Malnutrition and Allergies: Tipping the Immune Balance towards Health
Abstract
:1. Definition of Malnutrition
1.1. Malabsorption Due to Inflammation and Nutritional Immunity
1.2. Physiological Pathways of Nutrient Absorption
- (1)
- Direct bloodstream absorption: Simple sugars [37] (glucose, fructose), amino acids (from digested proteins), short-chain fatty acids SCFA and medium-chain fatty acids MCFA, water-soluble vitamin C, vitamin Bs (except B12) and folate [38], and most minerals (iron, calcium, potassium) are readily absorbed into the bloodstream and enter via the portal vein the liver.
- (2)
- Lymphatic absorption: Fat and fat-soluble compounds (including fat-soluble vitamin A, D, E, and K) are taken up via the lymphatic vessels [39]. These nutrients are emulsified in micelles composed of bile salts absorbed by the enterocytes and packed into chylomicrons [40,41]. Chylomicrons are composed primarily of triglycerides, phospholipids, cholesterol, and lipoproteins and enter the bloodstream near the collarbone.
- (3)
- Receptor-specific uptake: Digestion-resistant proteins serve as carriers for nutrients (iron [2,42,43,44,45,46,47,48,49,50,51], calcium/magnesium [52,53], vitamins [43,44,47], carbohydrates [54,55], phenolics [48,49,50,56], and lipids [57,58,59]). This receptor-specific uptake occurs often, typically exploiting the lacteals—the jejunal lymphatic vessels [60]—as shown with the absorption of milk proteins (whey) [61] and egg proteins [62,63] but also for plant-derived food, such as soy [64,65] and nut proteins [64,65].
2. General Immunological Implications of Malnutrition (Protein and Micronutrients): A Shift towards Th2
2.1. Malnutrition and the Thymus
2.2. The Importance of Allergenic Proteins: Saviors or Dangers
| Protein Families | Structure | Examples | Known Ligands | Origin | Function | Ref |
|---|---|---|---|---|---|---|
| Pathogenesis-related class seed storage protein | small protein with antiparallel beta-strands and alpha-helices | Bet v 1, Pru a 1, Mal d 1, Fra a 1 | phytohormones, siderophores, flavonoids, alkaloids | plants | Pathogenesis-related proteins (PRPs): signature genes for systemic acquired resistance in plants Microbicidic, Kunitz type of protease inhibitor | [154,155,156,157,158,159,160] |
| Ole e 1 Family | β-barrel fold, stabilized by 3 disulfide bond, heat stable | Ole e 1, Pla l 1, Che a 1 | 2+ metals | plants | Pollen tube development, leave senescence Activated under ROS induction, contribute to antioxidant production, plant defense responses. | [135,161,162,163,164] |
| nsLTPs seed storage proteins Prolamin superfamily | cysteine-rich alpha-helical; rich in proline and glutamine | Pru p 3, Ara h 9, Fra a 3 | Fatty acids, phospholipids | plants | Antimicrobial, lipid utilization, plant stress Regulate FAO, binds to calmodulin (central hub in calcium-dependent cellular regulation) | [154,165] |
| 2S albumin (conglutin) seed storage proteins prolamin superfamily | small cysteine-rich, alpha-helical protein | Ara h 2, Ber e 1, Ses I 1, Gly m 8 | phenolics | plants | Nutrient reservoir, regulate germination Antimicrobial, stress response [166] | [154,165] |
| Albumin 2S seed storage | hemopexin-like fold no disulphide bonds thermostabile, ß-propeller | heme, spermidine thiamine | plant | Stress response, antioxidative, agglutinate erythrocytes; peroxidase activity or heme binding Seed germination | [167,168,169] | |
| cereal prolamins prolamin superfamily | alpha-helical, conserved cystein-skeleton; rich in proline and glutamine | Tri a 19 (wheat), Sec c 20 Hor v 21 | copper, sugars, fats, phenolics | plants | Nutrient reservoir, regulate germination Antimicrobial, stress response | [166,170] |
| prolamin alpha-amylase inhibitors [171] | alpha-helical, cystein-rich | Tri a 28 Hor v 15 | calcium | plants | Antimicrobial, stress response Inhibit exogenous insect amylases | [172] |
| 7S/vicilin CUPIN | beta-barrel core [165] | Ara h 1, Jug r 2, Ses I 3 | copper, sugars, fats, phenolics | plants | Nutrient reservoir, regulate germination, Antimicrobial, stress response | [154,165,166] |
| 11S/legumin-like CUPIN | beta-barrel core | Ara h 3, Ber e 2, Ses i 6 | copper, sugars, fats, phenolics | plants | Nutrient reservoir, regulate germination Antimicrobial, stress response [166] | [154,165] |
| Lipocalins | symmetrical β-barrel fold, | Can f 1, Fel d 4, Bos d 5 | siderophores, phenolics, vitamin, heme products | animal | Stress response, microbicidic, nutritional immunity | [66,173,174] |
| Serum Albumin | globular, several long α helices | Fel d 2, Gal d 5, Can f 3, Equ c 3 | Cu2+, Zn2+, hormones, vitamins, minerals, drugs, hemin | animal | Carrier protein, nutritional immunity Negative acute phase protein Anti-inflammatory | [175,176,177] |
| Parvalbumin | calcium-binding, long α helices, EF-hand superfamily | Cyp c 1, Gad c 1 | Ca2+, phosphatidylcholine, phospatidylethanolamine | animal | Calcium buffer, immunomodulatory Protective against reactive oxygen species, antibacterial | [178,179] |
| Tropomyosin | two-chained, α-helical coiled coil protein | Bla g 7, Lep s 1, Der f 10 | actin | animal | Regulates stress fiber assembly Regulatea calcium-dependent interaction of actin/myosin during muscle contraction Host defense, immunomodulatory | [180,181,182] |
| Uteroglobin | homodimeric, alpha helical strucure linked by disulfide bonde | Fel d 1, Ory c 3 | phosphatidylcholine, phosphatidylinositol, polychlorinated, steroids, environmental toxins progesterone | animal | Anti-inflammatory, antioxidant Inhibitor of phospholipase A2 Increased vulnerability to oxygen toxicity in uteroglobin-knock-out mice, defects in uteroglobin are associated with a susceptibility to asthma; protects epithelial linings | [183,184] |
| NPC2 proteins MD-2-related lipid recognition family | immunoglobulin-like β-sandwich fold | Der p 2, Gly d 2, Tyr p 2 | lipids cholesterol other sterols, LPS | animal | Crucial for cholesterol transport and utilization | |
| Arginin Kinase | mainly α-helical | Bla g 9, Pen m 2, Der p 20 | ATP and L-arginine phosphoarginine | animal | Immunomodulatory, stress response Storage of phosphoarginine Cell signaling, apoptosis | [185,186,187] |
2.3. Iron Deficiency
- Iron deficiency/functional iron deficiency
- Anemia/absolute iron deficiency
2.3.1. Iron Deficiency Shifts the System toward Th2
2.3.2. Cell-Specific Alterations under Iron Deficiency
Macrophages
T Cells
IgE Antibodies
Epithelial Cells and Hair
Mast Cells and Eosinophils
2.4. Vitamin A Deficiency
2.4.1. Vitamin A Deficiency Results in Type 2 Inflammation
2.4.2. Cell-Specific Alterations under Vitamin A Deficiency
Macrophages
Lymphoid Cells
Epithelial Cells
Mast Cells
3. Malnutrition in Allergic Diseases
3.1. Iron Deficiency/Anemia and Atopic Diseases
3.1.1. Iron Interventions
3.1.2. Improving the Bioavailability of Iron
3.2. Vitamin A Deficiency and Atopic Diseases
Improving the Bioavailability of Vitamin A
4. Nutrition to Prevent Allergies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Valentino, D.; Sriram, K.; Shankar, P. Update on micronutrients in bariatric surgery. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Fakhimahmadi, A.; Hasanaj, I.; Hofstetter, G.; Pogner, C.; Gorfer, M.; Wiederstein, M.; Szepannek, N.; Bianchini, R.; Dvorak, Z.; Jensen, S.A.; et al. Nutritional Provision of Iron Complexes by the Major Allergen Alt a 1 to Human Immune Cells Decreases Its Presentation. Int. J. Mol. Sci. 2023, 24, 11934. [Google Scholar] [CrossRef]
- Crook, J.; Horgas, A.; Yoon, S.J.; Grundmann, O.; Johnson-Mallard, V. Insufficient Vitamin C Levels among Adults in the United States: Results from the NHANES Surveys, 2003–2006. Nutrients 2021, 13, 3910. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Reider, C.; Brooks, J.R.; Fulgoni, V.L., 3rd. Comparison of prevalence of inadequate nutrient intake based on body weight status of adults in the United States: An analysis of NHANES 2001-2008. J. Am. Coll. Nutr. 2015, 34, 126–134. [Google Scholar] [CrossRef]
- Higgins, K.A.; Bi, X.; Davis, B.J.; Barraj, L.M.; Scrafford, C.G.; Murphy, M.M. Adequacy of total usual micronutrient intakes among pregnant women in the United States by level of dairy consumption, NHANES 2003–2016. Nutr. Health 2022, 28, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Barker, L.A.; Gout, B.S.; Crowe, T.C. Hospital malnutrition: Prevalence, identification and impact on patients and the healthcare system. Int. J. Environ. Res. Public Health 2011, 8, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Lo Buglio, A.; Quiete, S.; Vendemiale, G. Malnutrition in Hospitalized Old Patients: Screening and Diagnosis, Clinical Outcomes, and Management. Nutrients 2022, 14, 910. [Google Scholar] [CrossRef] [PubMed]
- La Torre, M.; Ziparo, V.; Nigri, G.; Cavallini, M.; Balducci, G.; Ramacciato, G. Malnutrition and pancreatic surgery: Prevalence and outcomes. J. Surg. Oncol. 2013, 107, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Schreinemachers, P.; Shrestha, R.M.; Gole, B.; Bhattarai, D.R.; Ghimire, P.L.; Subedi, B.P.; Bruck, T.; Baliki, G.; Gautam, I.P.; Blake, C.E. Drivers of Food Choice among Children and Caregivers in Post-earthquake Nepal. Ecol. Food Nutr. 2021, 60, 826–846. [Google Scholar] [CrossRef]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Berni Canani, R.; O’Mahony, L.; Peroni, D.; Sokolowska, M.; Vassilopoulou, E.; Venter, C. Nutrition in chronic inflammatory conditions: Bypassing the mucosal block for micronutrients. Allergy 2024, 79, 353–383. [Google Scholar] [CrossRef]
- Ejaz, S.; Nasim, F.U.; Ashraf, M.; Ahmad, S. Serum Proteome Profiling to Identify Proteins Promoting Pathogenesis of Non-atopic Asthma. Protein Pept. Lett. 2018, 25, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.E.; Hansen, J.L.; Sauer, B.C.; Cheung, A.K.; Agarwal, A.; Greene, T. Heart Failure Hospitalization Risk associated with Iron Status in Veterans with CKD. Clin. J. Am. Soc. Nephrol. 2021, 16, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Guedes, M.; Muenz, D.; Zee, J.; Lopes, M.B.; Waechter, S.; Stengel, B.; Massy, Z.A.; Speyer, E.; Ayav, C.; Finkelstein, F.; et al. Serum biomarkers of iron stores are associated with worse physical health-related quality of life in nondialysis-dependent chronic kidney disease patients with or without anemia. Nephrol. Dial. Transplant. 2021, 36, 1694–1703. [Google Scholar] [CrossRef]
- Kalra, P.R.; Cleland, J.G.F.; Petrie, M.C.; Thomson, E.A.; Kalra, P.A.; Squire, I.B.; Ahmed, F.Z.; Al-Mohammad, A.; Cowburn, P.J.; Foley, P.W.X.; et al. Intravenous ferric derisomaltose in patients with heart failure and iron deficiency in the UK (IRONMAN): An investigator-initiated, prospective, randomised, open-label, blinded-endpoint trial. Lancet 2022, 400, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Cizman, B.; Carroll, K.; McMurray, J.J.V.; Perkovic, V.; Jha, V.; Johansen, K.L.; Lopes, R.D.; Macdougall, I.C.; Obrador, G.T.; et al. Efficacy and Safety of Daprodustat for Treatment of Anemia of Chronic Kidney Disease in Incident Dialysis Patients: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 592–602. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; von Haehling, S.; Kalra, P.R.; Court, E.; Bhandari, S.; McDonagh, T.; Cleland, J.G.F. Safety and Efficacy of Intravenous Ferric Derisomaltose Compared to Iron Sucrose for Iron Deficiency Anemia in Patients with Chronic Kidney Disease With and Without Heart Failure. Am. J. Cardiol. 2021, 152, 138–145. [Google Scholar] [CrossRef]
- Pisani, A.; Riccio, E.; Sabbatini, M.; Andreucci, M.; Del Rio, A.; Visciano, B. Effect of oral liposomal iron versus intravenous iron for treatment of iron deficiency anaemia in CKD patients: A randomized trial. Nephrol. Dial. Transplant. 2015, 30, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Liu, C.Y.; Lee, H.T.; Tsai, K.; Lin, Y.C.; Tarng, D.C.; Ho, C.H.; Lin, H.Y. Effects of intravenous iron saccharate on improving severe anemia in rheumatoid arthritis patients. Clin. Rheumatol. 2012, 31, 469–477. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Yuan, L.; Guo, L. Iron Deficiency, a Risk Factor of Thyroid Disorders in Reproductive-Age and Pregnant Women: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 629831. [Google Scholar] [CrossRef]
- Kisaoglu, H.; Baba, O.; Kalyoncu, M. Hematologic manifestations of juvenile systemic lupus erythematosus: An emphasis on anemia. Lupus 2022, 31, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Agarwal, P.; Wakhlu, A.; Kumar, A.; Mehrotra, R.; Mittal, S. Anaemia in Systemic Lupus Erythematosus Based on Iron Studies and Soluble Transferrin Receptor Levels. J. Clin. Diagn. Res. 2016, 10, EC08–EC11. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Chu, K.A.; Lin, M.C.; Chu, Y.H.; Hung, Y.M.; Wei, J.C. Newly diagnosed iron deficiency anemia and subsequent autoimmune disease: A matched cohort study in Taiwan. Curr. Med. Res. Opin. 2020, 36, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Maas, L.A.; Krishna, M.; Parian, A.M. Ironing It All Out: A Comprehensive Review of Iron Deficiency Anemia in Inflammatory Bowel Disease Patients. Dig. Dis. Sci. 2022, 68, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.; Sinopoulou, V.; Iheozor-Ejiofor, Z.; Iqbal, T.; Allen, P.; Hoque, S.; Engineer, J.; Akobeng, A.K. Interventions for treating iron deficiency anaemia in inflammatory bowel disease. Cochrane Database Syst. Rev. 2021, 1, CD013529. [Google Scholar] [CrossRef] [PubMed]
- Wyart, E.; Hsu, M.Y.; Sartori, R.; Mina, E.; Rausch, V.; Pierobon, E.S.; Mezzanotte, M.; Pezzini, C.; Bindels, L.B.; Lauria, A.; et al. Iron supplementation is sufficient to rescue skeletal muscle mass and function in cancer cachexia. EMBO Rep. 2022, 23, e53746. [Google Scholar] [CrossRef]
- Ludwig, H.; Evstatiev, R.; Kornek, G.; Aapro, M.; Bauernhofer, T.; Buxhofer-Ausch, V.; Fridrik, M.; Geissler, D.; Geissler, K.; Gisslinger, H.; et al. Iron metabolism and iron supplementation in cancer patients. Wien. Klin. Wochenschr. 2015, 127, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Escobar Alvarez, Y.; de Las Penas Bataller, R.; Perez Altozano, J.; Ros Martinez, S.; Sabino Alvarez, A.; Blasco Cordellat, A.; Brozos Vazquez, E.; Corral Jaime, J.; Garcia Escobar, I.; Beato Zambrano, C. SEOM clinical guidelines for anaemia treatment in cancer patients (2020). Clin. Transl. Oncol. 2021, 23, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Wright, K.; Vieira, M.C.; Chong, K.W.; Chatchatee, P.; Vlieg-Boerstra, B.J.; Groetch, M.; Dominguez-Ortega, G.; Heath, S.; Lang, A.; et al. International survey on growth indices and impacting factors in children with food allergies. J. Hum. Nutr. Diet. 2019, 32, 175–184. [Google Scholar] [CrossRef]
- Bartosik, T.; Jensen, S.A.; Afify, S.M.; Bianchini, R.; Hufnagl, K.; Hofstetter, G.; Berger, M.; Bastl, M.; Berger, U.; Rivelles, E.; et al. Ameliorating Atopy by Compensating Micronutritional Deficiencies in Immune Cells: A Double-Blind Placebo-Controlled Pilot Study. J. Allergy Clin. Immunol. Pract. 2022, 10, 1889–1902. [Google Scholar] [CrossRef]
- Petje, L.M.; Jensen, S.A.; Szikora, S.; Sulzbacher, M.; Bartosik, T.; Pjevac, P.; Hausmann, B.; Hufnagl, K.; Untersmayr, E.; Fischer, L.; et al. Functional iron-deficiency in women with allergic rhinitis is associated with symptoms after nasal provocation and lack of iron-sequestering microbes. Allergy 2021, 76, 2882–2886. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Kameda, M. Assessment of the Correlation Between Mother and Child Body Mass Index and Mother and Child Diet in Children With Food Allergies. J. Clin. Med. Res. 2019, 11, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, M.D.; Braaten, T.; Obstfelder, A.; Abelsen, B. Self-Reported Food Hypersensitivity: Prevalence, Characteristics, and Comorbidities in the Norwegian Women and Cancer Study. PLoS ONE 2016, 11, e0168653. [Google Scholar] [CrossRef]
- Mohn, E.S.; Johnson, E.J. Nutrient absorption in the human gastrointestinal tract. In Nanotechnology and Functional Foods; Wiley: Hoboken, NJ, USA, 2015; pp. 3–34. [Google Scholar]
- Basile, E.; Launico, M.; Sheer, A. Physiology, Nutrient Absorption. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Guillen, B.; Atherton, N.S. Short Bowel Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Steenbergen, J.M.; Lash, J.M.; Bohlen, H.G. Role of a lymphatic system in glucose absorption and the accompanying microvascular hyperemia. Am. J. Physiol. 1994, 267 Pt 1, G529–G535. [Google Scholar] [CrossRef]
- Rose, R.C. Intestinal absorption of water-soluble vitamins. Proc. Soc. Exp. Biol. Med. 1996, 212, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kvietys, P.R.; Granger, D.N. Role of intestinal lymphatics in interstitial volume regulation and transmucosal water transport. Ann. N. Y. Acad. Sci. 2010, 1207 (Suppl. S1), E29–E43. [Google Scholar] [CrossRef] [PubMed]
- Varsamis, N.; Christou, G.A.; Derdemezis, C.; Tselepis, A.; Kiortsis, D. The Associations of Dietary Vitamin K Intake and Circulating Vitamin 25(OH)D with Serum Lipoprotein Levels: The Vitamin Deficiency Matters. Horm. Metab. Res. 2023, 55, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, M.; Salomon, A.; Saupe, J.; Shearer, M.J. Transport of Vitamin K to Bone in Humans. J. Nutr. 1996, 126, 1192S–1196S. [Google Scholar] [CrossRef] [PubMed]
- Afify, S.M.; Pali-Scholl, I.; Hufnagl, K.; Hofstetter, G.; El-Bassuoni, M.A.-R.; Roth-Walter, F.; Jensen-Jarolim, E. Bovine Holo-Beta-Lactoglobulin Cross-Protects Against Pollen Allergies in an Innate Manner in BALB/c Mice: Potential Model for the Farm Effect. Front. Immunol. 2021, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Hufnagl, K.; Afify, S.M.; Braun, N.; Wagner, S.; Wallner, M.; Hauser, M.; Wiederstein, M.; Gadermaier, G.; Wildner, S.; Redegeld, F.A.; et al. Retinoic acid-loading of the major birch pollen allergen Bet v 1 may improve specific allergen immunotherapy: In silico, in vitro and in vivo data in BALB/c mice. Allergy 2020, 75, 2073–2077. [Google Scholar] [CrossRef]
- Hufnagl, K.; Ghosh, D.; Wagner, S.; Fiocchi, A.; Dahdah, L.; Bianchini, R.; Braun, N.; Steinborn, R.; Hofer, M.; Blaschitz, M.; et al. Retinoic acid prevents immunogenicity of milk lipocalin Bos d 5 through binding to its immunodominant T-cell epitope. Sci. Rep. 2018, 8, 1598. [Google Scholar] [CrossRef]
- Hufnagl, K.; Jensen-Jarolim, E. Vitamin A and D in allergy: From experimental animal models and cellular studies to human disease. Allergo J. Int. 2018, 27, 72–78. [Google Scholar] [CrossRef]
- Hufnagl, K.; Jensen-Jarolim, E. Does a carrot a day keep the allergy away? Immunol. Lett. 2019, 206, 54–58. [Google Scholar] [CrossRef]
- Hufnagl, K.; Kromp, L.; Bianchini, R.; Afify, S.M.; Wiederstein, M.; Redegeld, F.A.; Zuvalova, I.; Dvorak, Z.; Hofstetter, G.; Roth-Walter, F.; et al. Bet v 1 from birch pollen is a hypoallergen with vitamin D3 in the pocket. Allergy 2021, 76, 3801–3804. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Afify, S.M.; Pacios, L.F.; Blokhuis, B.R.; Redegeld, F.; Regner, A.; Petje, L.M.; Fiocchi, A.; Untersmayr, E.; Dvorak, Z.; et al. Cow’s milk protein beta-lactoglobulin confers resilience against allergy by targeting complexed iron into immune cells. J. Allergy Clin. Immunol. 2021, 147, 321–334 e324. [Google Scholar] [CrossRef] [PubMed]
- Roth-Walter, F.; Gomez-Casado, C.; Pacios, L.F.; Mothes-Luksch, N.; Roth, G.A.; Singer, J.; Diaz-Perales, A.; Jensen-Jarolim, E. Bet v 1 from Birch Pollen is a Lipocalin-like Protein acting as Allergen only when devoid of Iron by promoting Th2 lymphocytes. J. Biol. Chem. 2014, 289, 17416–17421. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Pacios, L.F.; Gomez-Casado, C.; Hofstetter, G.; Roth, G.A.; Singer, J.; Diaz-Perales, A.; Jensen-Jarolim, E. The major cow milk allergen Bos d 5 manipulates T-helper cells depending on its load with siderophore-bound iron. PLoS ONE 2014, 9, e104803. [Google Scholar] [CrossRef]
- Ghatak, S.K.; Majumdar, D.; Singha, A.; Sen, S.; Das, D.; Chakrabarti, A.; Mukhopadhyay, C.; Sen, K. Peanut protein sensitivity towards trace iron: A novel mode to ebb allergic response. Food Chem. 2015, 176, 308–313. [Google Scholar] [CrossRef]
- Zolfaghari Emameh, R.; Masoori, L.; Nosrati, H.; Falak, R.; Parkkila, S. Identification and characterization of the first fish parvalbumin-like protein data from a pathogenic fungal species, Trichophyton violaceum. Data Brief. 2020, 33, 106420. [Google Scholar] [CrossRef] [PubMed]
- Cantillo, J.F.; Puerta, L.; Lafosse-Marin, S.; Subiza, J.L.; Caraballo, L.; Fernandez-Caldas, E. Allergens involved in the cross-reactivity of Aedes aegypti with other arthropods. Ann. Allergy Asthma Immunol. 2017, 118, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.; Kitchen, B.J.; Winzor, D.J. Use of affinity chromatography for the quantitative study of acceptor-ligand interactions: The lactose synthetase system. Biochem. J. 1973, 135, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Kronman, M.J.; Bratcher, S.C. Conformational changes induced by zinc and terbium binding to native bovine alpha-lactalbumin and calcium-free alpha-lactalbumin. J. Biol. Chem. 1984, 259, 10887–10895. [Google Scholar] [CrossRef] [PubMed]
- Regner, A.; Szepannek, N.; Wiederstein, M.; Fakhimahmadi, A.; Paciosis, L.F.; Blokhuis, B.R.; Redegeld, F.A.; Hofstetter, G.; Dvorak, Z.; Jensen-Jarolim, E.; et al. Binding to Iron Quercetin Complexes Increases the Antioxidant Capacity of the Major Birch Pollen Allergen Bet v 1 and Reduces Its Allergenicity. Antioxidants 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gao, Y.; Sun, S.; Qu, Y.; Ji, S.; Wu, R.; Wu, J. Formation, characterization, and antigenicity of lecithin-beta-conglycinin complexes. Food Chem. 2023, 407, 135178. [Google Scholar] [CrossRef] [PubMed]
- Bublin, M.; Eiwegger, T.; Breiteneder, H. Do lipids influence the allergic sensitization process? J. Allergy Clin. Immunol. 2014, 134, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Matveevskaya, N.S.; Ignatova, A.A.; Toropygin, I.Y.; Ovchinnikova, T.V. Impact of Different Lipid Ligands on the Stability and IgE-Binding Capacity of the Lentil Allergen Len c 3. Biomolecules 2020, 10, 1668. [Google Scholar] [CrossRef]
- Rubas, W.; Grass, G.M. Gastrointestinal lymphatic absorption of peptides and proteins. Adv. Drug Deliv. Rev. 1991, 7, 15–69. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Berin, M.C.; Arnaboldi, P.; Escalante, C.R.; Dahan, S.; Rauch, J.; Jensen-Jarolim, E.; Mayer, L. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer’s patches. Allergy 2008, 63, 882–890. [Google Scholar] [CrossRef]
- Kilshaw, P.J.; Cant, A.J. The passage of maternal dietary proteins into human breast milk. Int. Arch. Allergy Appl. Immunol. 1984, 75, 8–15. [Google Scholar] [CrossRef]
- Wang, Y.; Ghoshal, S.; Ward, M.; de Villiers, W.; Woodward, J.; Eckhardt, E. Chylomicrons promote intestinal absorption and systemic dissemination of dietary antigen (ovalbumin) in mice. PLoS ONE 2009, 4, e8442. [Google Scholar] [CrossRef]
- Cohn, J.S.; Kamili, A.; Wat, E.; Chung, R.W.; Tandy, S. Reduction in intestinal cholesterol absorption by various food components: Mechanisms and implications. Atheroscler. Suppl. 2010, 11, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Tang, L.; de Villiers, W.J.; Cohen, D.; Woodward, J.; Finkelman, F.D.; Eckhardt, E.R. Dietary medium-chain triglycerides promote oral allergic sensitization and orally induced anaphylaxis to peanut protein in mice. J. Allergy Clin. Immunol. 2013, 131, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Roth-Walter, F. Iron-Deficiency in Atopic Diseases: Innate Immune Priming by Allergens and Siderophores. Front. Allergy 2022, 3, 859922. [Google Scholar] [CrossRef] [PubMed]
- Kalach, N.; Benhamou, P.H.; Campeotto, F.; Dupont, C. Anemia impairs small intestinal absorption measured by intestinal permeability in children. Eur. Ann. Allergy Clin. Immunol. 2007, 39, 20–22. [Google Scholar] [PubMed]
- Cousins, R.J. Toward a molecular understanding of zinc metabolism. Clin. Physiol. Biochem. 1986, 4, 20–30. [Google Scholar] [PubMed]
- Hennigar, S.R.; McClung, J.P. Hepcidin Attenuates Zinc Efflux in Caco-2 Cells. J. Nutr. 2016, 146, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Halsted, C.H.; Reisenauer, A.M.; Shane, B.; Tamura, T. Availability of monoglutamyl and polyglutamyl folates in normal subjects and in patients with coeliac sprue. Gut 1978, 19, 886–891. [Google Scholar] [CrossRef]
- Sobczynska-Malefora, A.; Delvin, E.; McCaddon, A.; Ahmadi, K.R.; Harrington, D.J. Vitamin B(12) status in health and disease: A critical review. Diagnosis of deficiency and insufficiency-clinical and laboratory pitfalls. Crit. Rev. Clin. Lab. Sci. 2021, 58, 399–429. [Google Scholar] [CrossRef] [PubMed]
- Kather, S.; Kacza, J.; Pfannkuche, H.; Bottcher, D.; Sung, C.H.; Steiner, J.M.; Gabel, G.; Dengler, F.; Heilmann, R.M. Expression of the cobalamin transporters cubam and MRP1 in the canine ileum-Upregulation in chronic inflammatory enteropathy. PLoS ONE 2024, 19, e0296024. [Google Scholar] [CrossRef]
- van den Berg, H.; van der Gaag, M.; Hendriks, H. Influence of lifestyle on vitamin bioavailability. Int. J. Vitam. Nutr. Res. 2002, 72, 53–59. [Google Scholar] [CrossRef]
- Weiss, D.; Brunk, D.K.; Goodman, D.A. Scottsdale Magnesium Study: Absorption, Cellular Uptake, and Clinical Effectiveness of a Timed-Release Magnesium Supplement in a Standard Adult Clinical Population. J. Am. Coll. Nutr. 2018, 37, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Turnlund, J.R.; Keyes, W.R.; Hudson, C.A.; Betschart, A.A.; Kretsch, M.J.; Sauberlich, H.E. A stable-isotope study of zinc, copper, and iron absorption and retention by young women fed vitamin B-6-deficient diets. Am. J. Clin. Nutr. 1991, 54, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.P.; Ross, A.C.; Stephensen, C.B.; Bohn, T.; Tanumihardjo, S.A. Metabolic Effects of Inflammation on Vitamin A and Carotenoids in Humans and Animal Models. Adv. Nutr. 2017, 8, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef] [PubMed]
- Docherty, K.F.; Welsh, P.; Verma, S.; De Boer, R.A.; O’Meara, E.; Bengtsson, O.; Kober, L.; Kosiborod, M.N.; Hammarstedt, A.; Langkilde, A.M.; et al. Iron Deficiency in Heart Failure and Effect of Dapagliflozin: Findings From DAPA-HF. Circulation 2022, 146, 980–994. [Google Scholar] [CrossRef]
- O’Brien, M.E.; Kupka, R.; Msamanga, G.I.; Saathoff, E.; Hunter, D.J.; Fawzi, W.W. Anemia is an independent predictor of mortality and immunologic progression of disease among women with HIV in Tanzania. J. Acquir. Immune Defic. Syndr. 2005, 40, 219–225. [Google Scholar] [CrossRef]
- Pries-Heje, M.M.; Hasselbalch, R.B.; Wiingaard, C.; Fosbol, E.L.; Glenthoj, A.B.; Ihlemann, N.; Gill, S.U.A.; Christiansen, U.; Elming, H.; Bruun, N.E.; et al. Severity of anaemia and association with all-cause mortality in patients with medically managed left-sided endocarditis. Heart 2022, 108, 882–888. [Google Scholar] [CrossRef]
- Baye, K.; Laillou, A.; Seyoum, Y.; Zvandaziva, C.; Chimanya, K.; Nyawo, M. Estimates of child mortality reductions attributed to vitamin A supplementation in sub-Saharan Africa: Scale up, scale back, or refocus? Am. J. Clin. Nutr. 2022, 116, 426–434. [Google Scholar] [CrossRef]
- Basu, S.; Khanna, P.; Srivastava, R.; Kumar, A. Oral vitamin A supplementation in very low birth weight neonates: A randomized controlled trial. Eur. J. Pediatr. 2019, 178, 1255–1265. [Google Scholar] [CrossRef]
- Glasziou, P.P.; Mackerras, D.E. Vitamin A supplementation in infectious diseases: A meta-analysis. BMJ 1993, 306, 366–370. [Google Scholar] [CrossRef]
- Wang, W.; Gao, J.; Li, N.; Han, S.; Wu, L.; Zhang, Y.; Han, T.; Shan, R.; Li, Y.; Sun, C.; et al. Dietary iron and vitamins in association with mortality. Clin. Nutr. 2021, 40, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, H.; Qi, G.; Tian, W. Nutrient deficiency patterns and all-cause and cardiovascular mortality in older adults with hypertension: A latent class analysis. BMC Public Health 2024, 24, 1551. [Google Scholar] [CrossRef] [PubMed]
- Beisel, W.R. History of nutritional immunology: Introduction and overview. J. Nutr. 1992, 122 (Suppl. S3), 591–596. [Google Scholar] [CrossRef] [PubMed]
- Simon, J. A Physiological Essay on the Thymus Gland. Br. Foreign Med. Rev. 1845, 20, 159–167. [Google Scholar]
- Savino, W.; Duraes, J.; Maldonado-Galdeano, C.; Perdigon, G.; Mendes-da-Cruz, D.A.; Cuervo, P. Thymus, undernutrition, and infection: Approaching cellular and molecular interactions. Front. Nutr. 2022, 9, 948488. [Google Scholar] [CrossRef] [PubMed]
- Lyra, J.S.; Madi, K.; Maeda, C.T.; Savino, W. Thymic extracellular matrix in human malnutrition. J. Pathol. 1993, 171, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Rytter, M.J.; Kolte, L.; Briend, A.; Friis, H.; Christensen, V.B. The immune system in children with malnutrition—A systematic review. PLoS ONE 2014, 9, e105017. [Google Scholar] [CrossRef]
- Nabukeera-Barungi, N.; Lanyero, B.; Grenov, B.; Friis, H.; Namusoke, H.; Mupere, E.; Michaelsen, K.F.; Molgaard, C.; Wiese, M.; Nielsen, D.S.; et al. Thymus size and its correlates among children admitted with severe acute malnutrition: A cross-sectional study in Uganda. BMC Pediatr. 2021, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Lunin, S.M.; Khrenov, M.O.; Novoselova, T.V.; Parfenyuk, S.B.; Novoselova, E.G. Thymulin, a thymic peptide, prevents the overproduction of pro-inflammatory cytokines and heat shock protein Hsp70 in inflammation-bearing mice. Immunol. Investig. 2008, 37, 858–870. [Google Scholar] [CrossRef]
- Novoselova, E.G.; Lunin, S.M.; Glushkova, O.V.; Khrenov, M.O.; Parfenyuk, S.B.; Zakharova, N.M.; Fesenko, E.E. Thymulin, free or bound to PBCA nanoparticles, protects mice against chronic septic inflammation. PLoS ONE 2018, 13, e0197601. [Google Scholar] [CrossRef]
- Chandra, R.K. Protein-energy malnutrition and immunological responses. J. Nutr. 1992, 122, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Pallaro, A.N.; Roux, M.E.; Slobodianik, N.H. Nutrition disorders and immunologic parameters: Study of the thymus in growing rats. Nutrition 2001, 17, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Woodward, B.; Chandra, R.K. Involution of thymic epithelium and low serum thymulin bioactivity in weanling mice subjected to severe food intake restriction or severe protein deficiency. Exp. Mol. Pathol. 1988, 48, 226–235. [Google Scholar] [CrossRef] [PubMed]
- de Castro, E.S.; Boyd, E.M. Organ weights and water content of rats fed protein-deficient diets. Bull. World Health Organ. 1968, 38, 971–977. [Google Scholar] [PubMed]
- Kuvibidila, S.; Warrier, R.P. Differential effects of iron deficiency and underfeeding on serum levels of interleukin-10, interleukin-12p40, and interferon-gamma in mice. Cytokine 2004, 26, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Kuvibidila, S.R.; Porretta, C.; Surendra Baliga, B.; Leiva, L.E. Reduced thymocyte proliferation but not increased apoptosis as a possible cause of thymus atrophy in iron-deficient mice. Br. J. Nutr. 2001, 86, 157–162. [Google Scholar] [CrossRef]
- Fraker, P.J.; Jardieu, P.; Cook, J. Zinc deficiency and immune function. Arch. Dermatol. 1987, 123, 1699–1701. [Google Scholar] [CrossRef]
- Petrault, I.; Zimowska, W.; Mathieu, J.; Bayle, D.; Rock, E.; Favier, A.; Rayssiguier, Y.; Mazur, A. Changes in gene expression in rat thymocytes identified by cDNA array support the occurrence of oxidative stress in early magnesium deficiency. Biochim. Biophys. Acta 2002, 1586, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Malpuech-Brugere, C.; Nowacki, W.; Gueux, E.; Kuryszko, J.; Rock, E.; Rayssiguier, Y.; Mazur, A. Accelerated thymus involution in magnesium-deficient rats is related to enhanced apoptosis and sensitivity to oxidative stress. Br. J. Nutr. 1999, 81, 405–411. [Google Scholar] [CrossRef]
- Kubena, K.S.; Cohill, D.T.; McMurray, D.N. Effect of varying levels of magnesium during gestation and lactation on humoral immune response and tissue minerals in rats. Ann. Nutr. Metab. 1989, 33, 7–14. [Google Scholar] [CrossRef]
- Uchio, R.; Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Ishigami, A. High dietary intake of vitamin C suppresses age-related thymic atrophy and contributes to the maintenance of immune cells in vitamin C-deficient senescence marker protein-30 knockout mice. Br. J. Nutr. 2015, 113, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Stoerk, H.C. Effects of calcium deficiency and pyridoxin deficiency on thymic atrophy (accidental involution). Proc. Soc. Exp. Biol. Med. 1946, 62, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Razali, N.; Hohjoh, H.; Inazumi, T.; Maharjan, B.D.; Nakagawa, K.; Konishi, M.; Sugimoto, Y.; Hasegawa, H. Induced Prostanoid Synthesis Regulates the Balance between Th1- and Th2-Producing Inflammatory Cytokines in the Thymus of Diet-Restricted Mice. Biol. Pharm. Bull. 2020, 43, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Skeie, E.; Tangvik, R.J.; Nymo, L.S.; Harthug, S.; Lassen, K.; Viste, A. Weight loss and BMI criteria in GLIM’s definition of malnutrition is associated with postoperative complications following abdominal resections–Results from a National Quality Registry. Clin. Nutr. 2020, 39, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, L.; Wang, H.; Wang, X.; Qu, G.; Cai, J.; Zhang, H. Malnutrition is associated with hyperinflammation and immunosuppression in COVID-19 patients: A prospective observational study. Nutr. Clin. Pract. 2021, 36, 863–871. [Google Scholar] [CrossRef]
- Pan, Y.P.; Kuo, H.C.; Lin, J.Y.; Chou, W.C.; Chang, P.H.; Ling, H.H.; Yeh, K.Y. Serum Cytokines Correlate with Pretreatment Body Mass Index-adjusted Body Weight Loss Grading and Cancer Progression in Patients with Stage III Esophageal Squamous Cell Carcinoma Undergoing Neoadjuvant Chemoradiotherapy Followed by Surgery. Nutr. Cancer 2024, 76, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Takele, Y.; Adem, E.; Getahun, M.; Tajebe, F.; Kiflie, A.; Hailu, A.; Raynes, J.; Mengesha, B.; Ayele, T.A.; Shkedy, Z.; et al. Malnutrition in Healthy Individuals Results in Increased Mixed Cytokine Profiles, Altered Neutrophil Subsets and Function. PLoS ONE 2016, 11, e0157919. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.; Gonzalez, C.; Flores, L.; Jimenez-Zamudio, L.; Graniel, J.; Ortiz, R. Assessment by flow cytometry of cytokine production in malnourished children. Clin. Diagn. Lab. Immunol. 2005, 12, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Sapartini, G.; Wong, G.W.K.; Indrati, A.R.; Kartasasmita, C.B.; Setiabudiawan, B. Stunting as a Risk Factor for Asthma: The Role of Vitamin D, Leptin, IL-4, and CD23. Medicina 2022, 58, 1236. [Google Scholar] [CrossRef]
- Mrimi, E.C.; Palmeirim, M.S.; Minja, E.G.; Long, K.Z.; Keiser, J. Correlation of Cytokines with Parasitic Infections, Undernutrition and Micronutrient Deficiency among Schoolchildren in Rural Tanzania: A Cross-Sectional Study. Nutrients 2023, 15, 1916. [Google Scholar] [CrossRef] [PubMed]
- Fock, R.A.; Vinolo, M.A.; Crisma, A.R.; Nakajima, K.; Rogero, M.M.; Borelli, P. Protein-energy malnutrition modifies the production of interleukin-10 in response to lipopolysaccharide (LPS) in a murine model. J. Nutr. Sci. Vitaminol. 2008, 54, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martinez, H.; Rodriguez, L.; Najera, O.; Cruz, D.; Miliar, A.; Dominguez, A.; Sanchez, F.; Graniel, J.; Gonzalez-Torres, M.C. Expression of cytokine mRNA in lymphocytes of malnourished children. J. Clin. Immunol. 2008, 28, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Steevels, T.A.; Hillyer, L.M.; Monk, J.M.; Fisher, M.E.; Woodward, B.D. Effector/memory T cells of the weanling mouse exhibit Type 2 cytokine polarization in vitro and in vivo in the advanced stages of acute energy deficit. J. Nutr. Biochem. 2010, 21, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.A.; Thoen, J.; Reseland, J.E.; Forre, O.; Kjeldsen-Kragh, J. Decreased CD4+ lymphocyte activation and increased interleukin-4 production in peripheral blood of rheumatoid arthritis patients after acute starvation. Clin. Rheumatol. 1999, 18, 394–401. [Google Scholar] [CrossRef]
- Hagel, I.; Lynch, N.R.; Di Prisco, M.C.; Sanchez, J.; Perez, M. Nutritional status and the IgE response against Ascaris lumbricoides in children from a tropical slum. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 562–565. [Google Scholar] [CrossRef] [PubMed]
- de Melo, J.F.; da Costa, T.B.; da Costa Lima, T.D.; Chaves, M.E.; Vayssade, M.; Nagel, M.D.; de Castro, C.M. Long-term effects of a neonatal low-protein diet in rats on the number of macrophages in culture and the expression/production of fusion proteins. Eur. J. Nutr. 2013, 52, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.A.; Houdijk, J.G.; Sakkas, P.; Bruce, A.D.; Mitchell, M.; Knox, D.P.; Kyriazakis, I. Dissecting the impact of protein versus energy host nutrition on the expression of immunity to gastrointestinal parasites during lactation. Int. J. Parasitol. 2011, 41, 711–719. [Google Scholar] [CrossRef]
- Fakhimahmadi, A.; Roth-Walter, F.; Hofstetter, G.; Wiederstein, M.; Jensen, S.A.; Berger, M.; Szepannek, N.; Bianchini, R.; Pali-Scholl, I.; Jensen-Jarolim, E.; et al. Mould allergen Alt a 1 spiked with the micronutrient retinoic acid reduces Th2 response and ameliorates Alternaria allergy in BALB/c mice. Allergy 2024, 79, 2144–2156. [Google Scholar] [CrossRef]
- Menezes, J.S.; Mucida, D.S.; Cara, D.C.; Alvarez-Leite, J.I.; Russo, M.; Vaz, N.M.; de Faria, A.M. Stimulation by food proteins plays a critical role in the maturation of the immune system. Int. Immunol. 2003, 15, 447–455. [Google Scholar] [CrossRef]
- Pearce, S.C.; Nisley, M.J.; Kerr, B.J.; Sparks, C.; Gabler, N.K. Effects of dietary protein level on intestinal function and inflammation in nursery pigs. J. Anim. Sci. 2024, 102, skae077. [Google Scholar] [CrossRef]
- Dewan, P.; Kaur, I.R.; Faridi, M.M.; Agarwal, K.N. Cytokine response to dietary rehabilitation with curd (Indian dahi) & leaf protein concentrate in malnourished children. Indian. J. Med. Res. 2009, 130, 31–36. [Google Scholar] [PubMed]
- Wang, L.C.; Chiang, B.L.; Huang, Y.M.; Shen, P.T.; Huang, H.Y.; Lin, B.F. Lower vitamin D levels in the breast milk is associated with atopic dermatitis in early infancy. Pediatr. Allergy Immunol. 2020, 31, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Grover, Z.; Ee, L.C. Protein energy malnutrition. Pediatr. Clin. N. Am. 2009, 56, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Missaoui, K.; Gonzalez-Klein, Z.; Pazos-Castro, D.; Hernandez-Ramirez, G.; Garrido-Arandia, M.; Brini, F.; Diaz-Perales, A.; Tome-Amat, J. Plant non-specific lipid transfer proteins: An overview. Plant Physiol. Biochem. 2022, 171, 115–127. [Google Scholar] [CrossRef]
- Shewry, P.R.; Beaudoin, F.; Jenkins, J.; Griffiths-Jones, S.; Mills, E.N. Plant protein families and their relationships to food allergy. Biochem. Soc. Trans. 2002, 30 Pt 6, 906–910. [Google Scholar] [CrossRef]
- Vassilopoulou, E.; Zuidmeer, L.; Akkerdaas, J.; Tassios, I.; Rigby, N.R.; Mills, E.N.; van Ree, R.; Saxoni-Papageorgiou, P.; Papadopoulos, N.G. Severe immediate allergic reactions to grapes: Part of a lipid transfer protein-associated clinical syndrome. Int. Arch. Allergy Immunol. 2007, 143, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Radauer, C.; Breiteneder, H. Evolutionary biology of plant food allergens. J. Allergy Clin. Immunol. 2007, 120, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Afify, S.M.; Regner, A.; Pacios, L.F.; Blokhuis, B.R.; Jensen, S.A.; Redegeld, F.A.; Pali-Scholl, I.; Hufnagl, K.; Bianchini, R.; Guethoff, S.; et al. Micronutritional supplementation with a holoBLG-based FSMP (food for special medical purposes)-lozenge alleviates allergic symptoms in BALB/c mice: Imitating the protective farm effect. Clin. Exp. Allergy 2022, 52, 426–441. [Google Scholar] [CrossRef] [PubMed]
- Chruszcz, M.; Chew, F.T.; Hoffmann-Sommergruber, K.; Hurlburt, B.K.; Mueller, G.A.; Pomes, A.; Rouvinen, J.; Villalba, M.; Wohrl, B.M.; Breiteneder, H. Allergens and their associated small molecule ligands-their dual role in sensitization. Allergy 2021, 76, 2367–2382. [Google Scholar] [CrossRef]
- Min, J.; Foo, A.C.Y.; Gabel, S.A.; Perera, L.; DeRose, E.F.; Pomes, A.; Pedersen, L.C.; Mueller, G.A. Structural and ligand binding analysis of the pet allergens Can f 1 and Fel d 7. Front. Allergy 2023, 4, 1133412. [Google Scholar] [CrossRef]
- Li, M.; Gustchina, A.; Alexandratos, J.; Wlodawer, A.; Wunschmann, S.; Kepley, C.L.; Chapman, M.D.; Pomes, A. Crystal structure of a dimerized cockroach allergen Bla g 2 complexed with a monoclonal antibody. J. Biol. Chem. 2008, 283, 22806–22814. [Google Scholar] [CrossRef] [PubMed]
- Stemeseder, T.; Freier, R.; Wildner, S.; Fuchs, J.E.; Briza, P.; Lang, R.; Batanero, E.; Lidholm, J.; Liedl, K.R.; Campo, P.; et al. Crystal structure of Pla l 1 reveals both structural similarity and allergenic divergence within the Ole e 1-like protein family. J. Allergy Clin. Immunol. 2017, 140, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Bakan, B.; Hamberg, M.; Larue, V.; Prange, T.; Marion, D.; Lascombe, M.B. The crystal structure of oxylipin-conjugated barley LTP1 highlights the unique plasticity of the hydrophobic cavity of these plant lipid-binding proteins. Biochem. Biophys. Res. Commun. 2009, 390, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, J.E.; Wimmer, R.; Larsen, J.N.; Spangfort, M.D.; Otzen, D.E. The major birch allergen, Bet v 1, shows affinity for a broad spectrum of physiological ligands. J. Biol. Chem. 2002, 277, 23684–23692. [Google Scholar] [CrossRef]
- Han, G.W.; Lee, J.Y.; Song, H.K.; Chang, C.; Min, K.; Moon, J.; Shin, D.H.; Kopka, M.L.; Sawaya, M.R.; Yuan, H.S.; et al. Structural basis of non-specific lipid binding in maize lipid-transfer protein complexes revealed by high-resolution X-ray crystallography1 1Edited by D. Rees. J. Mol. Biol. 2001, 308, 263–278. [Google Scholar] [CrossRef]
- Seutter von Loetzen, C.; Hoffmann, T.; Hartl, M.J.; Schweimer, K.; Schwab, W.; Rosch, P.; Hartl-Spiegelhauer, O. Secret of the major birch pollen allergen Bet v 1: Identification of the physiological ligand. Biochem. J. 2014, 457, 379–390. [Google Scholar] [CrossRef]
- Jacob, T.; von Loetzen, C.S.; Reuter, A.; Lacher, U.; Schiller, D.; Schobert, R.; Mahler, V.; Vieths, S.; Rosch, P.; Schweimer, K.; et al. Identification of a natural ligand of the hazel allergen Cor a 1. Sci. Rep. 2019, 9, 8714. [Google Scholar] [CrossRef] [PubMed]
- Casanal, A.; Zander, U.; Dupeux, F.; Valpuesta, V.; Marquez, J.A. Purification, crystallization and preliminary X-ray analysis of the strawberry allergens Fra a 1E and Fra a 3 in the presence of catechin. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69 Pt 5, 510–2514. [Google Scholar] [CrossRef]
- Vesic, J.; Stambolic, I.; Apostolovic, D.; Milcic, M.; Stanic-Vucinic, D.; Cirkovic Velickovic, T. Complexes of green tea polyphenol, epigalocatechin-3-gallate, and 2S albumins of peanut. Food Chem. 2015, 185, 309–317. [Google Scholar] [CrossRef]
- Structure of PR 10 Allergen Ara h 8.01 with Quercetin [Internet]. RCSB PDB. 2022. Available online: https://www.ebi.ac.uk/pdbe/entry/pdb/6aws (accessed on 19 January 2022).
- Hurlburt, B.K.; Offermann, L.R.; McBride, J.K.; Majorek, K.A.; Maleki, S.J.; Chruszcz, M. Structure and function of the peanut panallergen Ara h 8. J. Biol. Chem. 2013, 288, 36890–36901. [Google Scholar] [CrossRef]
- van Boxtel, E.L.; van den Broek, L.A.; Koppelman, S.J.; Vincken, J.P.; Gruppen, H. Peanut allergen Ara h 1 interacts with proanthocyanidins into higher molecular weight complexes. J. Agric. Food Chem. 2007, 55, 8772–8778. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Zhang, Y.; McClements, D.J.; Zhou, W.; Dai, T.; Wu, Z.; Chen, H. Investigation of peanut allergen-procyanidin non-covalent interactions: Impact on protein structure and in vitro allergenicity. Int. J. Biol. Macromol. 2024, 258 Pt 1, 128340. [Google Scholar] [CrossRef]
- Tong, P.; Gao, L.; Gao, J.; Li, X.; Wu, Z.; Yang, A.; Chen, H. Iron-induced chelation alleviates the potential allergenicity of ovotransferrin in a BALB/c mouse model. Nutr. Res. 2017, 47, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Song, C.Y.; Chen, W.L.; Yang, M.C.; Huang, J.P.; Mao, S.J. Epitope mapping of a monoclonal antibody specific to bovine dry milk: Involvement of residues 66-76 of strand D in thermal denatured beta-lactoglobulin. J. Biol. Chem. 2005, 280, 3574–3582. [Google Scholar] [CrossRef] [PubMed]
- Zurera-Cosano, G.; Moreno-Rojas, R.; Amaro-Lopez, M. Effect of processing on contents and relationships of mineral elements of milk. Food Chem. 1994, 51, 75–78. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Gomez-Casado, C.; Jensen-Jarolim, E.; Diaz Perales, A.; Pacios, L.F.; Singer, J. Method and Means for Diagnosing and Treating Allergy Using Lipocalin Levels. EP2894478A1, 13 January 2014. [Google Scholar]
- Buhot, N.; Douliez, J.P.; Jacquemard, A.; Marion, D.; Tran, V.; Maume, B.F.; Milat, M.L.; Ponchet, M.; Mikes, V.; Kader, J.C.; et al. A lipid transfer protein binds to a receptor involved in the control of plant defence responses. FEBS Lett. 2001, 509, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.F.; Uriel, N.; Teifoori, F.; Postigo, I.; Sunen, E.; Martinez, J. The major Alternaria alternata allergen, Alt a 1: A reliable and specific marker of fungal contamination in citrus fruits. Int. J. Food Microbiol. 2017, 257, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Roth-Walter, F.; Schmutz, R.; Mothes-Luksch, N.; Lemell, P.; Zieglmayer, P.; Zieglmayer, R.; Jensen-Jarolim, E. Clinical efficacy of sublingual immunotherapy is associated with restoration of steady-state serum lipocalin 2 after SLIT: A pilot study. World Allergy Organ. J. 2018, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, X.; Lu, C.; Zeng, X.; Li, Y.; Fu, D.; Wu, G. Non-specific lipid transfer proteins in plants: Presenting new advances and an integrated functional analysis. J. Exp. Bot. 2015, 66, 5663–5681. [Google Scholar] [CrossRef]
- Zehra, A.; Raytekar, N.A.; Meena, M.; Swapnil, P. Efficiency of microbial bio-agents as elicitors in plant defense mechanism under biotic stress: A review. Curr. Res. Microb. Sci. 2021, 2, 100054. [Google Scholar] [CrossRef]
- Liu, G.; Greenshields, D.L.; Sammynaiken, R.; Hirji, R.N.; Selvaraj, G.; Wei, Y. Targeted alterations in iron homeostasis underlie plant defense responses. J. Cell Sci. 2007, 120 Pt 4, 596–605. [Google Scholar] [CrossRef]
- Aglas, L.; Soh, W.T.; Kraiem, A.; Wenger, M.; Brandstetter, H.; Ferreira, F. Ligand Binding of PR-10 Proteins with a Particular Focus on the Bet v 1 Allergen Family. Curr. Allergy Asthma Rep. 2020, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Casañal, A.; Zander, U.; Muñoz, C.; Dupeux, F.; Luque, I.; Botella, M.A.; Schwab, W.; Valpuesta, V.; Marquez, J.A. The Strawberry Pathogenesis-related 10 (PR-10) Fra a Proteins Control Flavonoid Biosynthesis by Binding to Metabolic Intermediates. J. Biol. Chem. 2013, 288, 35322–35332. [Google Scholar] [CrossRef]
- Montejano-Ramírez, V.; Valencia-Cantero, E. Cross-Talk between Iron Deficiency Response and Defense Establishment in Plants. Int. J. Mol. Sci. 2023, 24, 6236. [Google Scholar] [CrossRef]
- Longsaward, R.; Viboonjun, U. Genome-wide identification of rubber tree pathogenesis-related 10 (PR-10) proteins with biological relevance to plant defense. Sci. Rep. 2024, 14, 1072. [Google Scholar] [CrossRef]
- Gasser, M.; Alloisio, N.; Fournier, P.; Balmand, S.; Kharrat, O.; Tulumello, J.; Carro, L.; Heddi, A.; Da Silva, P.; Normand, P.; et al. A Nonspecific Lipid Transfer Protein with Potential Functions in Infection and Nodulation. Mol. Plant Microbe Interact. 2022, 35, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.E.; Darwish, N.I.; Garcia-Sanchez, J.; Tyagi, N.; Trick, H.N.; McCormick, S.; Dill-Macky, R.; Tumer, N.E. A Lipid Transfer Protein has Antifungal and Antioxidant Activity and Suppresses Fusarium Head Blight Disease and DON Accumulation in Transgenic Wheat. Phytopathology 2021, 111, 671–683. [Google Scholar] [CrossRef]
- Dervisi, I.; Petropoulos, O.; Agalou, A.; Podia, V.; Papandreou, N.; Iconomidou, V.A.; Haralampidis, K.; Roussis, A. The SAH7 Homologue of the Allergen Ole e 1 Interacts with the Putative Stress Sensor SBP1 (Selenium-Binding Protein 1) in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 3580. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalez, M.; Gonzalez-Fernandez, E.; Fernandez-Gonzalez, D.; Rodriguez-Rajo, F.J. Secondary Outcomes of the Ole e 1 Proteins Involved in Pollen Tube Development: Impact on Allergies. Front. Plant Sci. 2020, 11, 974. [Google Scholar] [CrossRef]
- Jain, A. Seed Storage Protein, Functional Diversity and Association with Allergy. Allergies 2023, 3, 25–38. [Google Scholar] [CrossRef]
- Souza, P.F.N. The forgotten 2S albumin proteins: Importance, structure, and biotechnological application in agriculture and human health. Int. J. Biol. Macromol. 2020, 164, 4638–4649. [Google Scholar] [CrossRef] [PubMed]
- Gaur, V.; Qureshi, I.A.; Singh, A.; Chanana, V.; Salunke, D.M. Crystal Structure and Functional Insights of Hemopexin Fold Protein from Grass Pea. Plant Physiol. 2010, 152, 1842–1850. [Google Scholar] [CrossRef]
- Sharma, S.C.; Kumar, A.; Vashisht, S.; Salunke, D.M. High resolution structural and functional analysis of a hemopexin motif protein from Dolichos. Sci. Rep. 2019, 9, 19828. [Google Scholar] [CrossRef] [PubMed]
- Scarafoni, A.; Gualtieri, E.; Barbiroli, A.; Carpen, A.; Negri, A.; Duranti, M. Biochemical and Functional Characterization of an Albumin Protein Belonging to the Hemopexin Superfamily from Lens culinaris Seeds. J. Agric. Food Chem. 2011, 59, 9637–9644. [Google Scholar] [CrossRef] [PubMed]
- Phakela, K.; van Biljon, A.; Wentzel, B.; Guzman, C.; Labuschagne, M.T. Gluten protein response to heat and drought stress in durum wheat as measured by reverse phase-High performance liquid chromatography. J. Cereal Sci. 2021, 100, 103267. [Google Scholar] [CrossRef]
- Pastorello, E.A.; Farioli, L.; Conti, A.; Pravettoni, V.; Bonomi, S.; Iametti, S.; Fortunato, D.; Scibilia, J.; Bindslev-Jensen, C.; Ballmer-Weber, B.; et al. Wheat IgE-mediated food allergy in European patients: Alpha-amylase inhibitors, lipid transfer proteins and low-molecular-weight glutenins. Allergenic molecules recognized by double-blind, placebo-controlled food challenge. Int. Arch. Allergy Immunol. 2007, 144, 10–22. [Google Scholar] [CrossRef] [PubMed]
- El-latif, A.O.A.; Mohieldeen, N.; Salman, A.M.A.; Elpidina, E.N. Isolation and purification of α-amylase inhibitors and their in vitro and in vivo effects on Tribolium castaneum (Herbst) and Callosobruchus maculatus (F.). J. Plant Prot. Res. 2020, 60, 377–387. [Google Scholar] [CrossRef]
- Huang, S.W.; Lim, S.K.; Yu, Y.A.; Pan, Y.C.; Lien, W.J.; Mou, C.Y.; Hu, C.J.; Mou, K.Y. Overcoming the nutritional immunity by engineering iron-scavenging bacteria for cancer therapy. Elife 2024, 12, RP90798. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Pacios, L.F.; Bianchini, R.; Jensen-Jarolim, E. Linking iron-deficiency with allergy: Role of molecular allergens and the microbiome. Metallomics 2017, 9, 1676–1692. [Google Scholar] [CrossRef] [PubMed]
- Bal, W.; Sokołowska, M.; Kurowska, E.; Faller, P. Binding of transition metal ions to albumin: Sites, affinities and rates. Biochim. Biophys. Acta (BBA)-General. Subj. 2013, 1830, 5444–5455. [Google Scholar] [CrossRef]
- Nira, N.H.; Hoque, M.R.; Khan, S.R.; Ferdausee, M.; Momo, F.R. Status of C-reactive protein, Serum Albumin and Serum Zinc in Hospital Admitted Patients with Chronic Kidney Disease. Mymensingh Med. J. 2024, 33, 1–8. [Google Scholar] [PubMed]
- Gremese, E.; Bruno, D.; Varriano, V.; Perniola, S.; Petricca, L.; Ferraccioli, G. Serum Albumin Levels: A Biomarker to Be Repurposed in Different Disease Settings in Clinical Practice. J. Clin. Med. 2023, 12, 6017. [Google Scholar] [CrossRef] [PubMed]
- Permyakov, E.A.; Uversky, V.N. What Is Parvalbumin for? Biomolecules 2022, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- Permyakov, E.A.; Kreimer, D.I.; Kalinichenko, L.P.; Orlova, A.A.; Shnyrov, V.L. Interactions of parvalbumins with model phospholipid vesicles. Cell Calcium. 1989, 10, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, M.; Haseeb, M.; Hu, R.; Ali, H.; Memon, M.A.; Yan, R.; Xu, L.; Song, X.; Zhu, X.; Li, X. Tropomyosin: An Excretory/Secretory Protein from Haemonchus contortus Mediates the Immuno-Suppressive Potential of Goat Peripheral Blood Mononuclear Cells In Vitro. Vaccines 2020, 8, 109. [Google Scholar] [CrossRef]
- Gateva, G.; Kremneva, E.; Reindl, T.; Kotila, T.; Kogan, K.; Gressin, L.; Gunning, P.W.; Manstein, D.J.; Michelot, A.; Lappalainen, P. Tropomyosin Isoforms Specify Functionally Distinct Actin Filament Populations In Vitro. Curr. Biol. 2017, 27, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, C.; Rapetti, A.; Aliani, M.; Castagneto, C.; Corso, N.; Landi, M.; Lietti, D.; Murante, N.; Muratore, L.; Russello, M.; et al. Food allergy as defined by component resolved diagnosis. Recent Pat. Inflamm. Allergy Drug Discov. 2014, 8, 59–73. [Google Scholar] [CrossRef]
- Uhlen, M.; Karlsson, M.J.; Hober, A.; Svensson, A.S.; Scheffel, J.; Kotol, D.; Zhong, W.; Tebani, A.; Strandberg, L.; Edfors, F.; et al. The human secretome. Sci. Signal 2019, 12, eaaz0274. [Google Scholar] [CrossRef]
- SCGB1A1 [Internet]. The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000149021-SCGB1A1 (accessed on 6 July 2024).
- Qiu, Y.; Yang, X.; Wang, L.; Gao, K.; Jiang, Z. L-Arginine Inhibited Inflammatory Response and Oxidative Stress Induced by Lipopolysaccharide via Arginase-1 Signaling in IPEC-J2 Cells. Int. J. Mol. Sci. 2019, 20, 1800. [Google Scholar] [CrossRef]
- Gomez-Yanes, A.C.; Moreno-Cordova, E.N.; Garcia-Orozco, K.D.; Laino, A.; Islas-Osuna, M.A.; Lopez-Zavala, A.A.; Valenzuela, J.G.; Sotelo-Mundo, R.R. The Arginine Kinase from the Tick Rhipicephalus sanguineus Is an Efficient Biocatalyst. Catalysts 2022, 12, 1178. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, Z.; Gu, X.; Xie, Y.; He, R.; Xu, J.; Jing, B.; Peng, X.; Yang, G. Immunomodulatory effects of two recombinant arginine kinases in Sarcoptes Scabiei on host peripheral blood mononuclear cells. Front. Immunol. 2022, 13, 1035729. [Google Scholar] [CrossRef]
- Otero, G.A.; Pliego-Rivero, F.B.; Porcayo-Mercado, R.; Mendieta-Alcantara, G. Working memory impairment and recovery in iron deficient children. Clin. Neurophysiol. 2008, 119, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- West, K.P., Jr.; Shamim, A.A.; Mehra, S.; Labrique, A.B.; Ali, H.; Shaikh, S.; Klemm, R.D.; Wu, L.S.; Mitra, M.; Haque, R.; et al. Effect of maternal multiple micronutrient vs iron-folic acid supplementation on infant mortality and adverse birth outcomes in rural Bangladesh: The JiVitA-3 randomized trial. JAMA 2014, 312, 2649–2658. [Google Scholar] [CrossRef] [PubMed]
- Tada, A.; Nagai, T.; Koya, T.; Nakao, M.; Ishizaka, S.; Mizuguchi, Y.; Aoyagi, H.; Imagawa, S.; Tokuda, Y.; Takahashi, M.; et al. Applicability of new proposed criteria for iron deficiency in Japanese patients with heart failure. ESC Heart Fail. 2023, 10, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Schwarz, F.; Sadlon, A.; Abderhalden, L.A.; de Godoi Rezende Costa Molino, C.; Spahn, D.R.; Schaer, D.J.; Orav, E.J.; Egli, A.; Bischoff-Ferrari, H.A.; et al. Iron deficiency and biomarkers of inflammation: A 3-year prospective analysis of the DO-HEALTH trial. Aging Clin. Exp. Res. 2021, 34, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Huang, J.; Zhu, T.; Lin, S.; Hao, C.; Zhang, M. Functional iron deficiency anemia was associated with higher mortality in chronic kidney disease patients: The NHANES III follow-up study. Ren. Fail. 2023, 45, 2290926. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, C.; Reinhold, J.; Gruner-Hegge, N.; Kydd, A.; Bhagra, S.; Parameshwar, K.J.; Lewis, C.; Martinez, L.; Pettit, S.J. Prognostic value of three iron deficiency definitions in patients with advanced heart failure. Eur. J. Heart Fail. 2023, 25, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Schrage, B.; Rubsamen, N.; Schulz, A.; Munzel, T.; Pfeiffer, N.; Wild, P.S.; Beutel, M.; Schmidtmann, I.; Lott, R.; Blankenberg, S.; et al. Iron deficiency is a common disorder in general population and independently predicts all-cause mortality: Results from the Gutenberg Health Study. Clin. Res. Cardiol. 2020, 109, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Kulik-Rechberger, B.; Dubel, M. Iron deficiency, iron deficiency anaemia and anaemia of inflammation—An overview. Ann. Agric. Environ. Med. 2024, 31, 151–157. [Google Scholar] [CrossRef]
- Gedfie, S.; Getawa, S.; Melku, M. Prevalence and Associated Factors of Iron Deficiency and Iron Deficiency Anemia Among Under-5 Children: A Systematic Review and Meta-Analysis. Glob. Pediatr. Health 2022, 9, 2333794X221110860. [Google Scholar] [CrossRef]
- Baum, P.; Toyka, K.V.; Bluher, M.; Kosacka, J.; Nowicki, M. Inflammatory Mechanisms in the Pathophysiology of Diabetic Peripheral Neuropathy (DN)-New Aspects. Int. J. Mol. Sci. 2021, 22, 10835. [Google Scholar] [CrossRef] [PubMed]
- Nyakeriga, A.M.; Williams, T.N.; Marsh, K.; Wambua, S.; Perlmann, H.; Perlmann, P.; Grandien, A.; Troye-Blomberg, M. Cytokine mRNA expression and iron status in children living in a malaria endemic area. Scand. J. Immunol. 2005, 61, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Helmby, H.; Kullberg, M.; Troye-Blomberg, M. Expansion of IL-3-responsive IL-4-producing non-B non-T cells correlates with anemia and IL-3 production in mice infected with blood-stage Plasmodium chabaudi malaria. Eur. J. Immunol. 1998, 28, 2559–2570. [Google Scholar] [CrossRef]
- Jason, J.; Archibald, L.K.; Nwanyanwu, O.C.; Bell, M.; Jensen, R.J.; Gunter, E.; Buchanan, I.; Larned, J.; Kazembe, P.N.; Dobbie, H.; et al. The effects of iron deficiency on lymphocyte cytokine production and activation: Preservation of hepatic iron but not at all cost. Clin. Exp. Immunol. 2001, 126, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Pita-Rodriguez, G.M.; Chavez-Chong, C.; Lambert-Lamazares, B.; Montero-Diaz, M.; Selgas-Lizano, R.; Basabe-Tuero, B.; Alfonso-Sague, K.; Diaz-Sanchez, M.E. Influence of Inflammation on Assessing Iron-Deficiency Anemia in Cuban Preschool Children. MEDICC Rev. 2021, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Dhankar, N.; Gupta, R.; Jain, S.L.; Mandal, S.; Sarkar, B. Perturbation of monocyte subsets in iron-deficient children-a shift to a pro-inflammatory state? Allergol. Immunopathol. 2021, 49, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Munoz, C.; Olivares, M.; Schlesinger, L.; Lopez, M.; Letelier, A. Increased in vitro tumour necrosis factor-alpha production in iron deficiency anemia. Eur. Cytokine Netw. 1994, 5, 401–404. [Google Scholar] [PubMed]
- Thorson, J.A.; Smith, K.M.; Gomez, F.; Naumann, P.W.; Kemp, J.D. Role of iron in T cell activation: TH1 clones differ from TH2 clones in their sensitivity to inhibition of DNA synthesis caused by IgG Mabs against the transferrin receptor and the iron chelator deferoxamine. Cell. Immunol. 1991, 134, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; Etaati, Z.; Rezvani Joibari, M.; Sobhani, S.A.; Hosseni Tashnizi, S. Immune deviation in recurrent vulvovaginal candidiasis: Correlation with iron deficiency anemia. Iran. J. Immunol. 2013, 10, 118–126. [Google Scholar]
- Winn, N.C.; Volk, K.M.; Hasty, A.H. Regulation of tissue iron homeostasis: The macrophage “ferrostat”. JCI Insight 2020, 5, e132964. [Google Scholar] [CrossRef]
- Corna, G.; Campana, L.; Pignatti, E.; Castiglioni, A.; Tagliafico, E.; Bosurgi, L.; Campanella, A.; Brunelli, S.; Manfredi, A.A.; Apostoli, P.; et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica 2010, 95, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Lanser, L.; Plaikner, M.; Schroll, A.; Burkert, F.R.; Seiwald, S.; Fauser, J.; Petzer, V.; Bellmann-Weiler, R.; Fritsche, G.; Tancevski, I.; et al. Tissue iron distribution in patients with anemia of inflammation: Results of a pilot study. Am. J. Hematol. 2023, 98, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Lanser, L.; Plaikner, M.; Fauser, J.; Petzer, V.; Denicolo, S.; Haschka, D.; Neuwirt, H.; Stefanow, K.; Rudnicki, M.; Kremser, C.; et al. Tissue Iron Distribution in Anemic Patients with End-Stage Kidney Disease: Results of a Pilot Study. J. Clin. Med. 2024, 13, 3487. [Google Scholar] [CrossRef]
- Denz, H.; Huber, P.; Landmann, R.; Orth, B.; Wachter, H.; Fuchs, D. Association between the activation of macrophages, changes of iron metabolism and the degree of anaemia in patients with malignant disorders. Eur. J. Haematol. 1992, 48, 244–248. [Google Scholar] [CrossRef]
- Oexle, H.; Gnaiger, E.; Weiss, G. Iron-dependent changes in cellular energy metabolism: Influence on citric acid cycle and oxidative phosphorylation. Biochim. Biophys. Acta 1999, 1413, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.L. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr. 2001, 131, 568S–579S, discussion 580S. [Google Scholar] [CrossRef] [PubMed]
- Hallquist, N.A.; McNeil, L.K.; Lockwood, J.F.; Sherman, A.R. Maternal-iron-deficiency effects on peritoneal macrophage and peritoneal natural-killer-cell cytotoxicity in rat pups. Am. J. Clin. Nutr. 1992, 55, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Littwitz-Salomon, E.; Moreira, D.; Frost, J.N.; Choi, C.; Liou, K.T.; Ahern, D.K.; O’Shaughnessy, S.; Wagner, B.; Biron, C.A.; Drakesmith, H.; et al. Metabolic requirements of NK cells during the acute response against retroviral infection. Nat. Commun. 2021, 12, 5376. [Google Scholar] [CrossRef] [PubMed]
- Dickson, K.B.; Zhou, J. Role of reactive oxygen species and iron in host defense against infection. FBL 2020, 25, 1600–1616. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Adcock, I.M.; Benito-Villalvilla, C.; Bianchini, R.; Bjermer, L.; Caramori, G.; Cari, L.; Chung, K.F.; Diamant, Z.; Eguiluz-Gracia, I.; et al. Metabolic pathways in immune senescence and inflammaging: Novel therapeutic strategy for chronic inflammatory lung diseases. An EAACI position paper from the Task Force for Immunopharmacology. Allergy 2024, 79, 1089–1122. [Google Scholar] [CrossRef]
- Aly, S.S.; Fayed, H.M.; Ismail, A.M.; Abdel Hakeem, G.L. Assessment of peripheral blood lymphocyte subsets in children with iron deficiency anemia. BMC Pediatr. 2018, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Saha, K.; Mukhopadhyay, D.; Roy, S.; Raychaudhuri, G.; Chatterjee, M.; Mitra, P.K. Impact of iron deficiency anemia on cell-mediated and humoral immunity in children: A case control study. J. Nat. Sci. Biol. Med. 2014, 5, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Hileti, D.; Panayiotidis, P.; Hoffbrand, A.V. Iron chelators induce apoptosis in proliferating cells. Br. J. Haematol. 1995, 89, 181–187. [Google Scholar] [CrossRef]
- Ni, S.; Yuan, Y.; Kuang, Y.; Li, X. Iron Metabolism and Immune Regulation. Front. Immunol. 2022, 13, 816282. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.P.; Arezes, J.; Dias, V.; Oliveira, S.; Vieira, I.; Costa, M.; Vos, M.; Carlsson, A.; Rikers, Y.; Rangel, M.; et al. Physiological implications of NTBI uptake by T lymphocytes. Front. Pharmacol. 2014, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Regis, G.; Bosticardo, M.; Conti, L.; De Angelis, S.; Boselli, D.; Tomaino, B.; Bernabei, P.; Giovarelli, M.; Novelli, F. Iron regulates T-lymphocyte sensitivity to the IFN-gamma/STAT1 signaling pathway in vitro and in vivo. Blood 2005, 105, 3214–3221. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Pone, E.J.; Tran, D.C.; Patel, P.J.; Dao, L.; Xu, Z.; Casali, P. Iron inhibits activation-induced cytidine deaminase enzymatic activity and modulates immunoglobulin class switch DNA recombination. J. Biol. Chem. 2012, 287, 21520–21529. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.J.; Mano, H.; Aoki, K.; Hayashi, T.; Muto, A.; Nambu, Y.; Takahashi, K.; Itoh, K.; Taketani, S.; Nutt, S.L.; et al. Mitochondrial function provides instructive signals for activation-induced B-cell fates. Nat. Commun. 2015, 6, 6750. [Google Scholar] [CrossRef] [PubMed]
- Le Huong, T.; Brouwer, I.D.; Nguyen, K.C.; Burema, J.; Kok, F.J. The effect of iron fortification and de-worming on anaemia and iron status of Vietnamese schoolchildren. Br. J. Nutr. 2007, 97, 955–962. [Google Scholar] [CrossRef]
- Rizwan Ahmad, A.M.; Ahmed, W.; Iqbal, S.; Mushtaq, M.H.; Anis, R.A. Iron and prebiotic fortified flour improves the immune function of iron deficient women of childbearing age. Pak. J. Pharm. Sci. 2020, 33, 253–261. [Google Scholar]
- An, P.; Barron-Casella, E.A.; Strunk, R.C.; Hamilton, R.G.; Casella, J.F.; DeBaun, M.R. Elevation of IgE in children with sickle cell disease is associated with doctor diagnosis of asthma and increased morbidity. J. Allergy Clin. Immunol. 2011, 127, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Zhao, M.; Wang, Y.; Qu, Y.; Liu, H.; Wang, H.; Xing, L.; Shao, Z. Expression of BTK/p-BTK is different between CD5(+) and CD5(−) B lymphocytes from Autoimmune Hemolytic Anemia/Evans syndromes. Hematology 2019, 24, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Noureldin, M.S.; Shaltout, A.A. Anti-schistosomal IgE and its relation to gastrointestinal allergy in breast-fed infants of Schistosoma mansoni infected mothers. J. Egypt. Soc. Parasitol. 1998, 28, 539–550. [Google Scholar]
- Seka-Seka, J.; Brouh, Y.; Yapo-Crezoit, A.C.; Atseye, N.H. The role of serum immunoglobulin E in the pathogenesis of Plasmodium falciparum malaria in Ivorian children. Scand. J. Immunol. 2004, 59, 228–230. [Google Scholar] [CrossRef] [PubMed]
- MohanKumar, K.; Namachivayam, K.; Sivakumar, N.; Alves, N.G.; Sidhaye, V.; Das, J.K.; Chung, Y.; Breslin, J.W.; Maheshwari, A. Severe neonatal anemia increases intestinal permeability by disrupting epithelial adherens junctions. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G705–G716. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; She, E.; Gelbart, T.; Truksa, J.; Lee, P.; Xia, Y.; Khovananth, K.; Mudd, S.; Mann, N.; Moresco, E.M.; et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science 2008, 320, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Vanderford, D.A.; Greer, P.K.; Sharp, J.M.; Chichlowski, M.; Rouse, D.C.; Selim, M.A.; Hale, L.P. Alopecia in IL-10-deficient mouse pups is c-kit-dependent and can be triggered by iron deficiency. Exp. Dermatol. 2010, 19, 518–526. [Google Scholar] [CrossRef] [PubMed]
- St Pierre, S.A.; Vercellotti, G.M.; Donovan, J.C.; Hordinsky, M.K. Iron deficiency and diffuse nonscarring scalp alopecia in women: More pieces to the puzzle. J. Am. Acad. Dermatol. 2010, 63, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.C.; Syed, H.A.; Saleh, D. Telogen Effluvium. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lin, C.S.; Chan, L.Y.; Wang, J.H.; Chang, C.H. Diagnosis and treatment of female alopecia: Focusing on the iron deficiency-related alopecia. Tzu Chi Med. J. 2023, 35, 322–328. [Google Scholar] [CrossRef]
- Shalit, M.; Tedeschi, A.; Miadonna, A.; Levi-Schaffer, F. Desferal (desferrioxamine)--A novel activator of connective tissue-type mast cells. J. Allergy Clin. Immunol. 1991, 88, 854–860. [Google Scholar] [CrossRef]
- Mecheri, S.; Peltre, G.; Lapeyre, J.; David, B. Biological effect of transferrin on mast cell mediator release during the passive cutaneous anaphylaxis reaction: A possible inhibition mechanism involving iron. Ann. Inst. Pasteur Immunol. 1987, 138, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Theobald, K.; Gross-Weege, W.; Keymling, J.; Konig, W. Inhibition of histamine release in vitro by a blocking factor from human serum: Comparison with the iron binding proteins transferrin and lactoferrin. Agents Actions 1987, 20, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Theobald, K.; Gross-Weege, W.; Keymling, J.; Konig, W. Purification of serum proteins with inhibitory activity on the histamine release in vitro and/or in vivo. Int. Arch. Allergy Appl. Immunol. 1987, 82, 295–297. [Google Scholar] [CrossRef] [PubMed]
- He, S.; McEuen, A.R.; Blewett, S.A.; Li, P.; Buckley, M.G.; Leufkens, P.; Walls, A.F. The inhibition of mast cell activation by neutrophil lactoferrin: Uptake by mast cells and interaction with tryptase, chymase and cathepsin G. Biochem. Pharmacol. 2003, 65, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Takeuchi, T.; Shirakawa, T. Differentiation, distribution, and chemical state of intracellular trace elements in LAD2 mast cell line. Biol. Trace Elem. Res. 2005, 108, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Jain, A.K.; Agarwal, S.; Yadav, D. Iron Deficiency and Pruritus: A Cross-Sectional Analysis to Assess Its Association and Relationship. Indian J. Dermatol. 2021, 66, 705. [Google Scholar] [CrossRef]
- Guarneri, F.; Guarneri, C.; Cannavo, S.P. Oral iron therapy and chronic idiopathic urticaria: Sideropenic urticaria? Dermatol. Ther. 2014, 27, 223–226. [Google Scholar] [CrossRef]
- Maazi, H.; Shirinbak, S.; Bloksma, N.; Nawijn, M.C.; van Oosterhout, A.J. Iron administration reduces airway hyperreactivity and eosinophilia in a mouse model of allergic asthma. Clin. Exp. Immunol. 2011, 166, 80–86. [Google Scholar] [CrossRef]
- Wen, J.; Wang, C.; Xia, J.; Giri, M.; Guo, S. Relationship between serum iron and blood eosinophil counts in asthmatic adults: Data from NHANES 2011–2018. Front. Immunol. 2023, 14, 1201160. [Google Scholar] [CrossRef]
- Weigert, R.; Dosch, N.C.; Bacsik-Campbell, M.E.; Guilbert, T.W.; Coe, C.L.; Kling, P.J. Maternal pregnancy weight gain and cord blood iron status are associated with eosinophilia in infancy. J. Perinatol. 2015, 35, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Tam, E.; Keats, E.C.; Rind, F.; Das, J.K.; Bhutta, A.Z.A. Micronutrient Supplementation and Fortification Interventions on Health and Development Outcomes among Children Under-Five in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Vitamin A deficiency among children—Federated States of Micronesia, 2000. MMWR Morb. Mortal. Wkly. Rep. 2001, 50, 509–512.
- Jimenez, C.; Leets, I.; Puche, R.; Anzola, E.; Montilla, R.; Parra, C.; Aguilera, A.; Garcia-Casal, M.N. A single dose of vitamin A improves haemoglobin concentration, retinol status and phagocytic function of neutrophils in preschool children. Br. J. Nutr. 2010, 103, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.; de Benoist, B.; Dary, O.; Hurrell, R. Guidelines on Food Fortification with Micronutrients; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Reifen, R.; Nur, T.; Ghebermeskel, K.; Zaiger, G.; Urizky, R.; Pines, M. Vitamin A deficiency exacerbates inflammation in a rat model of colitis through activation of nuclear factor-kappaB and collagen formation. J. Nutr. 2002, 132, 2743–2747. [Google Scholar] [CrossRef] [PubMed]
- Green, M.H.; Ford, J.L.; Green, J.B. Development of a Compartmental Model to Investigate the Influence of Inflammation on Predictions of Vitamin A Total Body Stores by Retinol Isotope Dilution in Theoretical Humans. J. Nutr. 2021, 151, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Mayland, C.; Allen, K.R.; Degg, T.J.; Bennet, M. Micronutrient concentrations in patients with malignant disease: Effect of the inflammatory response. Ann. Clin. Biochem. 2004, 41 Pt 2, 138–141. [Google Scholar] [CrossRef]
- de Dios, O.; Navarro, P.; Ortega-Senovilla, H.; Herrero, L.; Gavela-Perez, T.; Soriano-Guillen, L.; Lasuncion, M.A.; Garces, C. Plasma Retinol Levels and High-Sensitivity C-Reactive Protein in Prepubertal Children. Nutrients 2018, 10, 1257. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, E.; Golgiri, F.; Janani, L.; Moradi, N.; Fallah, S.; Abiri, B.; Vafa, M. Randomized Study of the Effects of Zinc, Vitamin A, and Magnesium Co-supplementation on Thyroid Function, Oxidative Stress, and hs-CRP in Patients with Hypothyroidism. Biol. Trace Elem. Res. 2021, 199, 4074–4083. [Google Scholar] [CrossRef]
- Liu, N.; Kawahira, N.; Nakashima, Y.; Nakano, H.; Iwase, A.; Uchijima, Y.; Wang, M.; Wu, S.M.; Minamisawa, S.; Kurihara, H.; et al. Notch and retinoic acid signals regulate macrophage formation from endocardium downstream of Nkx2-5. Nat. Commun. 2023, 14, 5398. [Google Scholar] [CrossRef]
- Hiraga, H.; Chinda, D.; Maeda, T.; Murai, Y.; Ogasawara, K.; Muramoto, R.; Ota, S.; Hasui, K.; Sakuraba, H.; Ishiguro, Y.; et al. Vitamin A Promotes the Fusion of Autophagolysosomes and Prevents Excessive Inflammasome Activation in Dextran Sulfate Sodium-Induced Colitis. Int. J. Mol. Sci. 2023, 24, 8684. [Google Scholar] [CrossRef] [PubMed]
- Nurrahmah, Q.I.; Madhyastha, R.; Madhyastha, H.; Purbasari, B.; Maruyama, M.; Nakajima, Y. Retinoic acid abrogates LPS-induced inflammatory response via negative regulation of NF-kappa B/miR-21 signaling. Immunopharmacol. Immunotoxicol. 2021, 43, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Penkert, R.R.; Jones, B.G.; Hacker, H.; Partridge, J.F.; Hurwitz, J.L. Vitamin A differentially regulates cytokine expression in respiratory epithelial and macrophage cell lines. Cytokine 2017, 91, 1–5. [Google Scholar] [CrossRef]
- Zhu, Y.N.; Gu, X.L.; Wang, L.Y.; Guan, N.; Li, C.G. All-Trans Retinoic Acid Promotes M2 Macrophage Polarization in Vitro by Activating the p38MAPK/STAT6 Signaling Pathway. Immunol. Investig. 2023, 52, 298–318. [Google Scholar] [CrossRef] [PubMed]
- Pinos, I.; Yu, J.; Pilli, N.; Kane, M.A.; Amengual, J. Functional characterization of interleukin 4 and retinoic acid signaling crosstalk during alternative macrophage activation. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2023, 1868, 159291. [Google Scholar] [CrossRef] [PubMed]
- Carman, J.A.; Hayes, C.E. Abnormal regulation of IFN-gamma secretion in vitamin A deficiency. J. Immunol. 1991, 147, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Nashold, F.E.; Hayes, C.E. Vitamin A deficiency results in a priming environment conducive for Th1 cell development. Eur. J. Immunol. 1995, 25, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.Y.; Lee, J.M.; Jang, Y.S.; Kang, S.G.; Yoon, S.I.; Ko, H.J.; Lee, G.S.; Park, S.R.; Nagler, C.R.; Kim, P.H. Mechanism underlying the suppressor activity of retinoic acid on IL4-induced IgE synthesis and its physiological implication. Cell. Immunol. 2017, 322, 49–55. [Google Scholar] [CrossRef]
- Ruhl, R.; Garcia, A.; Schweigert, F.J.; Worm, M. Modulation of cytokine production by low and high retinoid diets in ovalbumin-sensitized mice. Int. J. Vitam. Nutr. Res. 2004, 74, 279–284. [Google Scholar] [CrossRef]
- Sun, C.M.; Hall, J.A.; Blank, R.B.; Bouladoux, N.; Oukka, M.; Mora, J.R.; Belkaid, Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007, 204, 1775–1785. [Google Scholar] [CrossRef]
- Spencer, S.P.; Wilhelm, C.; Yang, Q.; Hall, J.A.; Bouladoux, N.; Boyd, A.; Nutman, T.B.; Urban, J.F., Jr.; Wang, J.; Ramalingam, T.R.; et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 2014, 343, 432–437. [Google Scholar] [CrossRef]
- Mellody, K.T.; Bradley, E.J.; Mambwe, B.; Cotterell, L.F.; Kiss, O.; Halai, P.; Loftus, Z.; Bell, M.; Griffiths, T.W.; Griffiths, C.E.M.; et al. Multifaceted amelioration of cutaneous photoageing by (0.3%) retinol. Int. J. Cosmet. Sci. 2022, 44, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Y.; Cui, T.; Yang, T.; Liu, L.; Li, T.; Chen, J. Retinoic Acid Facilitates Toll-Like Receptor 4 Expression to Improve Intestinal Barrier Function through Retinoic Acid Receptor Beta. Cell. Physiol. Biochem. 2017, 42, 1390–1406. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.M.; Roop, D.; Huang, F.L. Vitamin A: A key nutrient for the maintenance of epithelial differentiation. Acta Vitaminol. Enzymol. 1985, 7, 13–20. [Google Scholar] [PubMed]
- Lotan, R. Squamous cell differentiation markers in normal, premalignant, and malignant epithelium: Effects of retinoids. J. Cell Biochem. Suppl. 1993, 17F, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Sundelin, J.; Busch, C.; Das, K.; Das, S.; Eriksson, U.; Jonsson, K.H.; Kampe, O.; Laurent, B.; Liljas, A.; Newcomer, M.; et al. Structure and tissue distribution of some retinoid-binding proteins. J. Investig. Dermatol. 1983, 81, 59s–63s. [Google Scholar] [CrossRef] [PubMed]
- Chopra, D.P.; Cooney, R.A.; Taylor, G.W. Effects of vitamin A deficiency on cell proliferation and morphology of trachea of the hamster. Cell Tissue Kinet. 1990, 23, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Ng-Blichfeldt, J.P.; Schrik, A.; Kortekaas, R.K.; Noordhoek, J.A.; Heijink, I.H.; Hiemstra, P.S.; Stolk, J.; Konigshoff, M.; Gosens, R. Retinoic acid signaling balances adult distal lung epithelial progenitor cell growth and differentiation. EBioMedicine 2018, 36, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, J.S.; Zou, W.J.; Tan, Q.; Xiao, Y.Z.; Luo, X.Y.; Gao, P.; Fu, Z.; Wang, H. Vitamin A deficiency exacerbates extrinsic atopic dermatitis development by potentiating type 2 helper T cell-type inflammation and mast cell activation. Clin. Exp. Allergy 2020, 50, 942–953. [Google Scholar] [CrossRef]
- Qi, C.; Tu, H.; Zhao, Y.; Zhou, J.; Chen, J.; Hu, H.; Yu, R.; Sun, J. Breast Milk-Derived Limosilactobacillus reuteri Prevents Atopic Dermatitis in Mice via Activating Retinol Absorption and Metabolism in Peyer’s Patches. Mol. Nutr. Food Res. 2023, 67, e2200444. [Google Scholar] [CrossRef]
- Cantwell, M.E.; Foreman, J.C. The actions of retinal and retinoic acid on histamine release from rat peritoneal mast cells. Eur. J. Pharmacol. 1989, 160, 43–51. [Google Scholar] [CrossRef]
- Larange, A.; Cheroutre, H. Retinoic Acid and Retinoic Acid Receptors as Pleiotropic Modulators of the Immune System. Annu. Rev. Immunol. 2016, 34, 369–394. [Google Scholar] [CrossRef]
- Shi, Z.; Ohno, H.; Satoh-Takayama, N. Dietary Derived Micronutrients Modulate Immune Responses Through Innate Lymphoid Cells. Front. Immunol. 2021, 12, 670632. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.H.; Hwang, J.; Kwon, R.; Lee, S.W.; Kim, M.S.; GBD 2019 Allergic Disorders Collaborators; Shin, J.I.; Yon, D.K. Global, regional, and national burden of allergic disorders and their risk factors in 204 countries and territories, from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Allergy 2023, 78, 2232–2254. [Google Scholar] [CrossRef]
- Miethe, S.; Guarino, M.; Alhamdan, F.; Simon, H.U.; Renz, H.; Dufour, J.F.; Potaczek, D.P.; Garn, H. Effects of obesity on asthma: Immunometabolic links. Pol. Arch. Intern. Med. 2018, 128, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.H.; Yeh, K.W.; Huang, J.L.; Su, K.W.; Chen, K.F.; Wu, C.C.; Tsai, M.H.; Hua, M.C.; Liao, S.L.; Lai, S.H.; et al. Longitudinal changes in body mass index Z-scores during infancy and risk of childhood allergies. J. Microbiol. Immunol. Infect. 2022, 55, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, V.V.; Skypala, I.J. Nutritional disorders prevalence among adults with immunoglobin E-mediated food allergy. Clin. Exp. Allergy 2024. [Google Scholar] [CrossRef] [PubMed]
- Dziechciarz, P.; Strozyk, A.; Horvath, A.; Cudowska, B.; Jedynak-Wasowicz, U.; Mol, N.; Jarocka-Cyrta, E.; Zawadzka-Krajewska, A.; Krauze, A. Nutritional status and feeding difficulties in children up to 2 years of age with cow’s milk allergy. J. Pediatr. Gastroenterol. Nutr. 2024, 79, 131–139. [Google Scholar] [CrossRef]
- Jasielska, M.; Buczynska, A.; Adamczyk, P.; Grzybowska-Chlebowczyk, U. Nutritional Status of Children with Newly Diagnosed Food Allergies. Children 2023, 10, 1687. [Google Scholar] [CrossRef]
- Calle, C.G.; Diaz-Vasquez, C.; Cordova-Calderon, W.; Gomez de la Torre, J.; Matos-Benavides, E.; Toribio-Dionicio, C. Clinical characteristics, laboratory findings, and tolerance acquisition in infants with cow’s milk protein allergy in a private center in Lima, Peru for the period 2021–2022. Immun. Inflamm. Dis. 2024, 12, e1246. [Google Scholar] [CrossRef]
- Chang, C.L.; Ali, G.B.; Lodge, C.J.; Abramson, M.J.; Erbas, B.; Tang, M.L.K.; Svanes, C.; Bui, D.S.; Dharmage, S.C.; Lowe, A.J. Associations between Body Mass Index Trajectories in the first two years of life and Allergic Rhinitis, Eczema and Food Allergy outcomes up to early adulthood. Pediatr. Allergy Immunol. 2022, 33, e13765. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulou, E.; Christoforou, C.; Andreou, E.; Heraclides, A. Effects of food allergy on the dietary habits and intake of primary schools’ Cypriot children. Eur. Ann. Allergy Clin. Immunol. 2017, 49, 181–185. [Google Scholar] [CrossRef]
- Meyer, R. Nutritional disorders resulting from food allergy in children. Pediatr. Allergy Immunol. 2018, 29, 689–704. [Google Scholar] [CrossRef]
- Mehta, H.; Ramesh, M.; Feuille, E.; Groetch, M.; Wang, J. Growth comparison in children with and without food allergies in 2 different demographic populations. J. Pediatr. 2014, 165, 842–848. [Google Scholar] [CrossRef]
- Low, D.W.; Jamil, A.; Md Nor, N.; Kader Ibrahim, S.B.; Poh, B.K. Food restriction, nutrition status, and growth in toddlers with atopic dermatitis. Pediatr. Dermatol. 2020, 37, 69–77. [Google Scholar] [CrossRef]
- Leung, T.F.; Wang, S.S.; Kwok, F.Y.; Leung, L.W.; Chow, C.M.; Hon, K.L. Assessment of dietary food and nutrient intake and bone density in children with eczema. Hong Kong Med. J. 2017, 23, 470–479. [Google Scholar] [CrossRef]
- Drury, K.E.; Schaeffer, M.; Silverberg, J.I. Association Between Atopic Disease and Anemia in US Children. JAMA Pediatr. 2016, 170, 29–34. [Google Scholar] [CrossRef]
- Rhew, K.; Oh, J.M. Association between atopic disease and anemia in pediatrics: A cross-sectional study. BMC Pediatr. 2019, 19, 455. [Google Scholar] [CrossRef] [PubMed]
- Rhew, K.; Brown, J.D.; Oh, J.M. Atopic Disease and Anemia in Korean Patients: Cross-Sectional Study with Propensity Score Analysis. Int. J. Environ. Res. Public Health 2020, 17, 1978. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sato, M.; Saito-Abe, M.; Miyaji, Y.; Shimada, M.; Sato, C.; Nishizato, M.; Kumasaka, N.; Mezawa, H.; Yamamoto-Hanada, K.; et al. Allergic Disorders and Risk of Anemia in Japanese Children: Findings from the Japan Environment and Children’s Study. Nutrients 2022, 14, 4335. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Chung, J.; Kim, M.K.; Kwon, S.O.; Cho, B.H. Antioxidant nutrient intakes and corresponding biomarkers associated with the risk of atopic dermatitis in young children. Eur. J. Clin. Nutr. 2010, 64, 245–252. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Vacca, C.; Pace, E.; Vuillemier, P.L.; Del Vecchio, L.; Berni Canani, R. Immunological and trace element study in 50 children with various diseases caused by food allergens and aeroallergens. Pediatr. Med. Chir. 1987, 9, 589–591. [Google Scholar]
- Ha, E.K.; Kim, J.H.; Lee, E.; Sung, M.; Jee, H.M.; Baek, H.S.; Shin, Y.H.; Lee, N.H.; Han, M.Y. Abnormal iron status is independently associated with reduced oscillometric lung function in schoolchildren. Clin. Respir. J. 2021, 15, 870–877. [Google Scholar] [CrossRef]
- Jardim-Botelho, A.; Martins, T.G.; Motta-Franco, J.; Meyer, R.; Fontes Vieira, S.C.; Protasio, B.F.; Santos Silva, M.L.; Pontes, R.S.; de Oliveira, M.B.B.; de Carvalho Barreto, I.D.; et al. Growth and Nutritional Biomarkers in Brazilian Infants with Cow’s Milk Allergy at Diagnosis and 18-Month Follow-Up: A Prospective Cohort Study. Pediatr. Gastroenterol. Hepatol. Nutr. 2023, 26, 355–369. [Google Scholar] [CrossRef]
- Nowak, S.; Wang, H.; Schmidt, B.; Jarvinen, K.M. Vitamin D and iron status in children with food allergy. Ann. Allergy Asthma Immunol. 2021, 127, 57–63. [Google Scholar] [CrossRef]
- Kvammen, J.A.; Thomassen, R.A.; Eskerud, M.B.; Rugtveit, J.; Henriksen, C. Micronutrient Status and Nutritional Intake in 0- to 2-Year-old Children Consuming a Cows’ Milk Exclusion Diet. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 831–837. [Google Scholar] [CrossRef]
- Lai, F.P.; Yang, Y.J. The prevalence and characteristics of cow’s milk protein allergy in infants and young children with iron deficiency anemia. Pediatr. Neonatol. 2018, 59, 48–52. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Hayes, H.; Gambling, L.; Craig, L.C.; Allan, K.; Prabhu, N.; Turner, S.W.; McNeill, G.; Erkkola, M.; Seaton, A.; et al. An exploratory study of the associations between maternal iron status in pregnancy and childhood wheeze and atopy. Br. J. Nutr. 2014, 112, 2018–2027. [Google Scholar] [CrossRef]
- Shaheen, S.O.; Macdonald-Wallis, C.; Lawlor, D.A.; Henderson, A.J. Haemoglobin concentrations in pregnancy and respiratory and allergic outcomes in childhood: Birth cohort study. Clin. Exp. Allergy 2017, 47, 1615–1624. [Google Scholar] [CrossRef]
- Quezada-Pinedo, H.G.; Mensink-Bout, S.M.; Reiss, I.K.; Jaddoe, V.W.V.; Vermeulen, M.J.; Duijts, L. Maternal iron status during early pregnancy and school-age, lung function, asthma, and allergy: The Generation R Study. Pediatr. Pulmonol. 2021, 56, 1771–1778. [Google Scholar] [CrossRef]
- Bedard, A.; Lewis, S.J.; Burgess, S.; Henderson, A.J.; Shaheen, S.O. Maternal iron status during pregnancy and respiratory and atopic outcomes in the offspring: A Mendelian randomisation study. BMJ Open Respir. Res. 2018, 5, e000275. [Google Scholar] [CrossRef]
- Fortes, C.; Mastroeni, S.; Mannooranparampil, T.J.; Di Lallo, D. Pre-natal folic acid and iron supplementation and atopic dermatitis in the first 6 years of life. Arch. Dermatol. Res. 2019, 311, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.O.; Newson, R.B.; Henderson, A.J.; Emmett, P.M.; Sherriff, A.; Cooke, M.; Team, A.S. Umbilical cord trace elements and minerals and risk of early childhood wheezing and eczema. Eur. Respir. J. 2004, 24, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Brigham, E.P.; McCormack, M.C.; Takemoto, C.M.; Matsui, E.C. Iron status is associated with asthma and lung function in US women. PLoS ONE 2015, 10, e0117545. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.P.; Krupani, S.; Stark, J.M.; Mosquera, R.A.; Waller, D.K.; Gonzales, T.; Brown, D.L.; Nguyen, T.T.; Jon, C.K.; Yadav, A. Validation of the breathmobile case identification survey for asthma screening in children with sickle cell disease. J. Asthma 2021, 58, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Vierucci, A.; De Martino, M.; Di Palma, A.; Novembre, E.; Rossi, M.E.; Resti, M.; Azzari, C. The multitransfused beta-thalassemic child: A model for the study of IgE response. Ann. Allergy 1986, 56, 158–161. [Google Scholar] [PubMed]
- De, A.; Agrawal, S.; Morrone, K.; Zhang, J.; Bjorklund, N.L.; Manwani, D.; Rastogi, D. Airway Inflammation and Lung Function in Sickle Cell Disease. Pediatr. Allergy Immunol. Pulmonol. 2019, 32, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.Y.; Huang, L.C.; Yu, H.R.; Kuo, K.C.; Chen, W.H.; Su, C.H.; Lee, C.P.; Chen, K.J.; Yang, Y.H.; Sheen, J.M. Pediatric thalassemic patients have higher incidence of asthma: A nationwide population-based retrospective cohort study. PLoS ONE 2021, 16, e0258727. [Google Scholar] [CrossRef] [PubMed]
- Pardalos, G.; Kanakoudi-Tsakalidis, F.; Malaka-Zafiriu, M.; Tsantali, H.; Athanasiou-Metaxa, M.; Kallinikos, G.; Papaevangelou, G. Iron-related disturbances of cell-mediated immunity in multitransfused children with thalassemia major. Clin. Exp. Immunol. 1987, 68, 138–145. [Google Scholar]
- Pandher, K.; Ghamrawi, R.I.; Heron, C.E.; Feldman, S.R. Controversial cardiovascular and hematologic comorbidities in atopic dermatitis. Arch. Dermatol. Res. 2021, 314, 317–324. [Google Scholar] [CrossRef]
- Rhew, K.; Choi, J.; Kim, K.; Choi, K.H.; Lee, S.H.; Park, H.W. Increased Risk of Anemia in Patients with Asthma. Clin. Epidemiol. 2023, 15, 31–38. [Google Scholar] [CrossRef]
- Krishna, M.T.; Subramanian, A.; Adderley, N.J.; Zemedikun, D.T.; Gkoutos, G.V.; Nirantharakumar, K. Allergic diseases and long-term risk of autoimmune disorders: Longitudinal cohort study and cluster analysis. Eur. Respir. J. 2019, 54, 1900476. [Google Scholar] [CrossRef]
- Shaheen, S.O.; Gissler, M.; Devereux, G.; Erkkola, M.; Kinnunen, T.I.; McArdle, H.; Sheikh, A.; Hemminki, E.; Nwaru, B.I. Maternal iron supplementation in pregnancy and asthma in the offspring: Follow-up of a randomised trial in Finland. Eur. Respir. J. 2020, 55, 1902335. [Google Scholar] [CrossRef] [PubMed]
- DellaValle, D.M.; Glahn, R.P.; Shaff, J.E.; O’Brien, K.O. Iron Absorption from an Intrinsically Labeled Lentil Meal Is Low but Upregulated in Women with Poor Iron Status. J. Nutr. 2015, 145, 2253–2257. [Google Scholar] [CrossRef]
- Gaitan, D.; Olivares, M.; Lonnerdal, B.; Brito, A.; Pizarro, F. Non-heme iron as ferrous sulfate does not interact with heme iron absorption in humans. Biol. Trace Elem. Res. 2012, 150, 68–73. [Google Scholar] [CrossRef]
- Walczyk, T.; Muthayya, S.; Wegmuller, R.; Thankachan, P.; Sierksma, A.; Frenken, L.G.; Thomas, T.; Kurpad, A.; Hurrell, R.F. Inhibition of iron absorption by calcium is modest in an iron-fortified, casein- and whey-based drink in Indian children and is easily compensated for by addition of ascorbic acid. J. Nutr. 2014, 144, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Hertrampf, E.; Olivares, M.; Walter, T.; Pizarro, F.; Heresi, G.; Llaguno, S.; Vega, V.; Cayazzo, M.; Chadud, P. [Iron-deficiency anemia in the nursing infant: Its elimination with iron-fortified milk]. Rev. Med. Chil. 1990, 118, 1330–1337. [Google Scholar] [PubMed]
- Berseth, C.L.; Van Aerde, J.E.; Gross, S.; Stolz, S.I.; Harris, C.L.; Hansen, J.W. Growth, efficacy, and safety of feeding an iron-fortified human milk fortifier. Pediatrics 2004, 114, e699–e706. [Google Scholar] [CrossRef] [PubMed]
- Chinnappan, A.; Sharma, A.; Agarwal, R.; Thukral, A.; Deorari, A.; Sankar, M.J. Fortification of Breast Milk With Preterm Formula Powder vs Human Milk Fortifier in Preterm Neonates: A Randomized Noninferiority Trial. JAMA Pediatr. 2021, 175, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Begin, F.; Santizo, M.C.; Peerson, J.M.; Torun, B.; Brown, K.H. Effects of bovine serum concentrate, with or without supplemental micronutrients, on the growth, morbidity, and micronutrient status of young children in a low-income, peri-urban Guatemalan community. Eur. J. Clin. Nutr. 2008, 62, 39–50. [Google Scholar] [CrossRef]
- Htet, M.K.; Fahmida, U.; Dillon, D.; Akib, A.; Utomo, B.; Thurnham, D.I. Is Iron Supplementation Influenced by Sub-Clinical Inflammation?: A Randomized Controlled Trial Among Adolescent Schoolgirls in Myanmar. Nutrients 2019, 11, 918. [Google Scholar] [CrossRef]
- Hoa, P.T.; Khan, N.C.; van Beusekom, C.; Gross, R.; Conde, W.L.; Khoi, H.D. Milk fortified with iron or iron supplementation to improve nutritional status of pregnant women: An intervention trial from rural Vietnam. Food Nutr. Bull. 2005, 26, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Tetens, I.; Larsen, T.M.; Kristensen, M.B.; Hels, O.; Jensen, M.; Morberg, C.M.; Thomsen, A.D.; Hojgaard, L.; Henriksen, M. The importance of dietary composition for efficacy of iron absorption measured in a whole diet that includes rye bread fortified with ferrous fumerate: A radioisotope study in young women. Br. J. Nutr. 2005, 94, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Giliberti, A.; Curcio, A.; Marchitto, N.; Di Lullo, L.; Paolozzi, F.; Nano, F.; Pironti, M.; Raimondi, G. Comparison of Ferric Sodium EDTA in Combination with Vitamin C, Folic Acid, Copper Gluconate, Zinc Gluconate, and Selenomethionine as Therapeutic Option for Chronic Kidney Disease Patients with Improvement in Inflammatory Status. Nutrients 2022, 14, 2116. [Google Scholar] [CrossRef] [PubMed]
- Smuts, C.M.; Matsungo, T.M.; Malan, L.; Kruger, H.S.; Rothman, M.; Kvalsvig, J.D.; Covic, N.; Joosten, K.; Osendarp, S.J.M.; Bruins, M.J.; et al. Effect of small-quantity lipid-based nutrient supplements on growth, psychomotor development, iron status, and morbidity among 6- to 12-mo-old infants in South Africa: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Mutumba, R.; Pesu, H.; Mbabazi, J.; Greibe, E.; Nexo, E.; Olsen, M.F.; Briend, A.; Molgaard, C.; Michaelsen, K.F.; Ritz, C.; et al. Effect of lipid-based nutrient supplements on micronutrient status and hemoglobin among children with stunting: Secondary analysis of a randomized controlled trial in Uganda. Am. J. Clin. Nutr. 2024, 119, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, M.; Kallio, M.J.; Siimes, M.A.; Ranki, A. Retinol concentrations after birth are inversely associated with atopic manifestations in children and young adults. Clin. Exp. Allergy 2007, 37, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Chakraborti, G.; Mukherjee, K.; Bhattacharjee, D.; Mallick, S.; Biswas, T. Retinol Levels in Serum and Chronic Skin Lesions of Atopic Dermatitis. Indian J. Dermatol. 2018, 63, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Daniluk, U.; Filimoniuk, A.; Kowalczuk-Kryston, M.; Alifier, M.; Karpinska, J.; Kaczmarski, M.G.; Lebensztejn, D.M. Association of antioxidants and vitamin D level with inflammation in children with atopic dermatitis. Int. J. Dermatol. 2019, 58, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Al Senaidy, A.M. Serum vitamin A and beta-carotene levels in children with asthma. J. Asthma 2009, 46, 699–702. [Google Scholar] [CrossRef]
- Wood, L.G.; Garg, M.L.; Smart, J.M.; Scott, H.A.; Barker, D.; Gibson, P.G. Manipulating antioxidant intake in asthma: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 534–543. [Google Scholar] [CrossRef]
- Allen, S.; Britton, J.R.; Leonardi-Bee, J.A. Association between antioxidant vitamins and asthma outcome measures: Systematic review and meta-analysis. Thorax 2009, 64, 610–619. [Google Scholar] [CrossRef]
- Defnet, A.E.; Shah, S.D.; Huang, W.; Shapiro, P.; Deshpande, D.A.; Kane, M.A. Dysregulated retinoic acid signaling in airway smooth muscle cells in asthma. FASEB J. 2021, 35, e22016. [Google Scholar] [CrossRef] [PubMed]
- Golebski, K.; Layhadi, J.A.; Sahiner, U.; Steveling-Klein, E.H.; Lenormand, M.M.; Li, R.C.Y.; Bal, S.M.; Heesters, B.A.; Vila-Nadal, G.; Hunewald, O.; et al. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity 2021, 54, 291–307.e7. [Google Scholar] [CrossRef]
- Xiang, J.; Wang, H.; Li, T. Comorbidity of Vitamin A and Vitamin D Deficiency Exacerbates the Severity of Atopic Dermatitis in Children. Dermatology 2019, 235, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, N.; Balde, A.; Lisse, I.M.; Eriksen, H.B.; Aaby, P.; Benn, C.S. Vitamin A supplementation and risk of atopy: Long-term follow-up of a randomized trial of vitamin A supplementation at six and nine months of age. BMC Pediatr. 2013, 13, 190. [Google Scholar] [CrossRef]
- McKeever, T.M.; Lewis, S.A.; Smit, H.; Burney, P.; Britton, J.; Cassano, P.A. Serum nutrient markers and skin prick testing using data from the Third National Health and Nutrition Examination Survey. J. Allergy Clin. Immunol. 2004, 114, 1398–1402. [Google Scholar] [CrossRef]
- Hathcock, J.N.; Hattan, D.G.; Jenkins, M.Y.; McDonald, J.T.; Sundaresan, P.R.; Wilkening, V.L. Evaluation of vitamin A toxicity. Am. J. Clin. Nutr. 1990, 52, 183–202. [Google Scholar] [CrossRef]
- Parr, C.L.; Magnus, M.C.; Karlstad, O.; Holvik, K.; Lund-Blix, N.A.; Haugen, M.; Page, C.M.; Nafstad, P.; Ueland, P.M.; London, S.J.; et al. Vitamin A and D intake in pregnancy, infant supplementation, and asthma development: The Norwegian Mother and Child Cohort. Am. J. Clin. Nutr. 2018, 107, 789–798. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim. Biophys. Acta 2012, 1821, 70–77. [Google Scholar] [CrossRef]
- Meza-Meza, M.R.; Ruiz-Ballesteros, A.I.; de la Cruz-Mosso, U. Functional effects of vitamin D: From nutrient to immunomodulator. Crit. Rev. Food Sci. Nutr. 2022, 62, 3042–3062. [Google Scholar] [CrossRef]
- Micronutrients IoMUPo. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- World Health, O. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Castenmiller, J.J.; West, C.E. Bioavailability and bioconversion of carotenoids. Annu. Rev. Nutr. 1998, 18, 19–38. [Google Scholar] [CrossRef]
- Kull, I.; Bergstrom, A.; Melen, E.; Lilja, G.; van Hage, M.; Pershagen, G.; Wickman, M. Early-life supplementation of vitamins A and D, in water-soluble form or in peanut oil, and allergic diseases during childhood. J. Allergy Clin. Immunol. 2006, 118, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Talaei, M.; Hughes, D.A.; Mahmoud, O.; Emmett, P.M.; Granell, R.; Guerra, S.; Shaheen, S.O. Dietary intake of vitamin A, lung function and incident asthma in childhood. Eur. Respir. J. 2021, 58, 2004407. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Murray, C.S.; Woodcock, A.; Simpson, A.; Custovic, A. Dietary antioxidant intake, allergic sensitization and allergic diseases in young children. Allergy 2009, 64, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Sasaki, S.; Ohya, Y.; Miyamoto, S.; Matsunaga, I.; Yoshida, T.; Hirota, Y.; Oda, H.; Osaka, M.; Child Health Study Group. Dietary intake of seaweed and minerals and prevalence of allergic rhinitis in Japanese pregnant females: Baseline data from the Osaka Maternal and Child Health Study. Ann. Epidemiol. 2006, 16, 614–621. [Google Scholar] [CrossRef]
- Nagel, G.; Nieters, A.; Becker, N.; Linseisen, J. The influence of the dietary intake of fatty acids and antioxidants on hay fever in adults. Allergy 2003, 58, 1277–1284. [Google Scholar] [CrossRef]
- Yang, A.R.; Kim, Y.N.; Lee, B.H. Dietary intakes and lifestyle patterns of Korean children and adolescents with atopic dermatitis: Using the fourth and fifth Korean National Health and Nutrition Examination Survey (KNHANES IV,V), 2007–2011. Ecol. Food Nutr. 2016, 55, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Toyran, M.; Kaymak, M.; Vezir, E.; Harmanci, K.; Kaya, A.; Ginis, T.; Kose, G.; Kocabas, C.N. Trace element levels in children with atopic dermatitis. J. Investig. Allergol. Clin. Immunol. 2012, 22, 341–344. [Google Scholar]
- Pereira de Jesus, S.; den Dekker, H.T.; de Jongste, J.C.; Reiss, I.K.; Steegers, E.A.; Jaddoe, V.W.V.; Duijts, L. Maternal hemoglobin and hematocrit levels during pregnancy and childhood lung function and asthma. The Generation R Study. Pediatr. Pulmonol. 2018, 53, 130–137. [Google Scholar] [CrossRef]
- Triche, E.W.; Lundsberg, L.S.; Wickner, P.G.; Belanger, K.; Leaderer, B.P.; Bracken, M.B. Association of maternal anemia with increased wheeze and asthma in children. Ann. Allergy Asthma Immunol. 2011, 106, 131–139.e1. [Google Scholar] [CrossRef]
- Rosenlund, H.; Magnusson, J.; Kull, I.; Hakansson, N.; Wolk, A.; Pershagen, G.; Wickman, M.; Bergstrom, A. Antioxidant intake and allergic disease in children. Clin. Exp. Allergy 2012, 42, 1491–1500. [Google Scholar] [CrossRef]
- Magdelijns, F.J.; Mommers, M.; Penders, J.; Smits, L.; Thijs, C. Folic acid use in pregnancy and the development of atopy, asthma, and lung function in childhood. Pediatrics 2011, 128, e135–e144. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, G.; Luo, M.; Lan, Q.; Shi, X.; Deng, H.; Wang, N.; Xu, X.; Zhang, C. Serum vitamin E levels and chronic inflammatory skin diseases: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0261259. [Google Scholar] [CrossRef] [PubMed]
- Petriashvili, M. Impact of Maternal Vitamin D Status on the Formation of Atopic Dermatitis in Young Children. Glob. Pediatr. Health 2021, 8, 2333794X211022916. [Google Scholar] [CrossRef]
- Lara-Corrales, I.; Huang, C.M.; Parkin, P.C.; Rubio-Gomez, G.A.; Posso-De Los Rios, C.J.; Maguire, J.; Pope, E. Vitamin D Level and Supplementation in Pediatric Atopic Dermatitis: A Randomized Controlled Trial. J. Cutan. Med. Surg. 2019, 23, 44–49. [Google Scholar] [CrossRef]
- Riccioni, G.; Bucciarelli, T.; Mancini, B.; Di Ilio, C.; Della Vecchia, R.; D’Orazio, N. Plasma lycopene and antioxidant vitamins in asthma: The PLAVA study. J. Asthma 2007, 44, 429–432. [Google Scholar] [CrossRef]
- Mills, K.; Lay, J.; Wu, W.; Robinette, C.; Kesic, M.J.; Dreskin, S.C.; Peden, D.B.; Hernandez, M. Vitamin E, gamma-tocopherol, diminishes ex vivo basophil response to dust mite allergen. Allergy 2014, 69, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Feketea, G.; Vassilopoulou, E.; Andreescu, O.; Berghea, E.C.; Pop, R.M.; Sabin, O.; Zdrenghea, M.; Bocsan, I.C. Vitamin D Level and Immune Modulation in Children with Recurrent Wheezing. Children 2024, 11, 219. [Google Scholar] [CrossRef]
- Petrov, V.I.; Shishimorov, I.N.; Perminov, A.A.; Nefedov, I.V. Influence of magnesium deficiency correction on the effectiveness of bronchial asthma pharmacotherapy in children. Eksp. Klin. Farmakol. 2014, 77, 23–27. [Google Scholar]
- Lipkin, G.; March, C.; Gowdey, J. Magnesium in Epidermis, Dermis, and Whole Skin of Normal and Atopic Subjects. J. Investig. Dermatol. 1964, 42, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Galland, L. Magnesium and immune function: An overview. Magnesium 1988, 7, 290–299. [Google Scholar]
- Yu, Z.; Xu, C.; Fang, C.; Zhang, F. Causal effect of iron status on lung function: A Mendelian randomization study. Front. Nutr. 2022, 9, 1025212. [Google Scholar] [CrossRef]
- Gomez, H.M.; Pillar, A.L.; Brown, A.C.; Kim, R.Y.; Ali, M.K.; Essilfie, A.T.; Vanders, R.L.; Frazer, D.M.; Anderson, G.J.; Hansbro, P.M.; et al. Investigating the Links between Lower Iron Status in Pregnancy and Respiratory Disease in Offspring Using Murine Models. Nutrients 2021, 13, 4461. [Google Scholar] [CrossRef]
- Esenboga, S.; Cetinkaya, P.G.; Sahiner, N.; Birben, E.; Soyer, O.; Sekerel, B.E.; Sahiner, U.M. Infantile atopic dermatitis: Serum vitamin D, zinc and TARC levels and their relationship with disease phenotype and severity. Allergol. Immunopathol. 2021, 49, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.S.; Ahn, I.S.; Byun, Y.S.; Yang, Y.S.; Kim, J.H.; Chung, B.Y.; Kim, H.O.; Park, C.W. Dietary pattern and nutrient intake of korean children with atopic dermatitis. Ann. Dermatol. 2014, 26, 570–575. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.H.; Ly, S.Y. Children with atopic dermatitis in Daejeon, Korea: Individualized nutrition intervention for disease severity and nutritional status. Asia Pac. J. Clin. Nutr. 2016, 25, 716–728. [Google Scholar] [CrossRef]
- Popescu, F.D. Cross-reactivity between aeroallergens and food allergens. World J. Methodol. 2015, 5, 31–50. [Google Scholar] [CrossRef]
- Worm, M.; Jappe, U.; Kleine-Tebbe, J.; Schäfer, C.; Reese, I.; Saloga, J.; Treudler, R.; Zuberbier, T.; Waßmann, A.; Fuchs, T.; et al. Food allergies resulting from immunological cross-reactivity with inhalant allergens: Guidelines from the German Society for Allergology and Clinical Immunology (DGAKI), the German Dermatology Society (DDG), the Association of German Allergologists (AeDA) and the Society for Pediatric Allergology and Environmental Medicine (GPA). Allergo J. Int. 2014, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Krikeerati, T.; Rodsaward, P.; Nawiboonwong, J.; Pinyopornpanish, K.; Phusawang, S.; Sompornrattanaphan, M. Revisiting Fruit Allergy: Prevalence across the Globe, Diagnosis, and Current Management. Foods 2023, 12, 4083. [Google Scholar] [CrossRef]
- Venter, C.; Agostoni, C.; Arshad, S.H.; Ben-Abdallah, M.; Du Toit, G.; Fleischer, D.M.; Greenhawt, M.; Glueck, D.H.; Groetch, M.; Lunjani, N.; et al. Dietary factors during pregnancy and atopic outcomes in childhood: A systematic review from the European Academy of Allergy and Clinical Immunology. Pediatr. Allergy Immunol. 2020, 31, 889–912. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Rifas-Shiman, S.L.; Platts-Mills, T.A.; Workman, L.; Sordillo, J.E.; Camargo, C.A., Jr.; Gillman, M.W.; Gold, D.R.; Litonjua, A.A. Peanut, milk, and wheat intake during pregnancy is associated with reduced allergy and asthma in children. J. Allergy Clin. Immunol. 2014, 133, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Willers, S.M.; Devereux, G.; Craig, L.C.; McNeill, G.; Wijga, A.H.; Abou El-Magd, W.; Turner, S.W.; Helms, P.J.; Seaton, A. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax 2007, 62, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Maslova, E.; Granstrom, C.; Hansen, S.; Petersen, S.B.; Strom, M.; Willett, W.C.; Olsen, S.F. Peanut and tree nut consumption during pregnancy and allergic disease in children-should mothers decrease their intake? Longitudinal evidence from the Danish National Birth Cohort. J. Allergy Clin. Immunol. 2012, 130, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Maslova, E.; Strom, M.; Oken, E.; Campos, H.; Lange, C.; Gold, D.; Olsen, S.F. Fish intake during pregnancy and the risk of child asthma and allergic rhinitis-longitudinal evidence from the Danish National Birth Cohort. Br. J. Nutr. 2013, 110, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Best, K.P.; Sullivan, T.; Palmer, D.; Gold, M.; Kennedy, D.J.; Martin, J.; Makrides, M. Prenatal Fish Oil Supplementation and Allergy: 6-Year Follow-up of a Randomized Controlled Trial. Pediatrics 2016, 137, e20154443. [Google Scholar] [CrossRef] [PubMed]
- Best, K.P.; Sullivan, T.R.; Palmer, D.J.; Gold, M.; Martin, J.; Kennedy, D.; Makrides, M. Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood-a longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ. J. 2018, 11, 10. [Google Scholar] [CrossRef]
- Palmer, D.J.; Sullivan, T.; Gold, M.S.; Prescott, S.L.; Heddle, R.; Gibson, R.A.; Makrides, M. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy 2013, 68, 1370–1376. [Google Scholar] [CrossRef]
- Hansen, S.; Strom, M.; Maslova, E.; Dahl, R.; Hoffmann, H.J.; Rytter, D.; Bech, B.H.; Henriksen, T.B.; Granstrom, C.; Halldorsson, T.I.; et al. Fish oil supplementation during pregnancy and allergic respiratory disease in the adult offspring. J. Allergy Clin. Immunol. 2017, 139, 104–111 e104. [Google Scholar] [CrossRef]
- Venter, C.; Palumbo, M.P.; Glueck, D.H.; Sauder, K.A.; O’Mahony, L.; Fleischer, D.M.; Ben-Abdallah, M.; Ringham, B.M.; Dabelea, D. The maternal diet index in pregnancy is associated with offspring allergic diseases: The Healthy Start study. Allergy 2022, 77, 162–172. [Google Scholar] [CrossRef]
- Milewska-Wrobel, D.; Lis-Swiety, A. Does maternal diet during pregnancy influence clinical and laboratory characteristics of infantile-onset atopic dermatitis? Eur. Ann. Allergy Clin. Immunol. 2020, 52, 277–279. [Google Scholar] [CrossRef]
- Garcia-Larsen, V.; Ierodiakonou, D.; Jarrold, K.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Ruparelia, A.; Devani, P.; Trivella, M.; et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: A systematic review and meta-analysis. PLoS Med. 2018, 15, e1002507. [Google Scholar] [CrossRef]
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R.; et al. EAACI guideline: Preventing the development of food allergy in infants and young children (2020 update). Pediatr. Allergy Immunol. 2021, 32, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.L.; Bacharier, L.B.; Bateman, E.; Boulet, L.P.; Brightling, C.; Buhl, R.; Brusselle, G.; Cruz, A.A.; Drazen, J.M.; Duijts, L.; et al. Key recommendations for primary care from the 2022 Global Initiative for Asthma (GINA) update. NPJ Prim. Care Respir. Med. 2023, 33, 7. [Google Scholar] [CrossRef]
- Fleischer, D.M.; Chan, E.S.; Venter, C.; Spergel, J.M.; Abrams, E.M.; Stukus, D.; Groetch, M.; Shaker, M.; Greenhawt, M. A Consensus Approach to the Primary Prevention of Food Allergy Through Nutrition: Guidance from the American Academy of Allergy, Asthma, and Immunology; American College of Allergy, Asthma, and Immunology; and the Canadian Society for Allergy and Clinical Immunology. J. Allergy Clin. Immunol. Pract. 2021, 9, 22–43 e24. [Google Scholar] [CrossRef]
- van Esch, B.; Porbahaie, M.; Abbring, S.; Garssen, J.; Potaczek, D.P.; Savelkoul, H.F.J.; van Neerven, R.J.J. The Impact of Milk and Its Components on Epigenetic Programming of Immune Function in Early Life and Beyond: Implications for Allergy and Asthma. Front. Immunol. 2020, 11, 2141. [Google Scholar] [CrossRef]
- Acevedo, N.; Alashkar Alhamwe, B.; Caraballo, L.; Ding, M.; Ferrante, A.; Garn, H.; Garssen, J.; Hii, C.S.; Irvine, J.; Llinas-Caballero, K.; et al. Perinatal and Early-Life Nutrition, Epigenetics, and Allergy. Nutrients 2021, 13, 724. [Google Scholar] [CrossRef]
- Suarez-Varela, M.M.; Alvarez, L.G.; Kogan, M.D.; Ferreira, J.C.; Martinez Gimeno, A.; Aguinaga Ontoso, I.; Gonzalez Diaz, C.; Arnedo Pena, A.; Dominguez Aurrecoechea, B.; Busquets Monge, R.M.; et al. Diet and prevalence of atopic eczema in 6 to 7-year-old schoolchildren in Spain: ISAAC phase III. J. Investig. Allergol. Clin. Immunol. 2010, 20, 469–475. [Google Scholar]
- Andrusaityte, S.; Grazuleviciene, R.; Petraviciene, I. Effect of diet and maternal education on allergies among preschool children: A case-control study. Environ. Res. 2017, 159, 374–380. [Google Scholar] [CrossRef]
- Loss, G.; Apprich, S.; Waser, M.; Kneifel, W.; Genuneit, J.; Buchele, G.; Weber, J.; Sozanska, B.; Danielewicz, H.; Horak, E.; et al. The protective effect of farm milk consumption on childhood asthma and atopy: The GABRIELA study. J. Allergy Clin. Immunol. 2011, 128, 766–773 e764. [Google Scholar] [CrossRef]
- Wijga, A.H.; Smit, H.A.; Kerkhof, M.; de Jongste, J.C.; Gerritsen, J.; Neijens, H.J.; Boshuizen, H.C.; Brunekreef, B. Association of consumption of products containing milk fat with reduced asthma risk in pre-school children: The PIAMA birth cohort study. Thorax 2003, 58, 567–572. [Google Scholar] [CrossRef]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Hirota, Y. Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy 2010, 65, 758–765. [Google Scholar] [CrossRef]
- Norback, D.; Zhao, Z.H.; Wang, Z.H.; Wieslander, G.; Mi, Y.H.; Zhang, Z. Asthma, eczema, and reports on pollen and cat allergy among pupils in Shanxi province, China. Int. Arch. Occup. Environ. Health 2007, 80, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, D.; Salameh, P.; Caillaud, D.; Charpin, D.; De Blay, F.; Kopferschmitt, C.; Lavaud, F.; Annesi-Maesano, I.; Baldi, I.; Raherison, C. Prevalence and association of asthma and allergic sensitization with dietary factors in schoolchildren: Data from the french six cities study. BMC Public Health 2015, 15, 993. [Google Scholar] [CrossRef] [PubMed]
- Hodge, L.; Salome, C.M.; Peat, J.K.; Haby, M.M.; Xuan, W.; Woolcock, A.J. Consumption of oily fish and childhood asthma risk. Med. J. Aust. 1996, 164, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Oien, T.; Storro, O.; Johnsen, R. Do early intake of fish and fish oil protect against eczema and doctor-diagnosed asthma at 2 years of age? A cohort study. J. Epidemiol. Community Health 2010, 64, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, F.; Priftis, K.N.; Papadimitriou, A.; Papadopoulos, M.; Roma, E.; Kapsokefalou, M.; Anthracopoulos, M.B.; Panagiotakos, D.B. Adherence to the Mediterranean type of diet is associated with lower prevalence of asthma symptoms, among 10-12 years old children: The PANACEA study. Pediatr. Allergy Immunol. 2011, 22, 283–289. [Google Scholar] [CrossRef]
- Vassilopoulou, E.; Comotti, A.; Douladiris, N.; Konstantinou, G.; Zuberbier, T.; Alberti, I.; Agostoni, C.; Berni Canani, R.; Bocsan, I.C.; Corsello, A.; et al. A systematic review and meta-analysis of nutritional and dietary interventions in randomized controlled trials for skin symptoms in children with atopic dermatitis and without food allergy: An EAACI task force report. Allergy 2024, 79, 1708–1724. [Google Scholar] [CrossRef] [PubMed]
- Lothian, J.B.; Grey, V.; Lands, L.C. Effect of whey protein to modulate immune response in children with atopic asthma. Int. J. Food Sci. Nutr. 2006, 57, 204–211. [Google Scholar] [CrossRef]
- Pontes, M.V.; Ribeiro, T.C.; Ribeiro, H.; de Mattos, A.P.; Almeida, I.R.; Leal, V.M.; Cabral, G.N.; Stolz, S.; Zhuang, W.; Scalabrin, D.M. Cow’s milk-based beverage consumption in 1- to 4-year-olds and allergic manifestations: An RCT. Nutr. J. 2016, 15, 19. [Google Scholar] [CrossRef]
- Bergmann, K.-C.; Raab, J.; Krause, L.; Becker, S.; Kugler, S.; Zuberbier, T.; Roth-Walter, F.; Jensen-Jaroli, E.; Kramer, M.F.; Graessel, A. Long-term benefits of targeted micronutrition with the holoBLG lozenge in house dust mite allergic patients. Allergo J. Int. 2022, 31, 161–171. [Google Scholar] [CrossRef]
- Bergmann, K.C.; Graessel, A.; Raab, J.; Banghard, W.; Krause, L.; Becker, S.; Kugler, S.; Zuberbier, T.; Ott, V.B.; Kramer, M.F.; et al. Targeted micronutrition via holo-BLG based on the farm effect in house dust mite allergic rhinoconjunctivitis patients–first evaluation in a standardized allergen exposure chamber. Allergo J. Int. 2021, 30, 141–191. [Google Scholar] [CrossRef]
- Abbring, S.; Kusche, D.; Roos, T.C.; Diks, M.A.P.; Hols, G.; Garssen, J.; Baars, T.; van Esch, B. Milk processing increases the allergenicity of cow’s milk-Preclinical evidence supported by a human proof-of-concept provocation pilot. Clin. Exp. Allergy 2019, 49, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
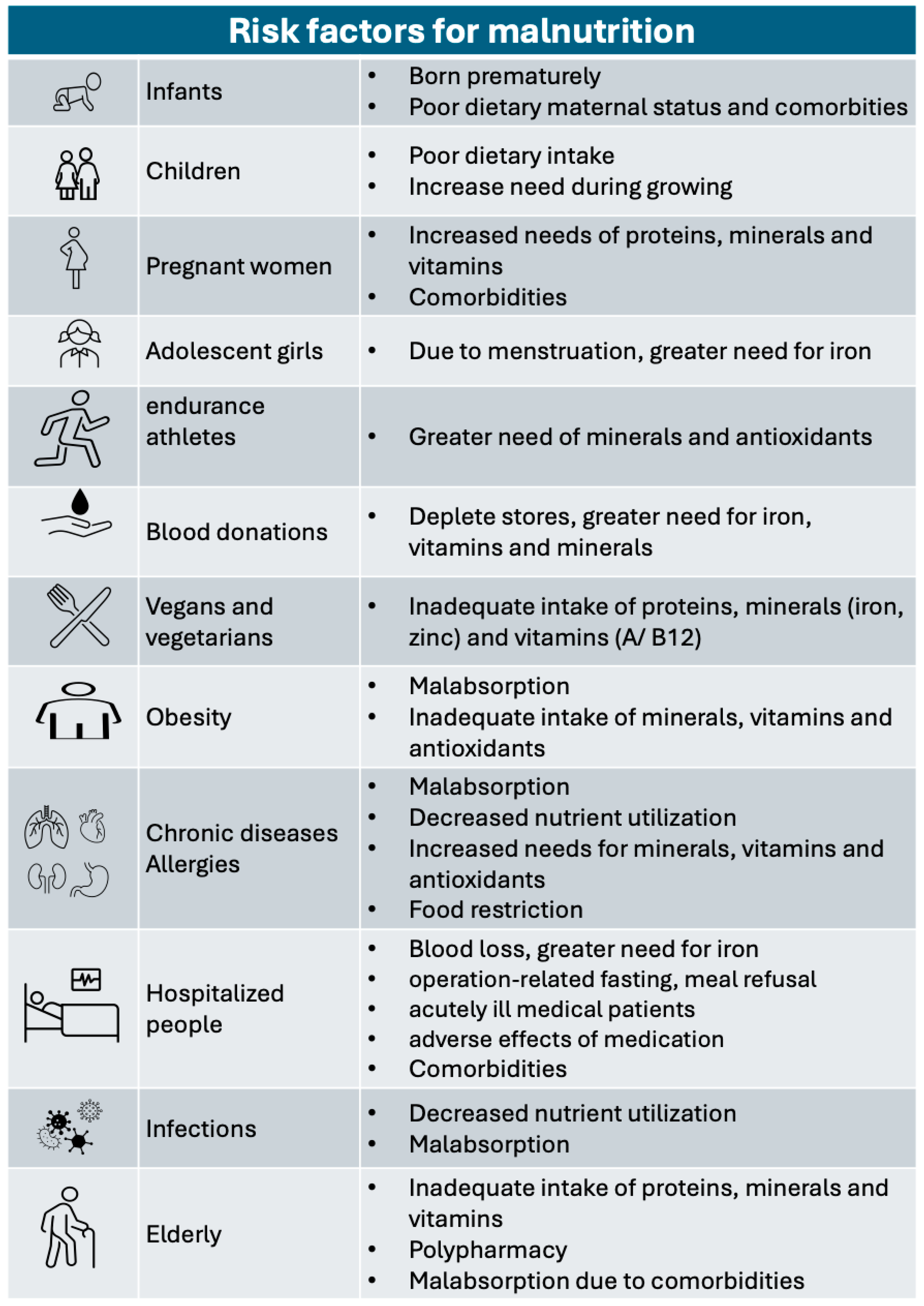
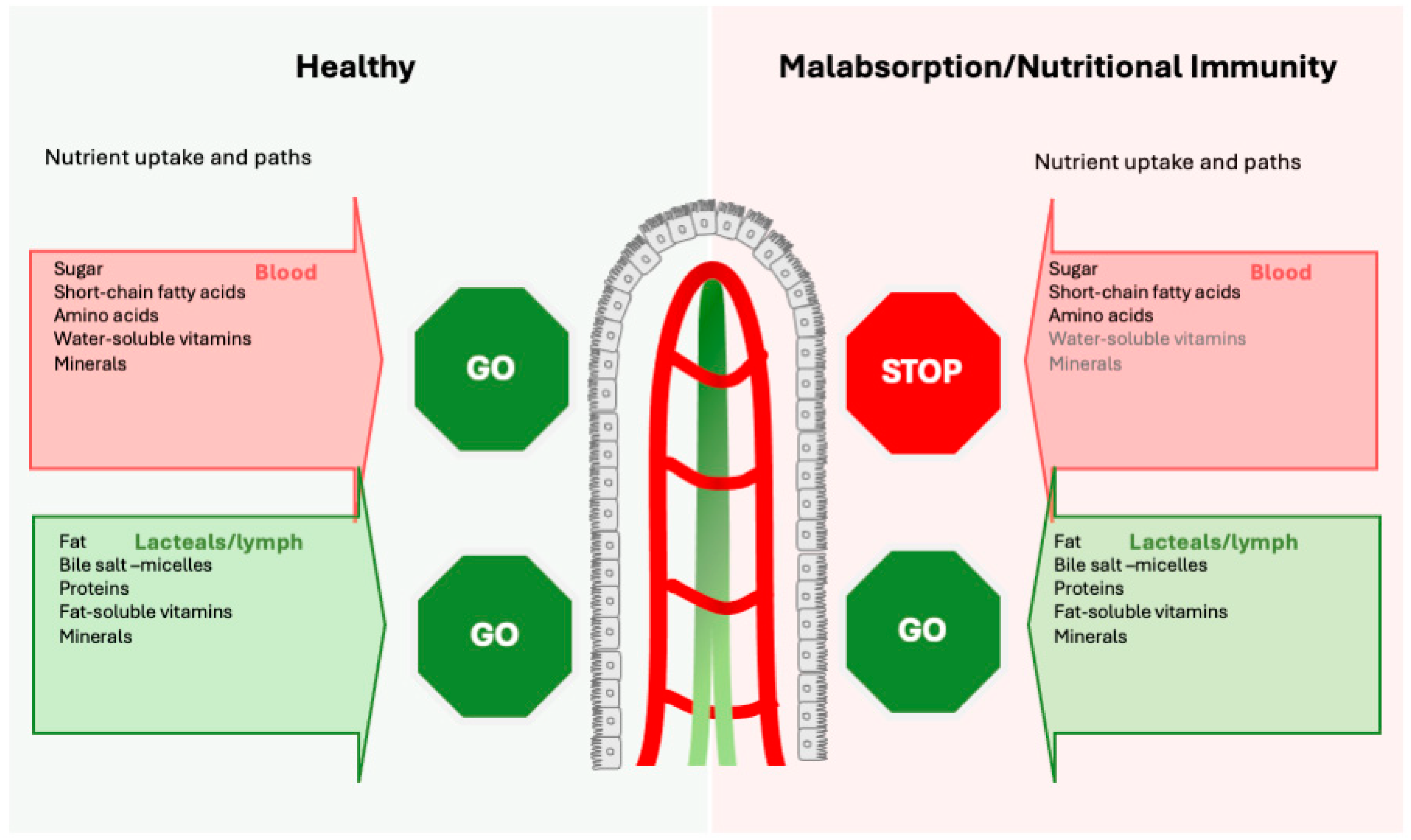
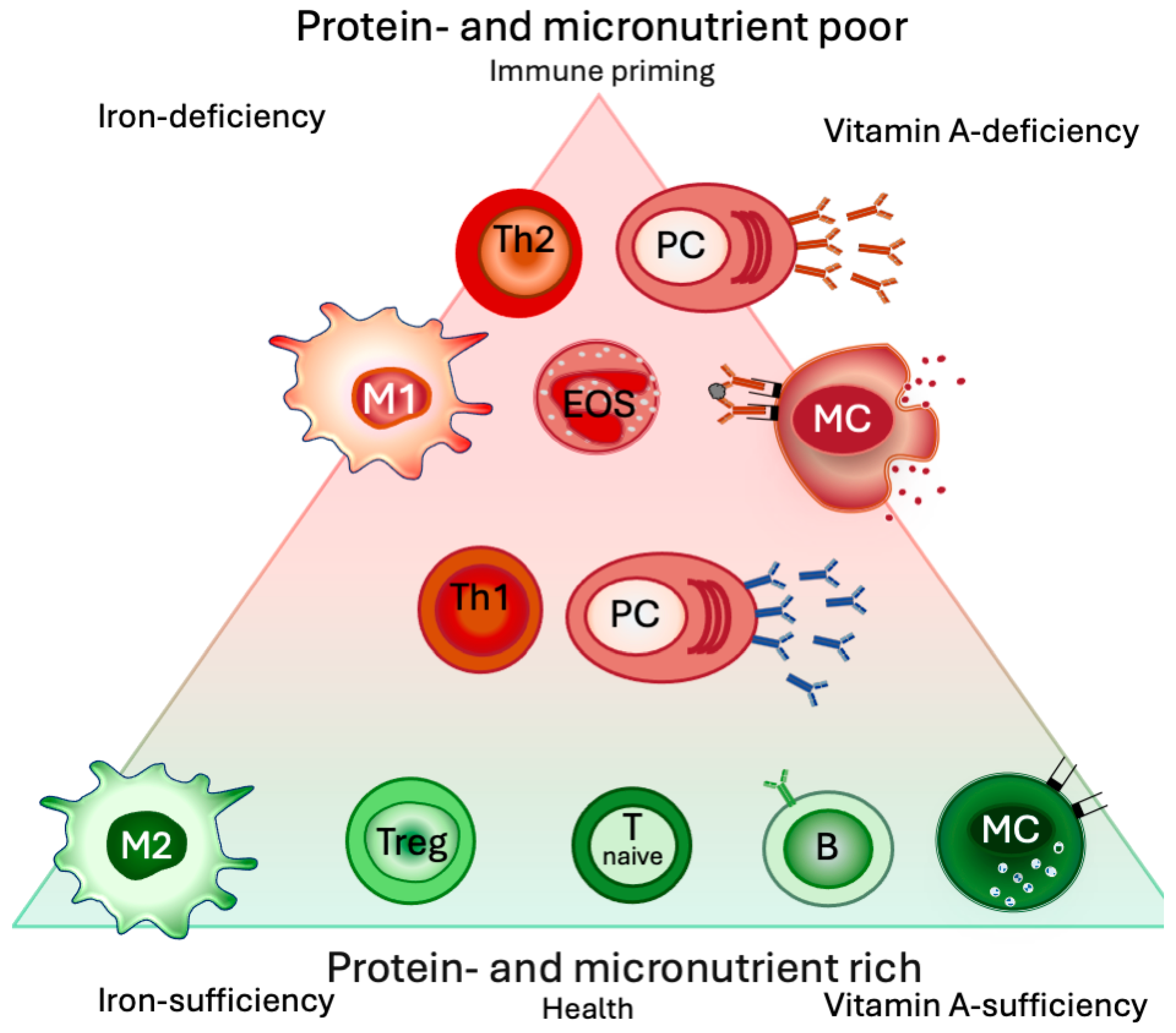
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassilopoulou, E.; Venter, C.; Roth-Walter, F. Malnutrition and Allergies: Tipping the Immune Balance towards Health. J. Clin. Med. 2024, 13, 4713. https://doi.org/10.3390/jcm13164713
Vassilopoulou E, Venter C, Roth-Walter F. Malnutrition and Allergies: Tipping the Immune Balance towards Health. Journal of Clinical Medicine. 2024; 13(16):4713. https://doi.org/10.3390/jcm13164713
Chicago/Turabian StyleVassilopoulou, Emilia, Carina Venter, and Franziska Roth-Walter. 2024. "Malnutrition and Allergies: Tipping the Immune Balance towards Health" Journal of Clinical Medicine 13, no. 16: 4713. https://doi.org/10.3390/jcm13164713





