Breast Reconstruction: The Oncoplastic Approach
Abstract
1. Introduction
1.1. The Development of Breast Oncologic Surgery
1.2. Oncoplastic Breast-Conserving Surgery (OBCS)
2. Oncoplastic Strategies and Classification
2.1. Preoperative Considerations on Oncoplastic Breast Surgery [32]
2.2. Timing of Surgery [32]
3. Surgical Techniques
3.1. Volume-Displacing Oncoplastic Techniques
3.1.1. Glandular Tissue Rearrangement
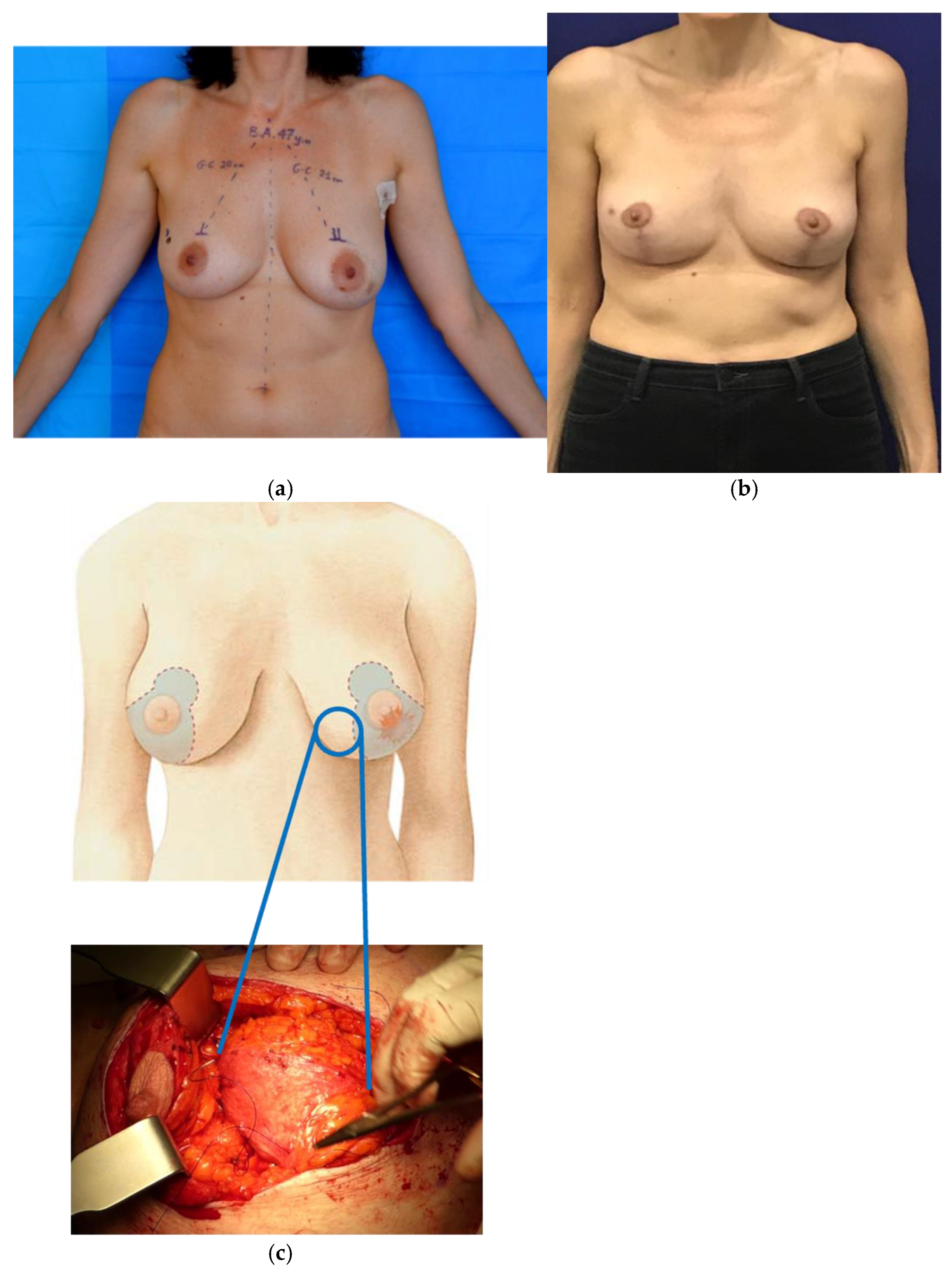
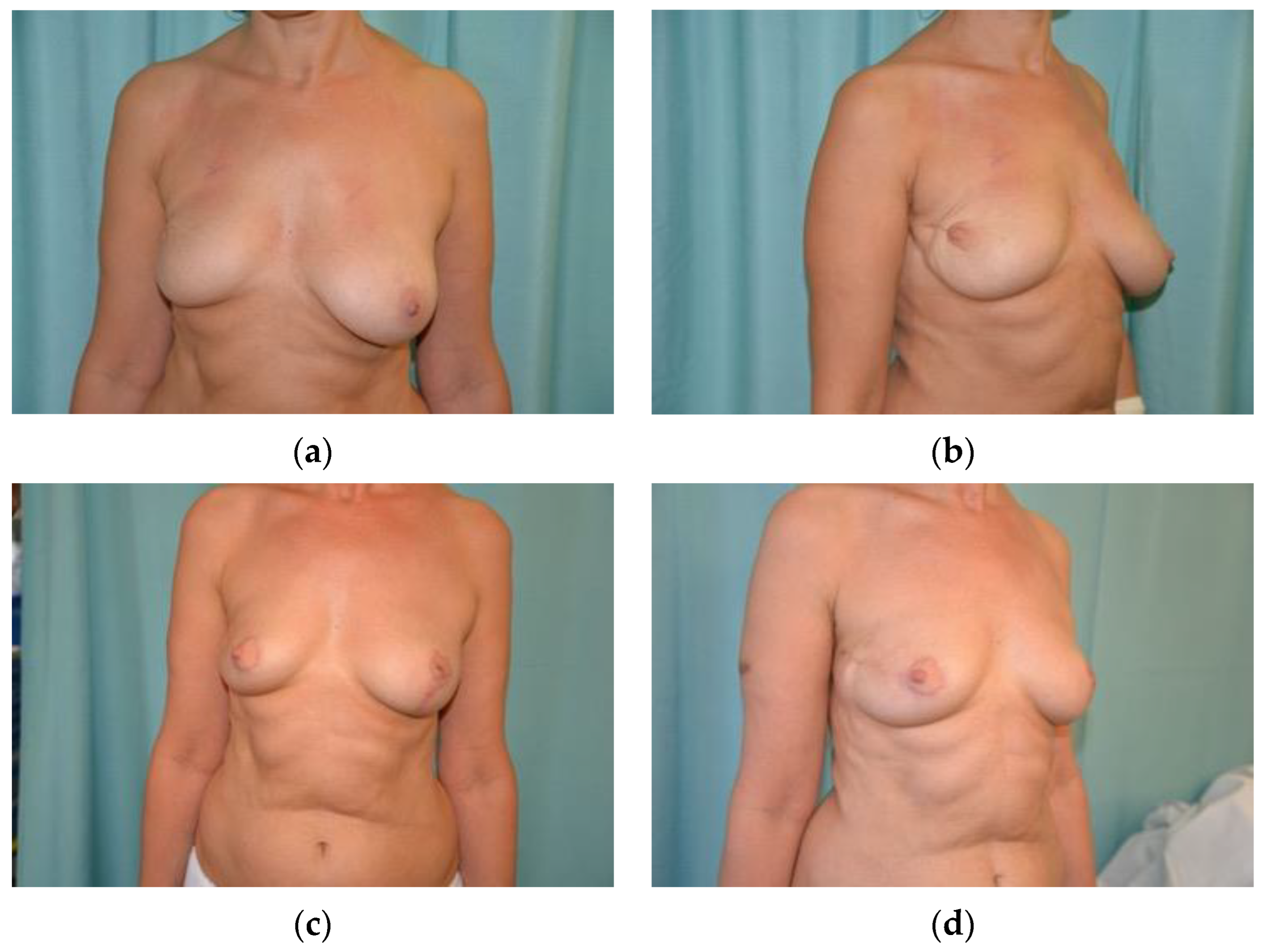
3.1.2. Oncoplastic Mastopexy and Reduction Mammoplasty
3.2. Volume Replacement Oncoplastic Techniques
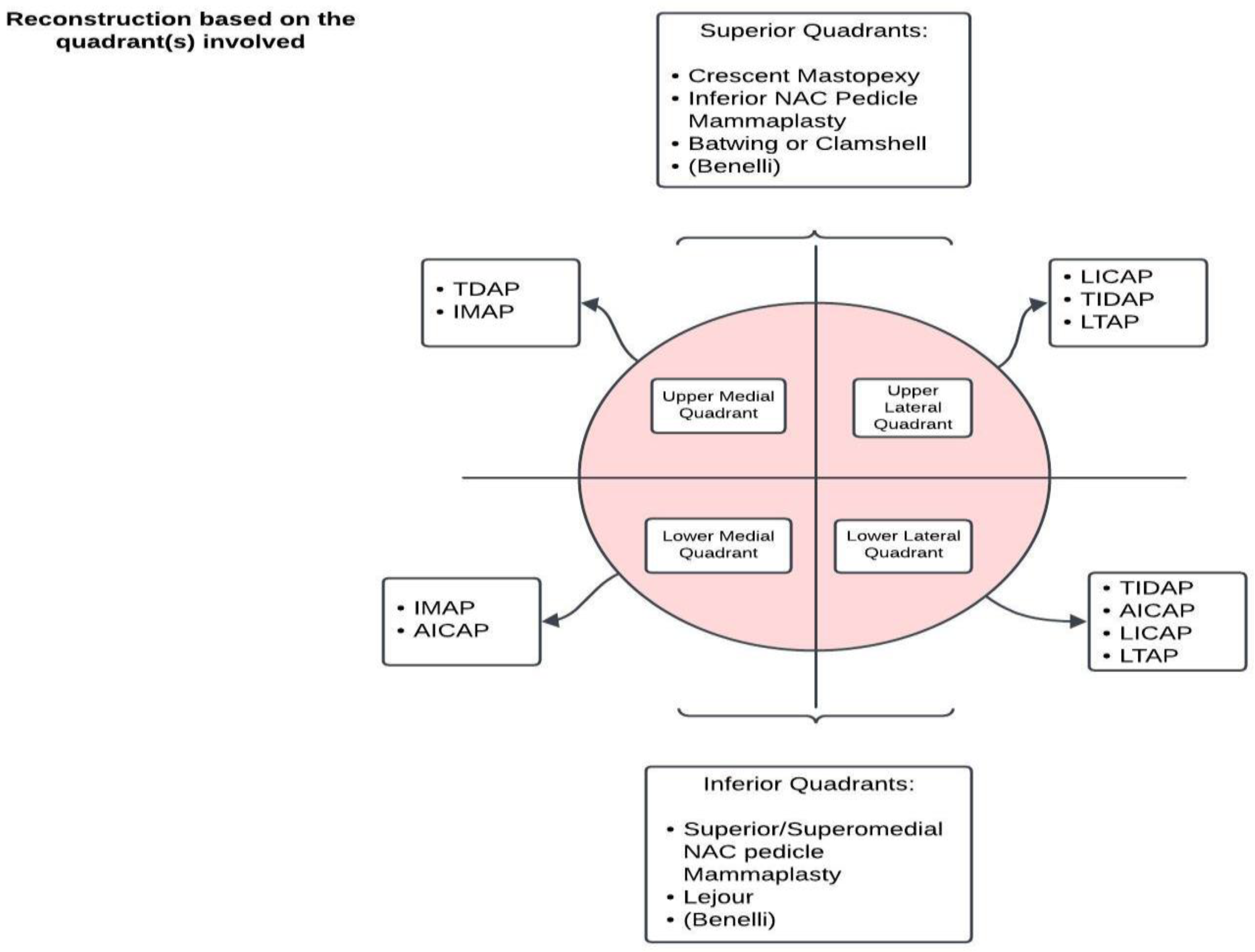
3.2.1. Regional Tissue Transfer [47]
3.2.2. Free Tissue Transfer
4. Fat Injections to the Breast: Lipomodeling
5. NAC Reconstruction
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Lancet. GLOBOCAN 2018: Counting the toll of cancer. Lancet 2018, 392, 985, Erratum in: Lancet 2018, 392, 1196. [Google Scholar] [CrossRef] [PubMed]
- Torras, I.; Cebrecos, I.; Castillo, H.; Mension, E. Evolution of breast conserving surgery-current implementation of oncoplastic techniques in breast conserving surgery: A literature review. Gland. Surg. 2024, 13, 412–425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jonczyk, M.M.; Jean, J.; Graham, R.; Chatterjee, A. Surgical trends in breast cancer: A rise in novel operative treatment options over a 12 year analysis. Breast Cancer Res. Treat. 2019, 173, 267–274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chatterjee, A., II; Asban, A.; Minasian, R.A.; Losken, A.; Graham, R.; Chen, L.; Czerniecki, B.J.; Fisher, C. A cost-utility analysis comparing oncoplastic breast surgery to standard lumpectomy in large breasted women. Adv. Breast Cancer Res. 2018, 7, 14. [Google Scholar] [CrossRef]
- Chatterjee, A.; Gass, J.; Burke, M.B.; Kopkash, K.; El-Tamer, M.B.; Holmes, D.R.; Clark, P.; Reiland, J. Results from the American Society of Breast Surgeons Oncoplastic Surgery Committee 2017 survey: Current practice and future directions. Ann. Surg. Oncol. 2018, 25, 2790–2794. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Vieira, R.A.; Facina, G.; Tiezzi, D.G.; Urban, C.A.; de Freitas Junior, R. Editorial: Oncoplastic surgery for breast cancer. Front. Oncol. 2024, 13, 1348964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moon, J.; Lee, J.; Lee, D.W.; Shin, H.J.; Lee, S.; Kang, Y.; Kim, N.Y.; Park, H.S. Impact of Body Composition on Postoperative Outcomes in Patients Undergoing Robotic Nipple-Sparing Mastectomy with Immediate Breast Reconstruction. Curr. Oncol. 2022, 29, 350–359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Angarita, F.A.; Castelo, M.; Englesakis, M.; McCready, D.R.; Cil, T.D. Robot-assisted nipple-sparing mastectomy: Systematic review. Br. J. Surg. 2020, 107, 1580–1594. [Google Scholar] [CrossRef]
- De Andrade Urban, C. New classification for oncoplastic procedures in surgical practice. Breast 2008, 17, 321–322. [Google Scholar] [CrossRef]
- Weber, W.P.; Morrow, M.; de Boniface, J.; Pusic, A.; Montagna, G.; Kappos, E.A.; Ritter, M. Knowledge gaps in oncoplastic breast surgery. Lancet Oncol. 2020, 21, e375–e385. [Google Scholar] [CrossRef]
- Clough, K.B.; Cuminet, J.; Fitoussi, A.; Nos, C.; Mosseri, V. Cosmetic sequelae after conservative treatment for breast cancer: Classification and results of surgical correction. Ann. Plast. Surg. 1998, 41, 471–481. [Google Scholar] [CrossRef]
- Cil, T.D.; Cordeiro, E. Complications of oncoplastic breast surgery involving soft tissue transfer versus breast-conserving surgery: An analysis of the NSQIP database. Ann. Surg. Oncol. 2016, 23, 3266–3271. [Google Scholar] [CrossRef]
- Bazzarelli, A.; Zhang, J.; Arnaout, A. Patient-reported satisfaction following oncoplastic breast-conserving therapy. In Proceedings of the American Society of Breast Surgeons Annual Meeting, Dallas, TX, USA, 13 April 2016. [Google Scholar]
- Chand, N.D.; Browne, V.; Paramanathan, N.; Peiris, L.J.; Laws, S.A.; Rainsbury, R.M. Patient-reported outcomes are better after oncoplastic breast conservation than after mastectomy and autologous reconstruction. Plast. Reconstr. Surg. 2017, 5, e1419. [Google Scholar] [CrossRef]
- Oliveira-Junior, I.; Silva, I.A.; Silva, F.C.B.; Silva, J.J.; Sarri, A.J.; Paiva, C.E.; da Costa Vieira, R.A. Oncoplastic Surgery in Breast-Conserving Treatment. Patient Profile and impact on quality of life. Breast Care 2021, 16, 243–253. [Google Scholar] [CrossRef]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.-H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, A.K.; Kon, P.S.; Oberg, K.C.; Miles, D.A. Aesthetic outcomes inpatients undergoing breast conservation therapy for the treatmentof localized breast cancer. Plast. Reconstr. Surg. 2004, 114, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Matory, W.E., Jr.; Wertheimer, M.; Fitzgerald, T.J.; Walton, R.L.; Love, S.; Matory, W.E. Aesthetic results following partial mastectomy andradiation therapy. Plast. Reconstr. Surg. 1990, 85, 739–746. [Google Scholar] [CrossRef]
- Clough, K.B.; Benyahi, D.; Nos, C.; Charles, C.; Sarfati, I. Oncoplastic surgery: Pushing the limits of breast-conserving surgery. Breast J. 2015, 21, 140–146. [Google Scholar] [CrossRef]
- Patel, K.; Bloom, J.; Nardello, S.; Cohen, S.; Reiland, J.; Chatterjee, A. An Oncoplastic Surgery Primer: Common Indications, Techniques, and Complications in Level 1 and 2 Volume Displacement Oncoplastic Surgery. Ann. Surg. Oncol. 2019, 26, 3063–3070. [Google Scholar] [CrossRef]
- Paulinelli, R.R.; Oliveira, V.M.; Bagnoli, F.; Chade, M.C.; Alves, K.L.; Freitas-Junior, R. Oncoplastic mammaplasty with geometric compensation—A technique for breast conservation. J. Surg. Oncol. 2014, 110, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, A.M.; Montag, E.; Gemperli, R. Current aspects of therapeutic reduction mammaplasty for immediate early breast cancer management: An update. World J. Clin. Oncol. 2014, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Borm, K.J.; Schonknecht, C.; Nestler, A.; Oechsner, M.; Waschulzik, B.; Combs, S.E.; Münch, S.; Niemeyer, M.; Duma, M.N. Outcomes of immediate oncoplastic surgery and adjuvant radiotherapy in breast cancer patients. BMC Cancer 2019, 19, 907. [Google Scholar] [CrossRef] [PubMed]
- Clough, K.B.; Kaufman, G.J.; Nos, C.; Buccimazza, I.; Sarfati, I.M. Improving breast cancer surgery: A classification and quadrant per quadrant atlas for oncoplastic surgery. Ann. Surg. Oncol. 2010, 17, 1375–1391. [Google Scholar] [CrossRef]
- Chatterjee, A.; Gass, J.; Patel, K.; Holmes, D.; Kopkash, K.; Peiris, L.; Peled, A.; Ryan, J.; El-Tamer, M.; Reiland, J. A Consensus Definition and Classification System of Oncoplastic Surgery Developed by the American Society of Breast Surgeons. Ann. Surg. Oncol. 2019, 26, 3436–3444. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.P.; Soysal, S.D.; El-Tamer, M.; Sacchini, V.; Knauer, M.; Tausch, C.; Hauser, N.; Günthert, A.; Harder, Y.; Kappos, E.A.; et al. First international consensus conference on standardization of oncoplastic breast conserving surgery. Breast Cancer Res. Treat. 2017, 165, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Wallwiener, D. Classifying breast cancer surgery: A novel, complexity-based system for oncological, oncoplastic and reconstructive procedures, and proof of principle by analysis of 1225 operations in 1166 patients. BMC Cancer. 2009, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.P.; Soysal, S.D.; Fulco, I.; Barandun, M.; Babst, D.; Kalbermatten, D.; Schaefer, D.J.; Oertli, D.; Kappos, E.A.; Haug, M. Standardization of oncoplastic breast conserving surgery. Eur. J. Surg. Oncol. 2017, 43, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.A.; Carrara, G.F.; Scapulatempo Neto, C.; Morini, M.A.; Brentani, M.M.; Folgueira, M.A. The role of oncoplastic breast conserving treatment for locally advanced breast tumors. A matching case-control study. Ann. Med. Surg. 2016, 10, 61–68. [Google Scholar] [CrossRef]
- Santos, G.; Urban, C.; Edelweiss, M.I.; Zucca-Matthes, G.; de Oliveira, V.M.; Arana, G.H.; Iera, M.; Rietjens, M.; de Lima, R.S.; Spautz, C.; et al. Long-Term Comparison of Aesthetical Outcomes After Oncoplastic Surgery and Lumpectomy in Breast Cancer Patients. Ann. Surg. Oncol. 2015, 22, 2500–2508. [Google Scholar] [CrossRef]
- Savalia, N.B.; Silverstein, M.J. Oncoplastic breast reconstruction: Patient selection and surgical techniques. J. Surg. Oncol. 2016, 113, 875–882. [Google Scholar] [CrossRef]
- Anderson, B.O.; Masetti, R.; Silverstein, M.J. Oncoplastic approaches to partial mastectomy: An overview of volume-displacement tech-niques. Lancet Oncol. 2005, 6, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Veiga, D.F.; Veiga-Filho, J.; Ribeiro, L.M.; Archangelo-Junior, I.; Mendes, D.A.; Andrade, V.O.; Caetano, L.V.; Campos, F.S.M.; Juliano, Y.; Ferreira, L.M. Evaluations of aesthetic outcomes of oncoplastic surgery by surgeons of different gender and specialty: A prospective controlled study. Breast 2011, 20, 407–412. [Google Scholar] [CrossRef]
- Losken, A.; Brown, C.A. How to optimise aesthetics for the partial mastectomy patient. Aesthet. Surg. J. 2020, 40 (Suppl. S2), S55–S65. [Google Scholar] [CrossRef] [PubMed]
- Munshi, A.; Kakkar, S.; Bhutani, R.; Jalali, R.; Budrukkar, A.; Dinshaw, K.A. Factors influencing cosmetic outcome in breast conservation. Clin. Oncol. (R. Coll Radiol.) 2009, 21, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.E.; Perez, C.A.; Halverson, K.J.; Kuske, R.R.; Philpott, G.W.; Garcia, D.M.; Mortimer, J.E.; Myerson, R.J.; Radford, D.; Rush, C. Factors influencing cosmetic results after conservation therapy for breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 753–764. [Google Scholar] [CrossRef]
- Foersterling, E.; Golatta, M.; Hennigs, A.; Schulz, S.; Rauch, G.; Schott, S.; Domschke, C.; Schuetz, F.; Sohn, C.; Heil, J. Predictors of early poor aesthetic outcome after breast-conserving surgery in patients with breast cancer: Initial results of a prospective cohort study at a single institution. J. Surg. Oncol. 2014, 110, 801–806. [Google Scholar] [CrossRef]
- Hennigs, A.; Hartmann, B.; Rauch, G.; Golatta, M.; Tabatabai, P.; Domschke, C.; Schott, S.; Schütz, F.; Sohn, C.; Heil, J. Long-term objective esthetic outcome after breast-conserving therapy. Breast Cancer Res. Treat. 2015, 153, 345–351. [Google Scholar] [CrossRef]
- Waljee, J.F.; Hu, E.S.; Newman, L.A.; Alderman, A.K. Predictors of breast asymmetry after breast-conserving operation for breast cancer. J. Am. Coll. Surg. 2008, 206, 274–280. [Google Scholar] [CrossRef]
- Kronowitz, S.J.; Kuerer, H.M.; Buchholz, T.A.; Valero, V.; Hunt, K.K. A management algorithm and practical oncoplastic surgical techniques for repairing partial mastectomy defects. Plast. Reconstr. Surg. 2008, 122, 1631–1647. [Google Scholar] [CrossRef]
- Egro, F.M.; Pinell-White, X.; Hart, A.M.; Marie, A.; Albert, L. The use of reduction mammaplasty with breast conservation therapy: An analysis of timing and outcomes. Plast. Reconstr. Surg. 2015, 135, 963e–971e. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Van Landuyt, K.; Monstrey, S.; Blondeel, P. Pedicledperforator flaps in breast reconstruction: A new concept. Br. J. PlastSurg. 2004, 57, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, A.J.; Khan, F.N. An intraoperative algorithm for use of the SIEAflap for breast reconstruction. Plast. Reconstr. Surg. 2007, 120, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, A.J.; Patel, K.M.; Cocilovo, C.; Nahabedian, M.Y.; Miraliakbari, R. The biplanar oncoplastic technique case series: A 2-year review. Gland. Surg. 2015, 4, 257–262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salibian, A.A.; Olson, B.; Shauly, O.; Patel, K.M. Oncoplastic breast reconstruction: Principles, current techniques, and future directions. J. Surg. Oncol. 2022, 126, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Chartier, C.; Safran, T.; Alhalabi, B.; Murphy, A.; Davison, P. Locoregional perforator flaps in breast reconstruction: An anatomic review & quadrant algorithm. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 1328–1341. [Google Scholar] [CrossRef] [PubMed]
- Kronowitz, S.J.; Feledy, J.A.; Hunt, K.K.; Kuerer, H.M.; Youssef, A.; Koutz, C.A.; Robb, G.L. Determining the optimal approach to breast reconstruction after partial mastectomy. PlastReconstr Surg. 2006, 117, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nahabedian, M.Y.; Patel, K.M.; Kaminsky, A.J.; Cocilovo, C.; Miraliakbari, R. Biplanar oncoplastic surgery: A novel approach to breast conserva-tion for small and medium sized breasts. Plast. Reconstr. Surg. 2013, 132, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Dayicioglu, D.; Khakpour, N.; Czerniecki, B.J. Oncoplastic Surgery: Keeping it simple with 5 essential volume displacement techniques for breast conservation in a patient with moderate- to large-sized breasts. Cancer Control 2017, 24, 1073274817729043. [Google Scholar] [CrossRef]
- Losken, A.; Hart, A.M.; Dutton, J.W.; Broecker, J.S.; Styblo, T.M.; Carlson, G.W. The expanded use of autoaugmentation techniques in oncoplastic breast surgery. Plast. Reconstr. Surg. 2018, 141, 10–19. [Google Scholar] [CrossRef]
- Wise, R.J. A preliminary report on a method of planning the mammaplasty. Plast Reconstr. Surg. (1946) 1956, 17, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Barnea, Y.; Inbal, A.; Barsuk, D.; Menes, T.; Zaretski, A.; Leshem, D.; Weiss, J.; Schneebaum, S.; Gur, E. Oncoplastic reduction using the vertical scar superior-medial pedicle pattern technique for immediate partial breast reconstruction. Can. J. Surg. 2014, 57, E134–E140. [Google Scholar] [CrossRef] [PubMed]
- Lejour, M. Vertical mammaplasty and liposuction of the breast. Plast. Reconstr. Surg. 1994, 94, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Losken, A.; Funderburk, C.D.; Duggal, C. The extended superomedial pedicle: Advancing mammaplasty techniques. Mod. Plast. Surg. 2013, 110, 82–89. [Google Scholar] [CrossRef][Green Version]
- Piper, M.L.; Esserman, L.J.; Sbitany, H.; Peled, A.W. Outcomes following oncoplastic reduction mammoplasty: A systematic review. Ann. PlastSurg. 2016, 76 (Suppl. S3), S222–S226. [Google Scholar] [CrossRef]
- Giacalone, P.L.; Dubon, O.; Roger, P.; El Gareh, N.; Rihaoui, S.; Daures, J.P. Doughnut mastopexy lumpectomy versus standard lumpectomy in breast cancer surgery: A prospective study. Eur. J. Surg. Oncol. 2007, 33, 301–306. [Google Scholar] [CrossRef]
- Chung, T.L.; Schnaper, L.; Silverman, R.P.; Holton, L.H., 3rd; Slezak, S. Anovel reconstructive technique following central lumpectomy. PlastReconstr Surg. 2006, 118, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, V.; Zurrida, S.; Luini, A.; Greco, M.; Zanini, V.; Callegari, M.; Grisotti, A.; Veronesi, P.; Catania, S. Central small size breast cancer: How to overcome the problem of nipple and areola involvement. Eur. J. Cancer 1993, 29A, 1093–1096. [Google Scholar] [CrossRef]
- Benelli, L. A new periareolar mammaplasty: The “round block” technique. Aesthetic Plast. Surg. 1990, 14, 93–100. [Google Scholar] [CrossRef]
- Rainsbury, R.M. Breast-sparing reconstruction with latissimus dorsi mini flaps. Eur. J. Surg. Oncol. 2002, 28, 891–895. [Google Scholar] [CrossRef]
- Hamdi, M.; Spano, A.; Landuyt, K.V.; D’Herde, K.; Blondeel, P.; Monstrey, S. The lateral intercostal artery perforators: Anatomical study and clinical application in breast surgery. Plast. Reconstr. Surg. 2008, 121, 389–396. [Google Scholar] [CrossRef]
- Hamdi, M.; Van Landuyt, K.; de Frene, B.; Roche, N.; Blondeel, P.; Monstrey, S. The versatility of the inter-costal artery perforator (ICAP) flaps. J. Plast. Reconstr. Aesthet. Surg. 2006, 59, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Eom, J.R.; Lee, J.W.; Lee, J.; Park, H.Y.; Yang, J.D. Utility of Two Surgical Techniques Using a Lateral Intercostal Artery Perforator Flap after Breast-Conserving Surgery: A Single-Center Retrospective Study. Plast. Reconstr. Surg. 2019, 143, 477e–487e. [Google Scholar] [CrossRef] [PubMed]
- Meybodi, F.; Cocco, A.M.; Messer, D.; Brown, A.; Kanesalingam, K.; Elder, E.; Hsu, J.; French, J. The modified lateral intercostal artery perforator flap. Plast. Reconstr. Surg. Glob. Open. 2019, 7, e2066. [Google Scholar] [CrossRef] [PubMed]
- Hakakian, C.S.B.; Lockhart, R.A.B.; Kulber, D.A.; Aronowitz, J.A. Lateral Intercostal Artery Perforator Flap in Breast Reconstruction: A Simplified Pedicle Permits an Expanded Role. Ann. Plast. Surg. 2016, 76, S184–S190. [Google Scholar] [CrossRef] [PubMed]
- McCulley, S.J.; Schaverien, M.V.; Tan, V.K.; Macmillan, R.D. Lateral thoracic artery perforator (LTAP) flap in partial breast reconstruction. J. PlastReconstr Aesthet. Surg. 2015, 68, 686–691.458. [Google Scholar] [CrossRef] [PubMed]
- Adler, N.; Seitz, I.A.; Song, D.H. Pedicled thoracodorsal artery perforator flap in breast reconstruction: Clinical experience. Eplasty 2009, 9, e24. [Google Scholar] [PubMed] [PubMed Central]
- Wei, F.; Mardini, S. Flaps and Reconstructive Surgery; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2, p. 447. [Google Scholar]
- Heitmann, C.; Pelzer, M.; Kuentscher, M.; Menke, H.; Germann, G. The extended latissimus dorsi flap revisited. Plast. Reconstr. Surg. 2003, 111, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, C.L.; Mendoza, M.M.; Sempere, L.N.; Sanz, J.S.; Torres, A.N.; Barraquer, E.L. Versatility of the pedicled thoracodorsal artery perforator (TDAP) flap in soft tissue reconstruction. Ann. Plast. Surg. 2007, 58, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, C.; Guerra, A.; Metzinger, S.W.; Levin, L.S.; Allen, R.J. The thoracodorsal artery perforator flap: Anatomic basis and clinical application. Ann. Plast. Surg. 2003, 51, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-López, C.; Ibanez, J.F.J.; Vilà, J.; Tomas, M.A.L.; Lopez, J.N.; Miguel, I.P.; Fernandez-Llamazares-Rodriguez, J.; Higueras-Suñe, C. Anterior intercostal artery perforator flap in immediate breast reconstruction: Anatomi-cal study and clinical application. Microsurgery 2017, 37, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Schaverien, M.V.; Kuerer, H.M.; Caudle, A.S.; Smith, B.D.; Hwang, R.F.; Robb, G.L. Outcomes of volume replacement oncoplastic breast-conserving surgery using chest wall perforator flaps: Comparison with volume displacement oncoplastic surgery and total breast reconstruction. Plast. Reconstr. Surg. 2020, 146, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Vesely, M.J.; Murray, D.J.; Novak, C.B.; Gullane, P.J.; Neligan, P.C. The internal mammary artery perforator flap: An anatomical study and a case report. Ann. Plast. Surg. 2007, 58, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Huizum, M.A.V.; Hage, J.J.; Oldenburg, H.A.; Hoornweg, M.J. Internal Mammary Artery Perforator Flap for Immediate Volume Replacement Following Wide Local Excision of Breast Cancer. Arch. Plast. Surg. 2017, 44, 502–508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rizzuto, R.P.; Allen, R.J. Reconstruction of a partial mastectomy defect with the superficial inferior epigastric artery (SIEA) flap. J. Reconstr. Microsurg. 2004, 20, 441–445. [Google Scholar] [CrossRef] [PubMed]
- McCulley, S.J.; Macmillan, R.D.; Rasheed, T. Transverse Upper Gracilis (TUG) flap for volume replacement in breast conserving surgery for medial breast tumours in small to medium sized breasts. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Molina, B.J.; Dayan, E.; Jablonka, E.M.; Okwali, M.; Kim, J.N.; Dayan, J.H.; Smith, M.L. Defining the Role of Free Flaps in Partial Breast Reconstruction. J. Reconstr. Microsurg. 2018, 34, 185–192. [Google Scholar] [CrossRef]
- Spiegel, A.J.; Eldor, L. Partial breast reconstruction with mini superficial inferior epigastric artery and mini deep inferior epigastric perforator flaps. Ann. Plast. Surg. 2010, 65, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Salibian, A.A.; Patel, K.M. Microsurgery in oncoplastic breast reconstruction. Gland. Surg. 2023, 12, 527–534. [Google Scholar] [CrossRef]
- Halim, A.S.; Alwi, A.A. Internal mammary perforators as recipient vessels for deep inferior epigastric perforator and muscle-sparing free transverse rectus abdominis musculocutaneous flap breast reconstruction in an Asian population. Ann. Plast. Surg. 2014, 73, 170–173. [Google Scholar] [CrossRef]
- Zaha, H.; Inamine, S.; Naito, T.; Nomura, H. Laparoscopically harvested omental flap for immediate breast reconstruction. Am. J. Surg. 2006, 192, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Delay, E.; Gosset, J.; Toussoun, G.; Delaporte, T.; Delbaere, M. Efficacy of lipomodelling for the management of sequelae of breast cancer conservative treatment. Ann. Chir. Plast. Esthet. 2008, 53, 153Y168. [Google Scholar]
- Spear, S.L.; Wilson, H.B.; Lockwood, M.D. Fat injection to correct contour de- formities in the reconstructed breast. Plast. Reconstr. Surg. 2005, 116, 1300Y1305. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, G.; Marchi, A.; Galiè, M.; Baroni, G.; Benati, D.; Krampera, M.; Pasini, A.; Sbarbati, A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose- derived adult stem cells. Plast. Reconstr. Surg. 2007, 119, 1409–1422. [Google Scholar] [CrossRef] [PubMed]
- Biazus, J.V.; Falcão, C.C.; Parizotto, A.C.; Stumpf, C.C.; Cavalheiro, J.A.C.; Schuh, F.; Cericatto, R.; Zucatto, Â.E.; Melo, M.P. Immediate reconstruction with autologous fat transfer following breast-conserving surgery. Breast J. 2015, 21, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Biazus, J.V.; Stumpf, C.C.; Melo, M.P.; Zucatto, A.E.; Cericatto, R.; Cavalheiro, J.A.; Damin, A.P. Breast-conserving surgery with immediate autologous fat grafting reconstruction: Oncologic outcomes. Aesthetic Plast. Surg. 2018, 42, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Khan, L.; Raine, C.; Dixon, J. Immediate lipofilling in breast conserving surgery. Eur J Surg Oncol (EJSO) 2017, 43, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, C.C.; Biazús, J.V.; Zucatto, F.S.; Cericatto, R.; Cavalheiro, J.A.; Damin, A.P.; Melo, M.P. Immediate reconstruction with autologous fat grafting: Influence in loco regional recurrence in breast cancer. Rev. Col. Bras. Cir. 2017, 44, 179–186. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, M.C.; Park, H.Y.; Yang, J.D. Oncoplastic volume replacement techniques according to the excised volume and tumor location in small-to moderate-sized breasts. Gland. Surg. 2014, 3, 14. [Google Scholar]
- Ahmed, Y.S.; El Maksoud, W.M.A.; Sultan, M.H.; El-Bakoury, E.A. Immediate Lipo-Filling as a Novel Technique for Volume Replacement in Oncoplastic Breast Conservative Surgery. Aesth Plast. Surg. 2022, 46, 1612–1621. [Google Scholar] [CrossRef]
- Sisti, A.; Grimaldi, L.; Tassinari, J.; Cuomo, R.; Fortezza, L.; Bocchiotti, M.A.; Roviello, F.; D’Aniello, C.; Nisi, G. Nipple-areola complex reconstruction techniques: A literature review. Eur. J. Surg. Oncol. (EJSO) 2016, 42, 441–465. [Google Scholar] [CrossRef] [PubMed]
- Boccola, M.; Savage, J.; Rozen, W.M.; Ashton, M.; Milner, C.; Rahdon, R.; Whitaker, I.S. Surgical Correction and Reconstruction of the Nipple-Areola Complex: Current Review of Techniques. J. Reconstr. Microsurg. 2010, 26, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Few, J.W.; Marcus, J.R.; Casas, L.A.; Aitken, M.E.; Redding, J. Long-term predictable nipple projection following reconstruction. Plast. Reconstr. Surg. 1999, 104, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Nimboriboonporn, A.; Chuthapisith, S. Nipple-areola complex reconstruction. Gland. Surg. 2014, 3, 35–42. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vindigni, V.; Marena, F.; Zanettin, C.; Bassetto, F. Breast Reconstruction: The Oncoplastic Approach. J. Clin. Med. 2024, 13, 4718. https://doi.org/10.3390/jcm13164718
Vindigni V, Marena F, Zanettin C, Bassetto F. Breast Reconstruction: The Oncoplastic Approach. Journal of Clinical Medicine. 2024; 13(16):4718. https://doi.org/10.3390/jcm13164718
Chicago/Turabian StyleVindigni, Vincenzo, Francesco Marena, Chiara Zanettin, and Franco Bassetto. 2024. "Breast Reconstruction: The Oncoplastic Approach" Journal of Clinical Medicine 13, no. 16: 4718. https://doi.org/10.3390/jcm13164718
APA StyleVindigni, V., Marena, F., Zanettin, C., & Bassetto, F. (2024). Breast Reconstruction: The Oncoplastic Approach. Journal of Clinical Medicine, 13(16), 4718. https://doi.org/10.3390/jcm13164718







