Icterus for Pancreatic Cancer in Japan, Using CA19-9 and Elastase-1 or Ultrasonography. Int. J. Pancreatol. Off. J. Int. Assoc. Pancreatol. 1991, 9, 119–124. [Google Scholar] [CrossRef]
- Locker, G.Y.; Hamilton, S.; Harris, J.; Jessup, J.M.; Kemeny, N.; Macdonald, J.S.; Somerfield, M.R.; Hayes, D.F.; Bast, R.C.; ASCO. ASCO 2006 Update of Recommendations for the Use of Tumor Markers in Gastrointestinal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 5313–5327. [Google Scholar] [CrossRef] [PubMed]
- CA 19-9 as a Biomarker in Advanced Pancreatic Cancer Patients Randomised to Gemcitabine plus Axitinib or Gemcitabine Alone | British Journal of Cancer. Available online: https://www.nature.com/articles/6605243 (accessed on 13 April 2024).
- Kim, J.-E.; Lee, K.T.; Lee, J.K.; Paik, S.W.; Rhee, J.C.; Choi, K.W. Clinical Usefulness of Carbohydrate Antigen 19-9 as a Screening Test for Pancreatic Cancer in an Asymptomatic Population. J. Gastroenterol. Hepatol. 2004, 19, 182–186. [Google Scholar] [CrossRef] [PubMed]
- CA 19-9: Reference Range, Interpretation, Collection and Panels. 2022. Available online: https://emedicine.medscape.com/article/2087513-overview?form=fpf (accessed on 13 April 2024).
- Koopmann, J.; Fedarko, N.S.; Jain, A.; Maitra, A.; Iacobuzio-Donahue, C.; Rahman, A.; Hruban, R.H.; Yeo, C.J.; Goggins, M. Evaluation of Osteopontin as Biomarker for Pancreatic Adenocarcinoma. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2004, 13, 487–491. [Google Scholar] [CrossRef]
- Kolb, A.; Kleeff, J.; Guweidhi, A.; Esposito, I.; Giese, N.A.; Adwan, H.; Giese, T.; Büchler, M.W.; Berger, M.R.; Friess, H. Osteopontin Influences the Invasiveness of Pancreatic Cancer Cells and Is Increased in Neoplastic and Inflammatory Conditions. Cancer Biol. Ther. 2005, 4, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Rychlíková, J.; Vecka, M.; Jáchymová, M.; Macášek, J.; Hrabák, P.; Zeman, M.; Vávrová, L.; Řoupal, J.; Krechler, T.; Ák, A. Osteopontin as a Discriminating Marker for Pancreatic Cancer and Chronic Pancreatitis. Cancer Biomark. Sect. Dis. Markers 2016, 17, 55–65. [Google Scholar] [CrossRef]
- Murphy, G. Tissue Inhibitors of Metalloproteinases. Genome Biol. 2011, 12, 233. [Google Scholar] [CrossRef]
- Zhai, L.-L.; Cai, C.-Y.; Wu, Y.; Tang, Z.-G. Correlation and Prognostic Significance of MMP-2 and TFPI-2 Differential Expression in Pancreatic Carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 682–691. [Google Scholar]
- Manne, A.; Esnakula, A.; Abushahin, L.; Tsung, A. Understanding the Clinical Impact of MUC5AC Expression on Pancreatic Ductal Adenocarcinoma. Cancers 2021, 13, 3059. [Google Scholar] [CrossRef]
- Andrianifahanana, M.; Moniaux, N.; Schmied, B.M.; Ringel, J.; Friess, H.; Hollingsworth, M.A.; Büchler, M.W.; Aubert, J.P.; Batra, S.K. Mucin (MUC) Gene Expression in Human Pancreatic Adenocarcinoma and Chronic Pancreatitis: A Potential Role of MUC4 as a Tumor Marker of Diagnostic Significance. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2001, 7, 4033–4040. [Google Scholar]
- Nagata, K.; Horinouchi, M.; Saitou, M.; Higashi, M.; Nomoto, M.; Goto, M.; Yonezawa, S. Mucin Expression Profile in Pancreatic Cancer and the Precursor Lesions. J. Hepatobiliary Pancreat. Surg. 2007, 14, 243–254. [Google Scholar] [CrossRef]
- Shekouh, A.R.; Thompson, C.C.; Prime, W.; Campbell, F.; Hamlett, J.; Herrington, C.S.; Lemoine, N.R.; Crnogorac-Jurcevic, T.; Buechler, M.W.; Friess, H.; et al. Application of Laser Capture Microdissection Combined with Two-Dimensional Electrophoresis for the Discovery of Differentially Regulated Proteins in Pancreatic Ductal Adenocarcinoma. Proteomics 2003, 3, 1988–2001. [Google Scholar] [CrossRef]
- Ohuchida, K.; Mizumoto, K.; Ishikawa, N.; Fujii, K.; Konomi, H.; Nagai, E.; Yamaguchi, K.; Tsuneyoshi, M.; Tanaka, M. The Role of S100A6 in Pancreatic Cancer Development and Its Clinical Implication as a Diagnostic Marker and Therapeutic Target. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 7785–7793. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.A.; Akita, H.; Takemasa, I.; Eguchi, H.; Pastural, E.; Nagano, H.; Monden, M.; Doki, Y.; Mori, M.; Jin, W.; et al. Metabolic System Alterations in Pancreatic Cancer Patient Serum: Potential for Early Detection. BMC Cancer 2013, 13, 416. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.A.; Chitou, B.; Zheng, Q.; Jayasinghe, D.; Jin, W.; Mochizuki, A.; Goodenowe, D.B. Pancreatic Cancer Serum Biomarker PC-594: Diagnostic Performance and Comparison to CA19-9. World J. Gastroenterol. 2015, 21, 6604–6612. [Google Scholar] [CrossRef] [PubMed]
- Koopmann, J.; Rosenzweig, C.N.W.; Zhang, Z.; Canto, M.I.; Brown, D.A.; Hunter, M.; Yeo, C.; Chan, D.W.; Breit, S.N.; Goggins, M. Serum Markers in Patients with Resectable Pancreatic Adenocarcinoma: Macrophage Inhibitory Cytokine 1 versus CA19-9. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 442–446. [Google Scholar] [CrossRef]
- Kemik, O.; Purisa, S.; Kemik, A.S.; Tuzun, S. Increase in the Circulating Level of Hepatocyte Growth Factor in Pancreatic Cancer Patients. Bratisl. Lek. Listy 2009, 110, 627–629. [Google Scholar] [PubMed]
- Mroczko, B.; Groblewska, M.; Gryko, M.; Kedra, B.; Szmitkowski, M. Diagnostic Usefulness of Serum Interleukin 6 (IL-6) and C-Reactive Protein (CRP) in the Differentiation between Pancreatic Cancer and Chronic Pancreatitis. J. Clin. Lab. Anal. 2010, 24, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Geng, Y.; Sun, M.; Chen, H.; Wang, P.; Chen, Z. Hyperfibrinogen Is Associated With the Systemic Inflammatory Response and Predicts Poor Prognosis in Advanced Pancreatic Cancer. Pancreas 2015, 44, 977–982. [Google Scholar] [CrossRef]
- Kharaziha, P.; Ceder, S.; Li, Q.; Panaretakis, T. Tumor Cell-Derived Exosomes: A Message in a Bottle. Biochim. Biophys. Acta 2012, 1826, 103–111. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Kalluri, R. Mechanisms Associated with Biogenesis of Exosomes in Cancer. Mol. Cancer 2019, 18, 52. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Angelini, D.F.; Di Raimo, R.; Falchi, M.; Battistini, L.; Fais, S. Microenvironmental pH and Exosome Levels Interplay in Human Cancer Cell Lines of Different Histotypes. Cancers 2018, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, C.; Zhou, T.; Liu, X.; Liu, X.; Li, X.; Chen, D. Role of Exosomal Proteins in Cancer Diagnosis. Mol. Cancer 2017, 16, 145. [Google Scholar] [CrossRef] [PubMed]
- Daamen, L.A.; Molenaar, I.Q.; Groot, V.P. Recent Advances and Future Challenges in Pancreatic Cancer Care: Early Detection, Liquid Biopsies, Precision Medicine and Artificial Intelligence. J. Clin. Med. 2023, 12, 7485. [Google Scholar] [CrossRef] [PubMed]
- Canto, M.I.; Almario, J.A.; Schulick, R.D.; Yeo, C.J.; Klein, A.; Blackford, A.; Shin, E.J.; Sanyal, A.; Yenokyan, G.; Lennon, A.M.; et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-Term Surveillance. Gastroenterology 2018, 155, 740–751.e2. [Google Scholar] [CrossRef] [PubMed]
- Naudin, S.; Viallon, V.; Hashim, D.; Freisling, H.; Jenab, M.; Weiderpass, E.; Perrier, F.; McKenzie, F.; Bueno-de-Mesquita, H.B.; Olsen, A.; et al. Healthy Lifestyle and the Risk of Pancreatic Cancer in the EPIC Study. Eur. J. Epidemiol. 2020, 35, 975–986. [Google Scholar] [CrossRef]
| Genetic Syndrome | Genes Affected | Risk of Pancreatic Cancer |
|---|---|---|
| Peutz–Jeghers Syndrome | STK11 | Relative Risk: 132; Cumulative Risk: 2.4% at age 40, 3.9% at age 50, 11.1% at age 60, and 25.6% at age 70 years |
| Lynch Syndrome | MLH1, MSH2, MSH6, PMS2 | Relative Risk: 5–9; Cumulative Risk: 1.3% by age 50 years, 3.7% by age 70 years |
| Hereditary Breast and Ovarian Cancer | BRCA1, BRCA2, PALB2 | BRCA1: Relative Risk: 2.26; BRCA2: Relative Risk: 3.51 |
| Familial Atypical Multiple Mole Melanoma | CDKN2A | Cumulative Risk: 17% by age 75 years |
| Li–Fraumeni Syndrome | TP53 | Relative Risk: 7.73 |
| Familial Adenomatous Polyposis | APC | Hazard Ratio: 6.45; Relative Risk: 4.46 |
| Ataxia–Telangiectasia | ATM | Relative Risk: 6.5 |
| Cystic Fibrosis | CFTR | Risk Ratio: 5.3 (95% CI: 2.4–10.1) |
| Hereditary Pancreatitis | PRSS1 | Standardized Incidence Ratio: 53 (95% CI: 23–105) Cumulative Risk: 40% by age 70 years |
| Risk Group | AGA Recommendation | ASGE Recommendation | CAPS Recommendation |
|---|---|---|---|
| Familial Pancreatic Cancer | Start screening at age 50 or 10 years younger than the initial age of onset | Start screening at age 50 or 10 years younger than the youngest relative | Start screening at age 50 or 10 years younger than the youngest relative |
| Peutz–Jeghers Syndrome | Start screening at age 35 | Start screening at age 35 | Start screening at age 40 or 10 years younger than youngest relative (Grade 2: probably do it); Start screening at age 30 or 35 (Grade 4: probably do not do it) |
| PRSS1 Mutation | Start screening at age 40 | Start screening at age 40 | Start screening at age 40 |
| CDKN2A Mutation | Start screening at age 40 | Start screening at age 40 | Start screening at age 40 |
| BRCA2 Mutation | Start screening at age 50 or 5 years earlier than high-risk individuals | Start screening at age 50 or 10 years younger than the youngest relative | Start screening at age 50 or 5 years earlier than high-risk individuals |
| ATM Mutation | Start screening at age 50 | Start screening at age 50 or 10 years younger than the youngest relative | Start screening at age 50 |
| PALB2 Mutation | Start screening at age 50 or 5 years earlier than high-risk individuals | Start screening at age 50 or 10 years younger than the youngest relative | Start screening at age 50 or 5 years earlier than high-risk individuals |
| Lynch Syndrome | Start screening at age 50 or 10 years younger than the youngest relative | Start screening at age 50 or 10 years younger than the youngest relative | Not specified |
| TP53 Mutation | Start screening at age 50 or younger depending on family history | Not specified | Not specified |
| Aspect | Efficacy | Limitations |
|---|---|---|
| Screening Modalities | EUS and MRI are primary methods, with EUS showing high sensitivity in detecting anomalies. | Accuracy varies, and no single method is universally effective. |
| Diagnostic Yield | EUS can detect 0 to 68.2 cases of pancreatic adenocarcinoma per 1000 individuals screened. | The yield for CT is lower, with 0 to 12 cases per 1000 individuals. |
| Sensitivity and Specificity | High sensitivity of screening methods ensures most malignant lesions are detected. | High sensitivity may also detect benign conditions, leading to false positives. |
| False-Positive Rates | - | False positives can cause psychological distress and unnecessary healthcare utilization. |
| Long-Term Outcomes | Some studies suggest potential survival benefits for high-risk individuals through screening. | Conflicting results on whether screening significantly improves overall survival rates. |
| Multi-Modal Approach | Description |
|---|---|
| Imaging Techniques | Combining CT scans, MRIs, and endoscopic ultrasound (EUS) to provide a comprehensive view of the pancreas and detect lesions. |
| Biomarker Analysis | Utilizing blood tests for biomarkers like CA 19-9 alongside imaging to improve the detection of pancreatic cancer. |
| Genetic Screening | Incorporating genetic testing for hereditary cancer syndromes that increase the risk of pancreatic cancer. |
| Artificial Intelligence | Applying AI algorithms to analyze imaging and biomarker data for enhanced accuracy in diagnosis. |
| Interdisciplinary Evaluation | A collaborative approach where gastroenterologists, oncologists, radiologists, and geneticists work together to interpret screening results. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, Y.; Dahiya, D.S.; Tiwari, A.; Kumar, H.; Gangwani, M.K.; Ali, H.; Hayat, U.; Alsakarneh, S.; Singh, S.; Malik, S.; et al. Advancements in Early Detection and Screening Strategies for Pancreatic Cancer: From Genetic Susceptibility to Novel Biomarkers. J. Clin. Med. 2024, 13, 4706. https://doi.org/10.3390/jcm13164706
Shah Y, Dahiya DS, Tiwari A, Kumar H, Gangwani MK, Ali H, Hayat U, Alsakarneh S, Singh S, Malik S, et al. Advancements in Early Detection and Screening Strategies for Pancreatic Cancer: From Genetic Susceptibility to Novel Biomarkers. Journal of Clinical Medicine. 2024; 13(16):4706. https://doi.org/10.3390/jcm13164706
Chicago/Turabian StyleShah, Yash, Dushyant Singh Dahiya, Angad Tiwari, Harendra Kumar, Manesh Kumar Gangwani, Hassam Ali, Umar Hayat, Saqr Alsakarneh, Sahib Singh, Sheza Malik, and et al. 2024. "Advancements in Early Detection and Screening Strategies for Pancreatic Cancer: From Genetic Susceptibility to Novel Biomarkers" Journal of Clinical Medicine 13, no. 16: 4706. https://doi.org/10.3390/jcm13164706






