Fistulizing Perianal Disease as a First Manifestation of Crohn’s Disease: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. In-And Exclusion Criteria
3. Outcomes
3.1. Study Selection and Data Extraction
3.2. Statistical Analysis
4. Results
4.1. Study Selection
4.2. Study Characteristics and Quality Assessment
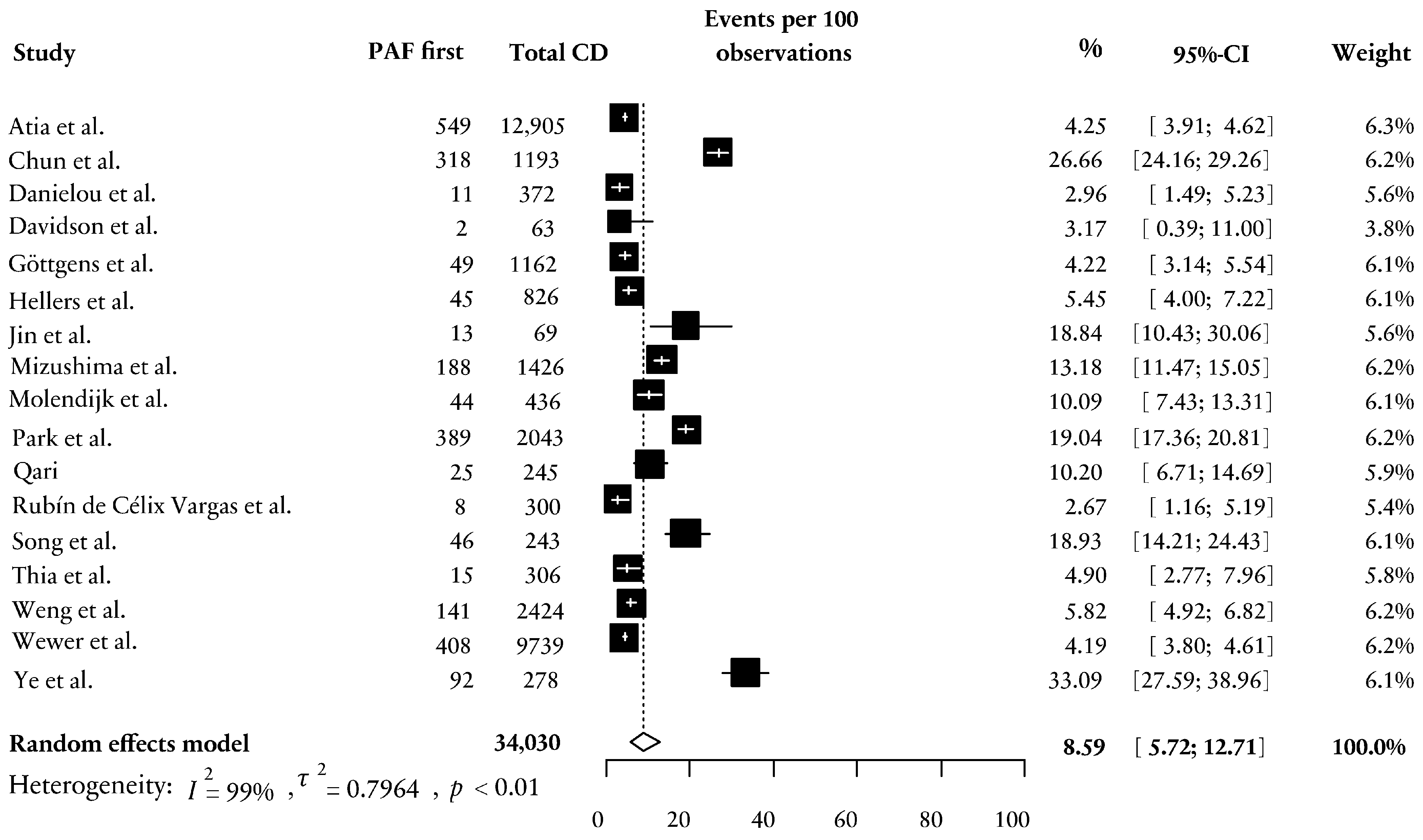
4.3. PAF First in Patients with CD and Time to Diagnosis of CD
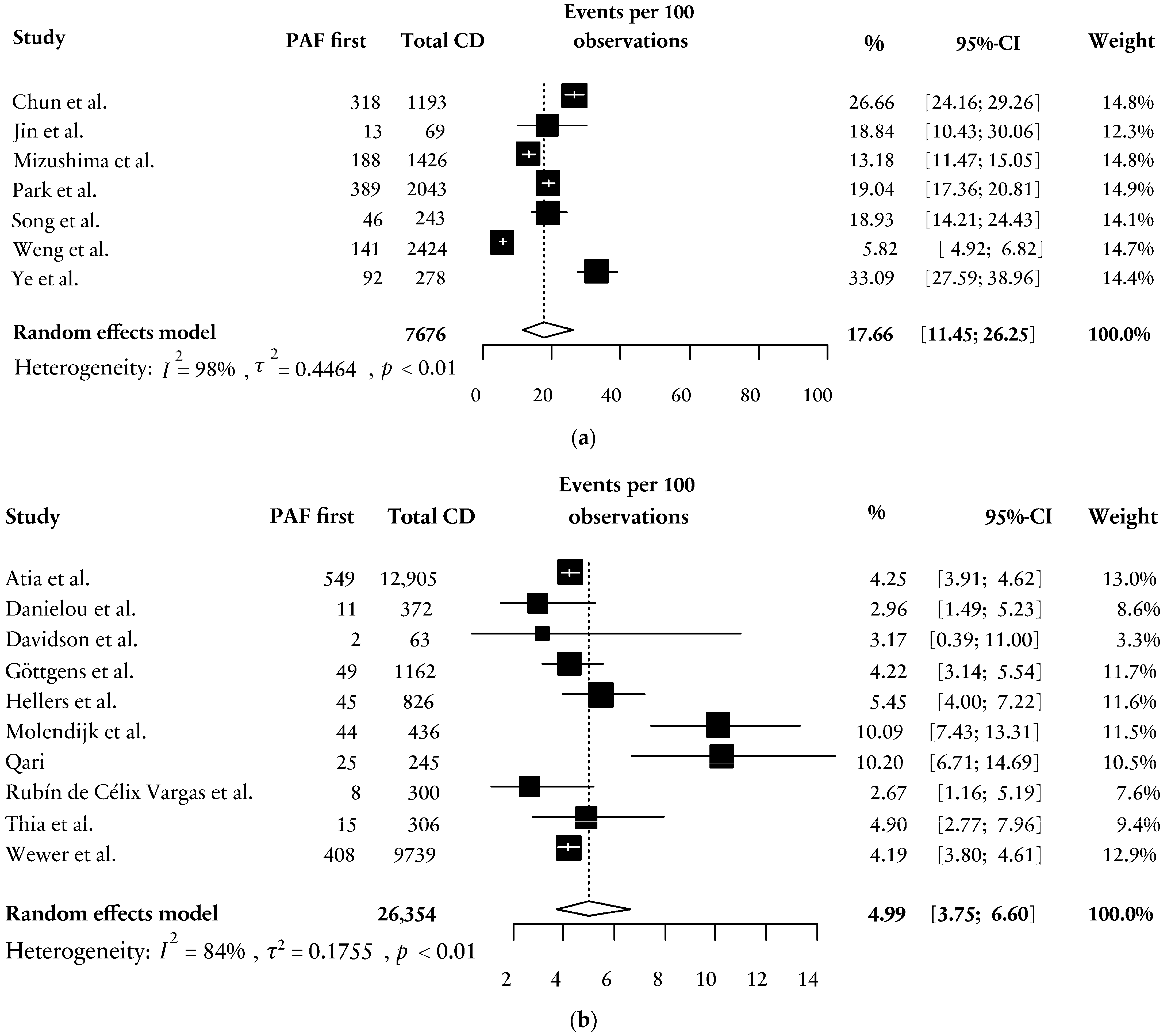
4.4. PAF First and Sex
4.5. PAF First and Ethnicity
4.6. PAF First and Age
4.7. Time to Diagnosis Correlated to Length of Follow-Up
4.8. Impact of Time to Diagnosis on Long-Term Clinical Outcomes
5. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adegbola, S.O.; Dibley, L.; Sahnan, K.; Wade, T.; Verjee, A.; Sawyer, R.; Mannick, S.; McCluskey, D.; Yassin, N.; Phillips, R.K.S.; et al. Burden of disease and adaptation to life in patients with Crohn’s perianal fistula: A qualitative exploration. Health Qual Life Outcomes 2020, 18, 370. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.; Velasco, A.L. Fistula-in-Ano. In StatPearls; StatPearls Publishing LLC: St. Petersburg, FL, USA, 2024. [Google Scholar]
- Gold, S.L.; Cohen-Mekelburg, S.; Schneider, Y.; Steinlauf, A. Perianal Fistulas in Patients with Crohn’s Disease, Part 2: Surgical, Endoscopic, and Future Therapies. Gastroenterol. Hepatol. 2018, 14, 521–528. [Google Scholar]
- Zhou, Z.; Ouboter, L.F.; Peeters, K.; Hawinkels, L.; Holman, F.; Pascutti, M.F.; Barnhoorn, M.C.; van der Meulen-de Jong, A.E. Crohn’s Disease-Associated and Cryptoglandular Fistulas: Differences and Similarities. J. Clin. Med. 2023, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Pemberton, J.H.; Sandborn, W.J. Diagnosis and treatment of perianal fistulas in Crohn disease. Ann. Intern. Med. 2001, 135, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.Y.; Levy, A.N.; Trivedi, H.D.; Chan, F.K.L.; Ng, S.C.; Ananthakrishnan, A.N. Ethnicity Influences Phenotype and Outcomes in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis of Population-based Studies. Clin. Gastroenterol. Hepatol. 2018, 16, 190–197.e111. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Kim, J.E.; Jung, J.H.; Choe, J.Y.; Kim, M.J.; Choe, Y.H.; Kim, S.; Koh, H.; Lee, Y.M.; Lee, J.H.; et al. Korean Children and Adolescents with Crohn’s Disease Are More Likely to Present with Perianal Fistulizing Disease at Diagnosis Compared to Their European Counterparts. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 49–62. [Google Scholar] [CrossRef] [PubMed]
- McKee, R.F.; Keenan, R.A. Perianal Crohn’s disease—Is it all bad news? Dis. Colon Rectum 1996, 39, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Pescatori, M.; Interisano, A.; Basso, L.; Arcanà, F.; Buffatti, P.; Di Bella, F.; Doldi, A.; Forcheri, V.; Gaetini, R.; Pera, A.; et al. Management of perianal Crohn’s disease. Results of a multicenter study in Italy. Dis. Colon Rectum 1995, 38, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Loftus, E.V., Jr.; Tremaine, W.J.; Panaccione, R.; Harmsen, W.S.; Zinsmeister, A.R.; Sandborn, W.J. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology 2002, 122, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, S.; Fasth, S.; Hultén, L. Anal fistulas in Crohn’s disease: Incidence and outcome of surgical treatment. Int. J. Color. Dis. 1992, 7, 214–218. [Google Scholar] [CrossRef]
- Scharl, M.; Rogler, G.; Biedermann, L. Fistulizing Crohn’s Disease. Clin. Transl. Gastroenterol. 2017, 8, e106. [Google Scholar] [CrossRef] [PubMed]
- Gecse, K.B.; Sebastian, S.; Hertogh, G.; Yassin, N.A.; Kotze, P.G.; Reinisch, W.; Spinelli, A.; Koutroubakis, I.E.; Katsanos, K.H.; Hart, A.; et al. Results of the Fifth Scientific Workshop of the ECCO [II]: Clinical Aspects of Perianal Fistulising Crohn’s Disease-the Unmet Needs. J. Crohn’s Colitis 2016, 10, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.Q.; Jiang, D.; Hoffman, S.N.; Guntaka, S.; Mays, J.L.; Wang, A.; Gomes, J.; Sorrentino, D. Impact of Diagnostic Delay and Associated Factors on Clinical Outcomes in a U.S. Inflammatory Bowel Disease Cohort. Inflamm. Bowel Dis. 2017, 23, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Chandan, J.S.; Harvey, P.R.; Bhala, N.; Ghosh, S.; Nirantharakumar, K.; Trudgill, N.J. The Risk of Inflammatory Bowel Disease in Subjects Presenting With Perianal Abscess: Findings From the THIN Database. J. Crohn’s Colitis 2019, 13, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, A.M.; Dehlavi, M.A.; Fournier, N.; Safroneeva, E.; Straumann, A.; Pittet, V.; Peyrin-Biroulet, L.; Michetti, P.; Rogler, G.; Vavricka, S.R. Diagnostic delay in Crohn’s disease is associated with a complicated disease course and increased operation rate. Am. J. Gastroenterol. 2013, 108, 1744–1753, quiz 1754.. [Google Scholar] [CrossRef] [PubMed]
- Beaugerie, L.; Seksik, P.; Nion-Larmurier, I.; Gendre, J.P.; Cosnes, J. Predictors of Crohn’s disease. Gastroenterology 2006, 130, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Freer, C.; Adegbola, S.; Elkady, S.; Parkes, M.; Hart, A.; Fearnhead, N.S.; Lobo, A.J.; Brown, S.R. Patients with perianal Crohn’s fistulas experience delays in accessing anti-TNF therapy due to slow recognition, diagnosis and integration of specialist services: Lessons learned from three referral centres. Color. Dis. 2018, 20, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Meima-van Praag, E.M.; van Rijn, K.L.; Wasmann, K.; Snijder, H.J.; Stoker, J.; D’Haens, G.R.; Gecse, K.B.; Gerhards, M.F.; Jansen, J.M.; Dijkgraaf, M.G.W.; et al. Short-term anti-TNF therapy with surgical closure versus anti-TNF therapy in the treatment of perianal fistulas in Crohn’s disease (PISA-II): A patient preference randomised trial. Lancet Gastroenterol. Hepatol. 2022, 7, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Meima-van Praag, E.M.; Becker, M.A.J.; van Rijn, K.L.; Wasmann, K.; Stoker, J.; D’Haens, G.; Ponsioen, C.Y.; Gecse, K.B.; Dijkgraaf, M.G.W.; Spinelli, A.; et al. Short-term anti-TNF therapy with surgical closure versus anti-TNF therapy alone for Crohn’s perianal fistulas (PISA-II): Long-term outcomes of an international, multicentre patient preference, randomised controlled trial. EClinicalMedicine 2023, 61, 102045. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Pedarla, V.; Null, K.D.; Cazzetta, S.E.; Khan, Q.R.; Schwartz, D.A. Health Care Costs and Resource Utilization Among Patients With Crohn’s Disease With and Without Perianal Fistula. Inflamm. Bowel Dis. 2022, 28, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, A.; Yanai, H.; Girardi, P.; Milicevic, S.; Carvello, M.; Maroli, A.; Avedano, L. The Impact of Crohn’s Perianal Fistula on Quality of Life: Results of an International Patient Survey. Crohn’s Colitis 360 2023, 5, otad036. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.F.; Gasche, C.; Geboes, K.; et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005, 19 (Suppl. A), 5a–36a. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Atia, O.; Focht, G.; Lujan, R.; Ledder, O.; Greenfeld, S.; Kariv, R.; Dotan, I.; Yanai, H.; Gabay, H.; Balicer, R.; et al. Perianal Crohn Disease Is More Common in Children and Is Associated With Complicated Disease Course Despite Higher Utilization of Biologics: A Population-based Study From The epidemiology group of the Israeli IBD Research Nucleus (epiIIRN). J. Pediatr. Gastroenterol. Nutr. 2022, 74, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Im, J.P.; Kim, J.W.; Lee, K.L.; Choi, C.H.; Kim, H.; Cheon, J.H.; Ye, B.D.; Kim, Y.H.; Kim, Y.S.; et al. Association of Perianal Fistulas with Clinical Features and Prognosis of Crohn’s Disease in Korea: Results from the CONNECT Study. Gut Liver 2018, 12, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Danielou, M.; Sarter, H.; Pariente, B.; Fumery, M.; Ley, D.; Mamona, C.; Barthoulot, M.; Charpentier, C.; Siproudhis, L.; Savoye, G.; et al. Natural History of Perianal Fistulising Lesions in Patients With Elderly-onset Crohn’s Disease: A Population-based Study. J. Crohn’s Colitis 2020, 14, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.M.; McLain, B.I.; Beasley, S.W.; Stokes, K.B. Perianal disease in childhood crohns-disease—Frequency, characteristics, and prognostic-significance. Pediatr. Surg. Int. 1992, 7, 174–176. [Google Scholar] [CrossRef]
- Göttgens, K.W.; Jeuring, S.F.; Sturkenboom, R.; Romberg-Camps, M.J.; Oostenbrug, L.E.; Jonkers, D.M.; Stassen, L.P.; Masclee, A.A.; Pierik, M.J.; Breukink, S.O. Time trends in the epidemiology and outcome of perianal fistulizing Crohn’s disease in a population-based cohort. Eur. J. Gastroenterol. Hepatol. 2017, 29, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Hellers, G.; Bergstrand, O.; Ewerth, S.; Holmström, B. Occurrence and outcome after primary treatment of anal fistulae in Crohn’s disease. Gut 1980, 21, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.S.; Park, J.H.; Lim, K.I.; Tchah, H.; Ryoo, E. Significance of Perianal Lesion in Pediatric Crohn Disease. Pediatr. Gastroenterol. Hepatol. Nutr. 2018, 21, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, T.; Ota, M.; Fujitani, Y.; Kanauchi, Y.; Iwakiri, R. Diagnostic Features of Perianal Fistula in Patients With Crohn’s Disease: Analysis of a Japanese Claims Database. Crohn’s Colitis 360 2021, 3, otab055. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, I.; Nuij, V.J.; van der Meulen-de Jong, A.E.; van der Woude, C.J. Disappointing durable remission rates in complex Crohn’s disease fistula. Inflamm. Bowel Dis. 2014, 20, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Qari, Y.A. Clinical Characteristics of Crohn’s Disease in a Cohort from Saudi Arabia. Saudi J. Med. Med. Sci. 2022, 10, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Rubín de Célix Vargas, C.; Algaba, A.; Guerra, I.; Serrano, Á.; Pérez-Viejo, E.; Aulló, C.; Bermejo, F. Resources used in the treatment of perianal Crohn’s disease and the results in a real-life cohort. Gastroenterol. Hepatol. 2018, 41, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Song, E.M.; Kim, N.; Lee, S.H.; Chang, K.; Hwang, S.W.; Park, S.H.; Yang, D.H.; Byeon, J.S.; Myung, S.J.; Yang, S.K.; et al. Clinical characteristics and long-term prognosis of elderly-onset Crohn’s disease. Scand. J. Gastroenterol. 2018, 53, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Thia, K.T.; Sandborn, W.J.; Harmsen, W.S.; Zinsmeister, A.R.; Loftus, E.V., Jr. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010, 139, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.T.; Lin, K.L.; Huang, Y.L.; Karki, C.; Hong, J.L.; Bennett, D.; Arnold Chan, K.; Wei, S.C. Epidemiology, Disease Course, and Clinical Outcomes of Perianal Fistulas and Fissures Crohn’s Disease: A Nationwide Population-Based Study in Taiwan. Crohn’s Colitis 360 2023, 5, otad035. [Google Scholar] [CrossRef] [PubMed]
- Wewer, M.D.; Zhao, M.; Nordholm-Carstensen, A.; Weimers, P.; Seidelin, J.B.; Burisch, J. The Incidence and Disease Course of Perianal Crohn’s Disease: A Danish Nationwide Cohort Study, 1997-2015. J. Crohn’s Colitis 2021, 15, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.D.; Yang, S.K.; Cho, Y.K.; Park, S.H.; Yang, D.H.; Yoon, S.M.; Kim, K.J.; Byeon, J.S.; Myung, S.J.; Yu, C.S.; et al. Clinical features and long-term prognosis of Crohn’s disease in Korea. Scand. J. Gastroenterol. 2010, 45, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Yang, S.K.; Park, S.K.; Kim, J.W.; Yang, D.H.; Jung, K.W.; Kim, K.J.; Ye, B.D.; Byeon, J.S.; Myung, S.J.; et al. Long-term prognosis of crohn’s disease and its temporal change between 1981 and 2012: A hospital-based cohort study from Korea. Inflamm. Bowel Dis. 2014, 20, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.; McCurdy, J.D.; Ma, C.; Jairath, V.; Singh, S. Epidemiology and Natural History of Perianal Crohn’s Disease: A Systematic Review and Meta-Analysis of Population-Based Cohorts. Inflamm. Bowel Dis. 2022, 28, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Pellino, G.; Sciaudone, G.; Selvaggi, F.; Riegler, G. Delayed diagnosis is influenced by the clinical pattern of Crohn’s disease and affects treatment outcomes and quality of life in the long term: A cross-sectional study of 361 patients in Southern Italy. Eur. J. Gastroenterol. Hepatol. 2015, 27, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Rencz, F.; Stalmeier, P.F.M.; Péntek, M.; Brodszky, V.; Ruzsa, G.; Gönczi, L.; Palatka, K.; Herszényi, L.; Schäfer, E.; Banai, J.; et al. Patient and general population values for luminal and perianal fistulising Crohn’s disease health states. Eur. J. Health Econ. 2019, 20 (Suppl. 1), 91–100. [Google Scholar] [CrossRef] [PubMed]
- Panes, J.; Reinisch, W.; Rupniewska, E.; Khan, S.; Forns, J.; Khalid, J.M.; Bojic, D.; Patel, H. Burden and outcomes for complex perianal fistulas in Crohn’s disease: Systematic review. World J. Gastroenterol. 2018, 24, 4821–4834. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Fiorino, G.; Mary, J.Y.; Lakatos, P.L.; D’Haens, G.; Moja, L.; D’Hoore, A.; Panes, J.; Reinisch, W.; Sandborn, W.J.; et al. Development of Red Flags Index for Early Referral of Adults with Symptoms and Signs Suggestive of Crohn’s Disease: An IOIBD Initiative. J. Crohn’s Colitis 2015, 9, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Lim, J.; Chang, H.S.; Lee, I.; Li, Y.; Liu, J.; Song, K. Association of TNFSF15 with Crohn’s disease in Koreans. Am. J. Gastroenterol. 2008, 103, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.H.; Yang, S.K.; Song, K.; Hong, M.; Park, S.H.; Lee, H.S.; Kim, J.B.; Lee, H.J.; Park, S.K.; Jung, K.W.; et al. TNFSF15 is an independent predictor for the development of Crohn’s disease-related complications in Koreans. J. Crohn’s Colitis 2014, 8, 1315–1326. [Google Scholar] [CrossRef]
- Goddard, G.R.; Lim, I.I.P.; Cheng, Y.C.; Velazco, C.S.; Jenkins, T.; Rosen, N.G.; Kotagal, M.; Garrison, A.P.; Falcone, R.; Rymeski, B.; et al. A child presents with perianal symptoms—How often is this Crohn’s disease? J. Pediatr. Surg. 2021, 56, 1618–1622. [Google Scholar] [CrossRef]
- Roskam, M.; de Meij, T.; Gemke, R.; Bakx, R. Perianal Abscesses in Infants Are Not Associated With Crohn’s Disease in a Surgical Cohort. J. Crohn’s Colitis 2020, 14, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Heyman, M.B.; Kirschner, B.S.; Gold, B.D.; Ferry, G.; Baldassano, R.; Cohen, S.A.; Winter, H.S.; Fain, P.; King, C.; Smith, T.; et al. Children with early-onset inflammatory bowel disease (IBD): Analysis of a pediatric IBD consortium registry. J. Pediatr. 2005, 146, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Shi, H.Y.; Tang, W.; Law, C.C.; Sung, J.J.; Chan, F.K.; Ng, S.C. Systematic Review and Meta-analysis: Phenotype and Clinical Outcomes of Older-onset Inflammatory Bowel Disease. J. Crohn’s Colitis 2016, 10, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Aniwan, S.; Scott Harmsen, W.; Tremaine, W.J.; Lightner, A.L.; Faubion, W.A.; Loftus, E.V. Update on the Natural Course of Fistulizing Perianal Crohn’s Disease in a Population-Based Cohort. Inflamm. Bowel Dis. 2019, 25, 1054–1060. [Google Scholar] [CrossRef] [PubMed]


| Author | Year | Country | Study Design | Single or Multicenter | Study Span (y) | Data Source | CD Patients (n) | Subtypes CD Cohort | Subtypes CD Cohort (n, %) | Age (y) | Male (n, %) | PAF Prior to CD Diagnosis (n, %) | Diagnostic Delay (Median) | Follow-Up (Median) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atia et al. [29] | 2022 | Israel | Retrospective population-based cohort study | Multicenter | 2005–2019 | epi-IIRN | 12,905 | (1) Adults; (2) children | (1) 10,719 (83) (2) 2186 (17) | (1) 34.4 (13.8) *; (2) 14.1 (3.5) * | (1) 6646 (62); (2) 1661 (76) | (1) 473/10,719 (4.4); (2) 76/2186 (3.5) | NR | 7.8 y [IQR 4.2–11.0] |
| Chun et al. [30] | 2018 | Korea | Retrospective cohort study | Multicenter | 1982–2008 | CONNECT study database | 1193 | NA | NA | 26.9 (11.9) * | 840 (70.4) | 318/1193 (26.7) | NR | 8.77 y (1.0 to 25.8) ** |
| Danielou et al. [31] | 2020 | France | Retrospective population-based cohort study | Multicenter | 1988–2006 | EPIMAD registry | 372 | NA | NA | 70.1 [65.2–76.4] | 142 (38.2) | 11/372 (3) | NR | 6 y [IQR 3–10] |
| Davidson et al. [32] | 1992 | Australia | Retrospective cohort study | Single center | 1971–1987 | Medical records | 63 | NA | NA | 12 [3–16] | 11 (17.5) | 2/63 (3.2) | NR | NR |
| Göttgens et al. [33] | 2016 | The Netherlands | Retrospective population-based cohort study | Multicenter | 1991–2011 | IBDSL registry | 1162 | (1) Without PAF/RVF; (2) only PAF; (3) RVF | (1) 995 (85.6); (2) 150 (12.9); (3) 17 (1.5) | (1) 38.5 (16.3) *; (2) 32.3 (12.5) *; (3) 37.3 (15.1) * | (1) 375 (37.7); (2) 59 (39.3); (3) 0 (0) | 49/1162 (4.2) | 0.8 y [0.2–2.7] | 8.7 y (5.7) ** |
| Hellers et al. [34] | 1980 | Sweden | Retrospective cohort study | Multicenter | 1955–1974 | Medical records | 826 | NA | NA | NR | 379 (45.9) | 45/826 (5.4) | (1) >2 y prior to CD diagnosis (19); (2) 6 m > and >2 y (26) | 9.4 y (0.5–22.5) ** |
| Jin et al. [35] | 2018 | Korea | Retrospective cohort study | Single center | 2000–2014 | Medical records | 69 | (1) CD with perianal lesions; (2) CD without perianal lesions | (1) 54 (78.2); (2) 15 (21.7) | 15.4 | 51 (73.9) | 13/69 (18.8) | 14 m | NR |
| Mizushima et al. [36] | 2021 | Japan | Retrospective cohort study | Multicenter | 2013–2019 | JMDC Co., Ltd., claims database | 1426 | (1) PAF after CD; (2) CD after PAF; (3) PAF + CD †; (4) CD only | (1) 43 (3.0); (2) 188 (13.2); (3) 43 (3.0); (4) 1152 (80.8) | (1) 27.1 (12.7) *; (2) 25.7 (10.5) *; (3) 26.9 (13.6) *; (4) 35.9 (15.7) * | (1) 39 (90.7); (2) 169 (89.9); (3) 36 (83.7) (4) 757 (65.7) | 188/1426 (13.2) | 10.8 m (15.8) ** | ≥12 m |
| Molendijk et al. [37] | 2014 | The Netherlands | Retrospective cohort study | Single center | 1980–2000 | Medical records | 436 | NA | NA | 22.8 [4.0–68.7] * | NR | 44/436 (10.1) * | NR | NR |
| Park et al. [45] | 2014 | Korea | Retrospective cohort study | Single center | 1981–2012 | Medical records | 2043 | (1) 1981–2000; (2) 2001–2005; (3) 2006–2012 | (1) 363 (17.8); (2) 611 (29.9); (3) 1069 (52.3) | 23 [9–75] * | 1462 (71.6) | (1) 70/363 (19.3); (2) 118/611 (19.3); (3) 201/1069 (18.8) | NR | 80 m (1–381) |
| Qari [38] | 2022 | Saudi Arabia | Retrospective cohort study | Single center | 2012–2018 | Medical records | 245 | NA | NA | 26.3 [14–73] * | 125 (51) | 25/245 (10.2) | NR | NR |
| Rubín de Célix Vargas et al. [39] | 2018 | Spain | Retrospective cohort study | Single center | 2004–2016 | Medical records | 300 | NA | NA | NR | 36 (12) | 8/300 (2.7) | NR | NR |
| Song et al. [40] | 2018 | Korea | Retrospective matched case-control study | Single center | 1989–2016 | Asan IBD registry | 243 | (1) Elderly onset; (2) Middle-age onset; (3) Young onset | (1) 27 (11.1); (2) 108 (44.4); (3) 108 (44.4) | 26.0 [21.0–34.1] * | 161 (66.3) | (1) 2/27 (7.4); (2) 15/108 (13.9); (3) 29/108 (26.9) | NR | 67.8 m [IQR 40.5–120.8] |
| Thia et al. [41] | 2010 | USA | Retrospective cohort study | Multicenter | 1970–2004 | Medical records (Olmsted County Database) | 306 | NA | NA | 30.2 [3–142]* | 150 (49) | 15/306 (4.9) | 38 m [range, 3–142] | 8.4 y [2 d–35.9 y] |
| Weng et al. [42] | 2023 | Taiwan | Retrospective cohort study | Multicenter | 2000–2017 | Taiwan’s National Health Insurance Research Database | 2424 | (1) With pCDl; (2) without pCD | (1) 358 (14.8); (2) 2066 (85.2) | (1) 33.7 (14.9); (2) 44.9 (21.8) | (1) 284 (79.3); (2) 1248 (60.4) | 141/2424 (5.8) | (1) 1266 [756–2237]; (2) 1611 d (1213) ** | Not clearly reported |
| Wewer et al. [43] | 2021 | Denmark | Retrospective cohort study | Multicenter | 1997–2015 | National Patient Registry | 9739 | (1) Without pCD; (2) with pCD | (1) 7927 (81.4); (2) 1812 (18.6) | (1) 39.6 [27.0–57.0] *; (2) 32.8 [24.1–46.2] * | (1) 3317 (41.8); (2) 903 (49.8) | 408/9739 (4.2) | NR | 8.2 y [4.1–13.3] |
| Ye et al. [44] | 2010 | Korea | Retrospective cohort study | Single center | 1991–2007 | Medical records | 278 | NA | NA | 23 [9–74] * | 191 (68.7) | 92/278 (33.1) | 32 [2–361] m | 71 m [1–210] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munster, L.J.; Mönnink, G.L.E.; van Dieren, S.; Mundt, M.W.; D’Haens, G.R.A.M.; Bemelman, W.A.; Buskens, C.J.; van der Bilt, J.D.W. Fistulizing Perianal Disease as a First Manifestation of Crohn’s Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 4734. https://doi.org/10.3390/jcm13164734
Munster LJ, Mönnink GLE, van Dieren S, Mundt MW, D’Haens GRAM, Bemelman WA, Buskens CJ, van der Bilt JDW. Fistulizing Perianal Disease as a First Manifestation of Crohn’s Disease: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(16):4734. https://doi.org/10.3390/jcm13164734
Chicago/Turabian StyleMunster, Liesbeth Jozefien, Giulia Louise Emilia Mönnink, Susan van Dieren, Marco William Mundt, Geert Renaat Alfons Maria D’Haens, Willem Adrianus Bemelman, Christianne Johanna Buskens, and Jarmila Dagmara Wendelien van der Bilt. 2024. "Fistulizing Perianal Disease as a First Manifestation of Crohn’s Disease: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 16: 4734. https://doi.org/10.3390/jcm13164734





