Contemporary Multimodality Imaging for Diagnosis and Management of Fabry Cardiomyopathy
Abstract
:1. Introduction
2. Cardiac Manifestations of FD
2.1. Left Ventricular and Septal Hypertrophy
2.2. Cardiac Arrhythmias
2.3. Valvulopathy
2.4. Microvascular Ischemia
3. Cardiac Evaluations and Monitoring
3.1. Electrocardiographic Monitoring
3.2. Echocardiography
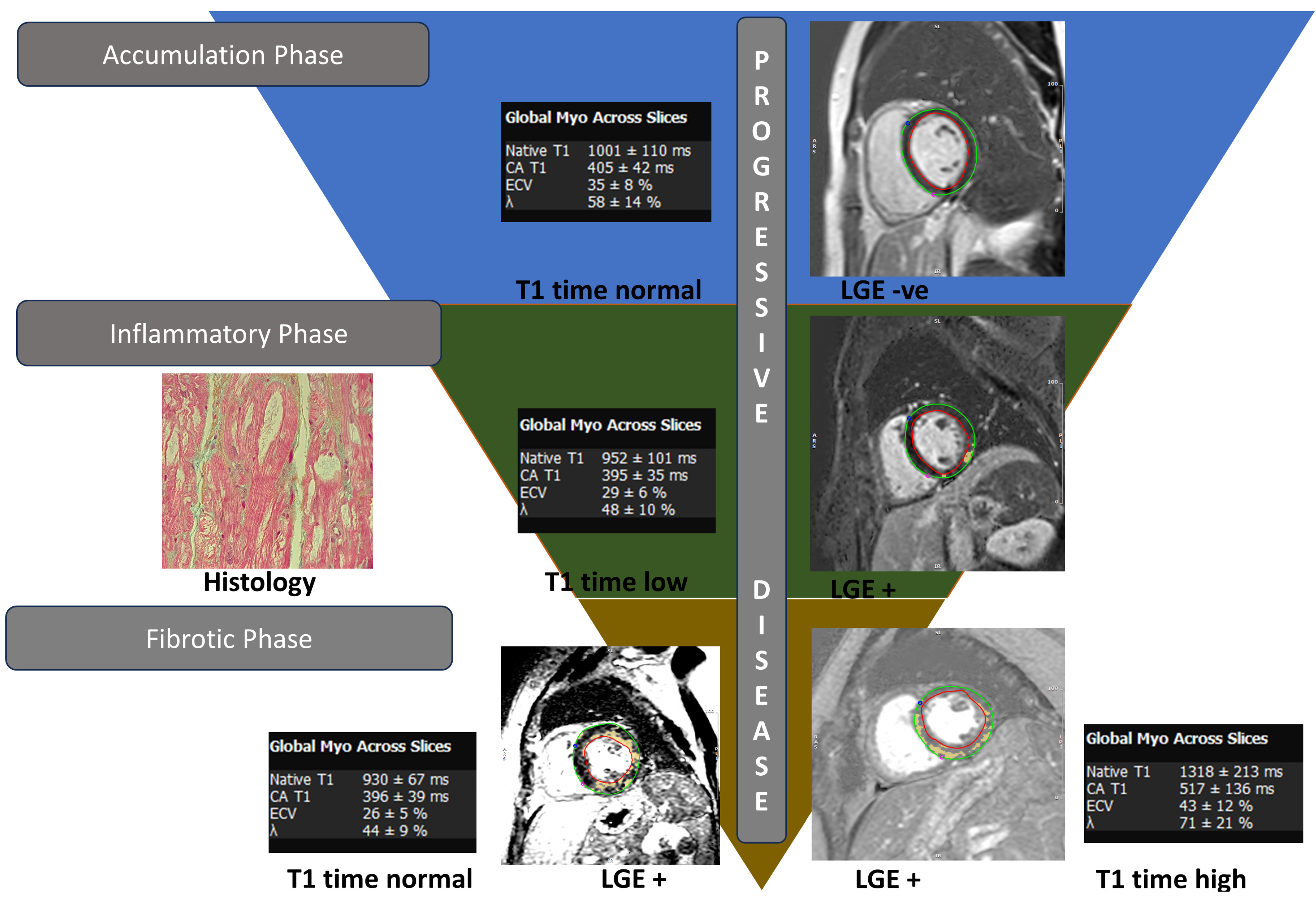
3.3. Cardiac Magnetic Resonance Imaging
3.4. Nuclear Scintigraphy and Positron Emission Tomography
4. Management
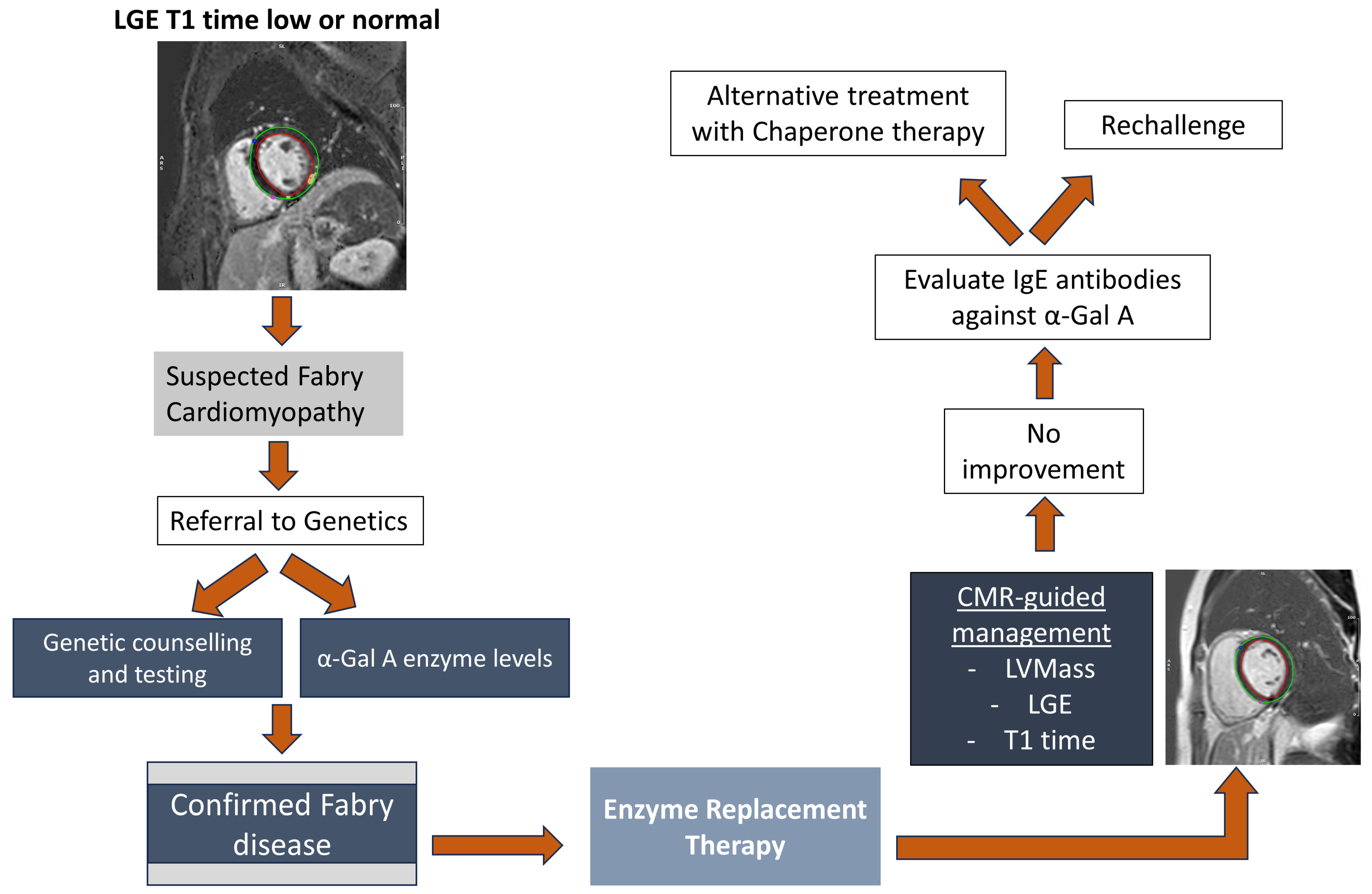
4.1. Treatment of Fabry Disease
4.1.1. Enzyme Replacement Therapy
4.1.2. Chaperone Therapy
4.2. Management of Cardiac Symptoms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kornreich, R.; Bishop, D.F.; Desnick, R.J. The gene encoding alpha-galactosidase A and gene rearrangements causing Fabry disease. Trans. Assoc. Am. Phys. 1989, 102, 30–43. [Google Scholar] [PubMed]
- Echevarria, L.; Benistan, K.; Toussaint, A.; Dubourg, O.; Hagege, A.A.; Eladari, D.; Jabbour, F.; Beldjord, C.; De Mazancourt, P.; Germain, D.P. X-chromosome inactivation in female patients with Fabry disease. Clin. Genet. 2016, 89, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Hughes, D.A. Fabry Disease. In GeneReviews(®); Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Sanders, K.A.; Gavrilov, D.K.; Oglesbee, D.; Raymond, K.M.; Tortorelli, S.; Hopwood, J.J.; Lorey, F.; Majumdar, R.; Kroll, C.A.; McDonald, A.M.; et al. A Comparative Effectiveness Study of Newborn Screening Methods for Four Lysosomal Storage Disorders. Int. J. Neonatal Screen. 2020, 6, 44. [Google Scholar] [CrossRef]
- Tower-Rader, A.; Jaber, W.A. Multimodality Imaging Assessment of Fabry Disease. Circ. Cardiovasc. Imaging 2019, 12, e009013. [Google Scholar] [CrossRef]
- Torra, R. Renal manifestations in Fabry disease and therapeutic options. Kidney Int. 2008, 74, S29–S32. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Abiose, A.; Bichet, D.G.; Cabrera, G.; Charrow, J.; Germain, D.P.; Hopkin, R.J.; Jovanovic, A.; Linhart, A.; Maruti, S.S.; et al. Time to treatment benefit for adult patients with Fabry disease receiving agalsidase β: Data from the Fabry Registry. J. Med. Genet. 2016, 53, 495–502. [Google Scholar] [CrossRef]
- Pieroni, M.; Moon, J.C.; Arbustini, E.; Barriales-Villa, R.; Camporeale, A.; Vujkovac, A.C.; Elliott, P.M.; Hagege, A.; Kuusisto, J.; Linhart, A.; et al. Cardiac Involvement in Fabry Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 922–936. [Google Scholar] [CrossRef]
- Rob, D.; Marek, J.; Dostalova, G.; Linhart, A. Heart failure in Fabry disease revisited: Application of current heart failure guidelines and recommendations. ESC Heart Fail. 2022, 9, 4043–4052. [Google Scholar] [CrossRef]
- Wu, J.C.; Ho, C.Y.; Skali, H.; Abichandani, R.; Wilcox, W.R.; Banikazemi, M.; Packman, S.; Sims, K.; Solomon, S.D. Cardiovascular manifestations of Fabry disease: Relationships between left ventricular hypertrophy, disease severity, and alpha-galactosidase A activity. Eur. Heart J. 2010, 31, 1088–1097. [Google Scholar] [CrossRef]
- Frustaci, A.; Morgante, E.; Russo, M.A.; Scopelliti, F.; Grande, C.; Verardo, R.; Franciosa, P.; Chimenti, C. Pathology and function of conduction tissue in Fabry disease cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2015, 8, 799–805. [Google Scholar] [CrossRef]
- Chimenti, C.; Hamdani, N.; Boontje, N.M.; DeCobelli, F.; Esposito, A.; Bronzwaer, J.G.; Stienen, G.J.; Russo, M.A.; Paulus, W.J.; Frustaci, A.; et al. Myofilament degradation and dysfunction of human cardiomyocytes in Fabry disease. Am. J. Pathol. 2008, 172, 1482–1490. [Google Scholar] [CrossRef]
- Baig, S.; Edward, N.C.; Kotecha, D.; Liu, B.; Nordin, S.; Kozor, R.; Moon, J.C.; Geberhiwot, T.; Steeds, R.P. Ventricular arrhythmia and sudden cardiac death in Fabry disease: A systematic review of risk factors in clinical practice. EP Eur. 2018, 20, f153–f161. [Google Scholar] [CrossRef] [PubMed]
- Linhart, A.; Germain, D.P.; Olivotto, I.; Akhtar, M.M.; Anastasakis, A.; Hughes, D.; Namdar, M.; Pieroni, M.; Hagège, A.; Cecchi, F.; et al. An expert consensus document on the management of cardiovascular manifestations of Fabry disease. Eur. J. Heart Fail. 2020, 22, 1076–1096. [Google Scholar] [CrossRef] [PubMed]
- Barbey, F.; Qanadli, S.D.; Juli, C.; Brakch, N.; Palaček, T.; Rizzo, E.; Jeanrenaud, X.; Eckhardt, B.; Linhart, A. Aortic remodelling in Fabry disease. Eur. Heart J. 2009, 31, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Peter, C.; Zhao, T.; Deegan, P.; Rusk, R. 18 Prevalence of aortic root dilatation in patients with fabry disease: A single centre experience. Heart 2021, 107, A14–A15. [Google Scholar] [CrossRef]
- Dobrowolski, M.K.; Marczak, M.; Dąbrowski, R. Aortic dissection four months after SARS-CoV-2 infection in a patient with Fabry disease whose targeted treatment was stopped 2 months earlier. Kardiol. Pol. 2022, 80, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Umar, H.; Ochoa-Ferraro, A.; Warfield, A.; Lewis, N.; Geberhiwot, T.; Steeds, R. Atherosclerosis in Fabry Disease—A Contemporary Review. J. Clin. Med. 2021, 10, 4422. [Google Scholar] [CrossRef]
- Namdar, M. Electrocardiographic Changes and Arrhythmia in Fabry Disease. Front. Cardiovasc. Med. 2016, 3, 7. [Google Scholar] [CrossRef]
- O’Mahony, C.; Coats, C.; Cardona, M.; Garcia, A.; Calcagnino, M.; Murphy, E.; Lachmann, R.; Mehta, A.; Hughes, D.; Elliott, P.M. Incidence and predictors of anti-bradycardia pacing in patients with Anderson-Fabry disease. Europace 2011, 13, 1781–1788. [Google Scholar] [CrossRef]
- Weidemann, F.; Maier, S.K.; Störk, S.; Brunner, T.; Liu, D.; Hu, K.; Seydelmann, N.; Schneider, A.; Becher, J.; Canan-Kühl, S.; et al. Usefulness of an Implantable Loop Recorder to Detect Clinically Relevant Arrhythmias in Patients with Advanced Fabry Cardiomyopathy. Am. J. Cardiol. 2016, 118, 264–274. [Google Scholar] [CrossRef]
- Calcagnino, M.; O’Mahony, C.; Coats, C.; Cardona, M.; Garcia, A.; Janagarajan, K.; Mehta, A.; Hughes, D.; Murphy, E.; Lachmann, R.; et al. Exercise-induced left ventricular outflow tract obstruction in symptomatic patients with Anderson-Fabry disease. J. Am. Coll. Cardiol. 2011, 58, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, M.; Chimenti, C.; Ricci, R.; Sale, P.; Russo, M.A.; Frustaci, A. Early detection of Fabry cardiomyopathy by tissue Doppler imaging. Circulation 2003, 107, 1978–1984. [Google Scholar] [CrossRef] [PubMed]
- Saccheri, M.C.; Cianciulli, T.F.; Lax, J.A.; Gagliardi, J.A.; Cáceres, G.L.; Quarin, A.E.; Kisinovsky, I.; Rozenfeld, P.A.; Reisin, R.C. Two-dimensional speckle tracking echocardiography for early detection of myocardial damage in young patients with Fabry disease. Echocardiography 2013, 30, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Collier, P.; Phelan, D.; Klein, A. A Test in Context: Myocardial Strain Measured by Speckle-Tracking Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Krämer, J.; Niemann, M.; Liu, D.; Hu, K.; Machann, W.; Beer, M.; Wanner, C.; Ertl, G.; Weidemann, F. Two-dimensional speckle tracking as a non-invasive tool for identification of myocardial fibrosis in Fabry disease. Eur. Heart J. 2013, 34, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Shanks, M.; Thompson, R.B.; Paterson, I.D.; Putko, B.; Khan, A.; Chan, A.; Becher, H.; Oudit, G.Y. Systolic and diastolic function assessment in fabry disease patients using speckle-tracking imaging and comparison with conventional echocardiographic measurements. J. Am. Soc. Echocardiogr. 2013, 26, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Pichette, M.; Serri, K.; Pagé, M.; Di, L.Z.; Bichet, D.G.; Poulin, F. Impaired Left Atrial Function in Fabry Disease: A Longitudinal Speckle-Tracking Echocardiography Study. J. Am. Soc. Echocardiogr. 2017, 30, 170–179.e2. [Google Scholar] [CrossRef]
- Linhart, A.; Palecek, T.; Bultas, J.; Ferguson, J.J.; Hrudová, J.; Karetová, D.; Zeman, J.; Ledvinová, J.; Poupetová, H.; Elleder, M.; et al. New insights in cardiac structural changes in patients with Fabry’s disease. Am. Heart J. 2000, 139, 1101–1108. [Google Scholar] [CrossRef]
- Tsuruda, T.; Higashi, Y.; Gi, T.; Nakao, S. Pericardial effusion in the course of Fabry disease cardiomyopathy: A case report. Eur. Heart J. Case Rep. 2021, 5, ytab407. [Google Scholar] [CrossRef] [PubMed]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients with Hypertrophic Cardiomyopathy. Circulation 2020, 142, e558–e631. [Google Scholar] [CrossRef] [PubMed]
- Hundley, W.G.; Bluemke, D.A.; Finn, J.P.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Ho, V.B.; Jerosch-Herold, M.; Kramer, C.M.; Manning, W.J.; et al. ACCF/ACR/AHA/NASCI/SCMR 2010 Expert Consensus Document on Cardiovascular Magnetic Resonance. Circulation 2010, 121, 2462–2508. [Google Scholar] [CrossRef]
- Cobelli, F.D.; Esposito, A.; Belloni, E.; Pieroni, M.; Perseghin, G.; Chimenti, C.; Frustaci, A.; Maschio, A.D. Delayed-Enhanced Cardiac MRI for Differentiation of Fabry’s Disease from Symmetric Hypertrophic Cardiomyopathy. Am. J. Roentgenol. 2009, 192, W97–W102. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.C.; Reed, E.; Sheppard, M.N.; Elkington, A.G.; Ho, S.Y.; Burke, M.; Petrou, M.; Pennell, D.J. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2004, 43, 2260–2264. [Google Scholar] [CrossRef] [PubMed]
- Nappi, C.; Altiero, M.; Imbriaco, M.; Nicolai, E.; Giudice, C.A.; Aiello, M.; Diomiaiuti, C.T.; Pisani, A.; Spinelli, L.; Cuocolo, A. First experience of simultaneous PET/MRI for the early detection of cardiac involvement in patients with Anderson-Fabry disease. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1025–1031. [Google Scholar] [CrossRef]
- Roller, F.C.; Brose, A.; Richter, M.; Schüssler, A.; Harth, S.; Tanislav, C.; Krombach, G.A. Value of Left Ventricular Feature Tracking Strain Analysis for Detection of Early Cardiac Involvement in Fabry Disease (FD). J. Clin. Med. 2021, 10, 3734. [Google Scholar] [CrossRef]
- Mathur, S.; Dreisbach, J.G.; Karur, G.R.; Iwanochko, R.M.; Morel, C.F.; Wasim, S.; Nguyen, E.T.; Wintersperger, B.J.; Hanneman, K. Loss of base-to-apex circumferential strain gradient assessed by cardiovascular magnetic resonance in Fabry disease: Relationship to T1 mapping, late gadolinium enhancement and hypertrophy. J. Cardiovasc. Magn. Reson. 2019, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Hanneman, K.; Karur, G.R.; Wasim, S.; Morel, C.F.; Iwanochko, R.M. Prognostic Significance of Cardiac Magnetic Resonance Imaging Late Gadolinium Enhancement in Fabry Disease. Circulation 2018, 138, 2579–2581. [Google Scholar] [CrossRef]
- Vijapurapu, R.; Nordin, S.; Baig, S.; Liu, B.; Rosmini, S.; Augusto, J.; Tchan, M.; Hughes, D.A.; Geberhiwot, T.; Moon, J.C.; et al. Global longitudinal strain, myocardial storage and hypertrophy in Fabry disease. Heart 2019, 105, 470–476. [Google Scholar] [CrossRef]
- Krämer, J.; Niemann, M.; Störk, S.; Frantz, S.; Beer, M.; Ertl, G.; Wanner, C.; Weidemann, F. Relation of burden of myocardial fibrosis to malignant ventricular arrhythmias and outcomes in Fabry disease. Am. J. Cardiol. 2014, 114, 895–900. [Google Scholar] [CrossRef]
- Deva, D.P.; Hanneman, K.; Li, Q.; Ng, M.Y.; Wasim, S.; Morel, C.; Iwanochko, R.M.; Thavendiranathan, P.; Crean, A.M. Cardiovascular magnetic resonance demonstration of the spectrum of morphological phenotypes and patterns of myocardial scarring in Anderson-Fabry disease. J. Cardiovasc. Magn. Reson. 2016, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Hanneman, K.; Karur, G.R.; Wasim, S.; Wald, R.M.; Iwanochko, R.M.; Morel, C.F. Left Ventricular Hypertrophy and Late Gadolinium Enhancement at Cardiac MRI Are Associated with Adverse Cardiac Events in Fabry Disease. Radiology 2020, 294, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Augusto, J.B.; Nordin, S.; Vijapurapu, R.; Baig, S.; Bulluck, H.; Castelletti, S.; Alfarih, M.; Knott, K.; Captur, G.; Kotecha, T.; et al. Myocardial Edema, Myocyte Injury, and Disease Severity in Fabry Disease. Circ. Cardiovasc. Imaging 2020, 13, e010171. [Google Scholar] [CrossRef] [PubMed]
- Sado, D.M.; White, S.K.; Piechnik, S.K.; Banypersad, S.M.; Treibel, T.; Captur, G.; Fontana, M.; Maestrini, V.; Flett, A.S.; Robson, M.D.; et al. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ. Cardiovasc. Imaging 2013, 6, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.-R.; Hung, S.-C.; Chang, F.-P.; Yu, W.-C.; Sung, S.-H.; Hsu, C.-L.; Dzhagalov, I.; Yang, C.-F.; Chu, T.-H.; Lee, H.-J.; et al. Later Onset Fabry Disease, Cardiac Damage Progress in Silence: Experience With a Highly Prevalent Mutation. J. Am. Coll. Cardiol. 2016, 68, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, F.; Beer, M.; Kralewski, M.; Siwy, J.; Kampmann, C. Early detection of organ involvement in Fabry disease by biomarker assessment in conjunction with LGE cardiac MRI: Results from the SOPHIA study. Mol. Genet. Metab. 2019, 126, 169–182. [Google Scholar] [CrossRef]
- Niemann, M.; Herrmann, S.; Hu, K.; Breunig, F.; Strotmann, J.; Beer, M.; Machann, W.; Voelker, W.; Ertl, G.; Wanner, C.; et al. Differences in Fabry cardiomyopathy between female and male patients: Consequences for diagnostic assessment. JACC Cardiovasc. Imaging 2011, 4, 592–601. [Google Scholar] [CrossRef]
- Sado, D.M.; Flett, A.S.; Banypersad, S.M.; White, S.K.; Maestrini, V.; Quarta, G.; Lachmann, R.H.; Murphy, E.; Mehta, A.; Hughes, D.A.; et al. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart 2012, 98, 1436–1441. [Google Scholar] [CrossRef]
- Umer, M.; Kalra, D.K. Cardiac MRI in Fabry disease. Front. Cardiovasc. Med. 2022, 9, 1075639. [Google Scholar] [CrossRef]
- Hazari, H.; Belenkie, I.; Kryski, A.; White, J.A.; Oudit, G.Y.; Thompson, R.; Fung, T.; Dehar, N.; Khan, A. Comparison of Cardiac Magnetic Resonance Imaging and Echocardiography in Assessment of Left Ventricular Hypertrophy in Fabry Disease. Can. J. Cardiol. 2018, 34, 1041–1047. [Google Scholar] [CrossRef]
- Thompson, R.B.; Chow, K.; Khan, A.; Chan, A.; Shanks, M.; Paterson, I.; Oudit, G.Y. T1 mapping with cardiovascular MRI is highly sensitive for fabry disease independent of hypertrophy and sex. Circ. Cardiovasc. Imaging 2013, 6, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Nordin, S.; Kozor, R.; Bulluck, H.; Castelletti, S.; Rosmini, S.; Abdel-Gadir, A.; Baig, S.; Mehta, A.; Hughes, D.; Moon, J.C. Cardiac Fabry Disease with Late Gadolinium Enhancement Is a Chronic Inflammatory Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 1707–1708. [Google Scholar] [CrossRef] [PubMed]
- Augusto, J.B.; Davies, R.H.; Bhuva, A.N.; Knott, K.D.; Seraphim, A.; Alfarih, M.; Lau, C.; Hughes, R.K.; Lopes, L.R.; Shiwani, H.; et al. Diagnosis and risk stratification in hypertrophic cardiomyopathy using machine learning wall thickness measurement: A comparison with human test-retest performance. Lancet Digit. Health 2021, 3, e20–e28. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, C.; Morgante, E.; Tanzilli, G.; Mangieri, E.; Critelli, G.; Gaudio, C.; Russo, M.A.; Frustaci, A. Angina in fabry disease reflects coronary small vessel disease. Circ. Heart Fail. 2008, 1, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Altarescu, G.; Moore, D.F.; Pursley, R.; Campia, U.; Goldstein, S.; Bryant, M.; Panza, J.A.; Schiffmann, R. Enhanced endothelium-dependent vasodilation in Fabry disease. Stroke 2001, 32, 1559–1562. [Google Scholar] [CrossRef] [PubMed]
- Tomberli, B.; Cecchi, F.; Sciagrà, R.; Berti, V.; Lisi, F.; Torricelli, F.; Morrone, A.; Castelli, G.; Yacoub, M.H.; Olivotto, I. Coronary microvascular dysfunction is an early feature of cardiac involvement in patients with Anderson–Fabry disease. Eur. J. Heart Fail. 2013, 15, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Imbriaco, M.; Nappi, C.; Ponsiglione, A.; Pisani, A.; Dell’Aversana, S.; Nicolai, E.; Spinelli, L.; Aiello, M.; Diomiaiuti, C.T.; Riccio, E.; et al. Hybrid positron emission tomography-magnetic resonance imaging for assessing different stages of cardiac impairment in patients with Anderson-Fabry disease: AFFINITY study group. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, L.; Imbriaco, M.; Nappi, C.; Nicolai, E.; Giugliano, G.; Ponsiglione, A.; Diomiaiuti, T.C.; Riccio, E.; Duro, G.; Pisani, A.; et al. Early Cardiac Involvement Affects Left Ventricular Longitudinal Function in Females Carrying α-Galactosidase A Mutation. Circ. Cardiovasc. Imaging 2018, 11, e007019. [Google Scholar] [CrossRef]
- Imbriaco, M.; Pellegrino, T.; Piscopo, V.; Petretta, M.; Ponsiglione, A.; Nappi, C.; Puglia, M.; Dell’Aversana, S.; Riccio, E.; Spinelli, L.; et al. Cardiac sympathetic neuronal damage precedes myocardial fibrosis in patients with Anderson-Fabry disease. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2266–2273. [Google Scholar] [CrossRef]
- Yamamoto, S.; Suzuki, H.; Sugimura, K.; Tatebe, S.; Aoki, T.; Miura, M.; Yaoita, N.; Sato, H.; Kozu, K.; Ota, H.; et al. Focal Reduction in Cardiac (123)I-Metaiodobenzylguanidine Uptake in Patients With Anderson-Fabry Disease. Circ. J. Off. J. Jpn. Circ. Soc. 2016, 80, 2550–2551. [Google Scholar] [CrossRef]
- Spinelli, L.; Imbriaco, M.; Giugliano, G.; Nappi, C.; Gaudieri, V.; Riccio, E.; Pisani, A.; Trimarco, B.; Cuocolo, A. Focal reduction in left ventricular 123I-metaiodobenzylguanidine uptake and impairment in systolic function in patients with Anderson-Fabry disease. J. Nucl. Cardiol. 2021, 28, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, M.; Namdar, M.; Olivotto, I.; Desnick, R.J. Anderson–Fabry disease management: Role of the cardiologist. Eur. Heart J. 2024, 45, 1395–1409. [Google Scholar] [CrossRef] [PubMed]
- Biegstraaten, M.; Arngrímsson, R.; Barbey, F.; Boks, L.; Cecchi, F.; Deegan, P.B.; Feldt-Rasmussen, U.; Geberhiwot, T.; Germain, D.P.; Hendriksz, C.; et al. Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: The European Fabry Working Group consensus document. Orphanet J. Rare Dis. 2015, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Germain, D.P.; Desnick, R.J.; Politei, J.; Mauer, M.; Burlina, A.; Eng, C.; Hopkin, R.J.; Laney, D.; Linhart, A.; et al. Fabry disease revisited: Management and treatment recommendations for adult patients. Mol. Genet. Metab. 2018, 123, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.P.; Charrow, J.; Desnick, R.J.; Guffon, N.; Kempf, J.; Lachmann, R.H.; Lemay, R.; Linthorst, G.E.; Packman, S.; Scott, C.R.; et al. Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J. Med. Genet. 2015, 52, 353–358. [Google Scholar] [CrossRef]
- Beck, M.; Hughes, D.; Kampmann, C.; Larroque, S.; Mehta, A.; Pintos-Morell, G.; Ramaswami, U.; West, M.; Wijatyk, A.; Giugliani, R. Long-term effectiveness of agalsidase alfa enzyme replacement in Fabry disease: A Fabry Outcome Survey analysis. Mol. Genet. Metab. Rep. 2015, 3, 21–27. [Google Scholar] [CrossRef]
- Weidemann, F.; Niemann, M.; Störk, S.; Breunig, F.; Beer, M.; Sommer, C.; Herrmann, S.; Ertl, G.; Wanner, C. Long-term outcome of enzyme-replacement therapy in advanced Fabry disease: Evidence for disease progression towards serious complications. J. Intern. Med. 2013, 274, 331–341. [Google Scholar] [CrossRef]
- Germain, D.P.; Hughes, D.A.; Nicholls, K.; Bichet, D.G.; Giugliani, R.; Wilcox, W.R.; Feliciani, C.; Shankar, S.P.; Ezgu, F.; Amartino, H.; et al. Treatment of Fabry’s Disease with the Pharmacologic Chaperone Migalastat. N. Engl. J. Med. 2016, 375, 545–555. [Google Scholar] [CrossRef]
- Burban, A.; Pucyło, S.; Sikora, A.; Opolski, G.; Grabowski, M.; Kołodzińska, A. Hypertrophic Cardiomyopathy versus Storage Diseases with Myocardial Involvement. Int. J. Mol. Sci. 2023, 24, 13239. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Hirayama, M.; Hirota, Y.; Asa, E.; Seki, J.; Tanaka, Y. Drug-induced phospholipidosis is caused by blockade of mannose 6-phosphate receptor-mediated targeting of lysosomal enzymes. Biochem. Biophys. Res. Commun. 2008, 377, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Pintavorn, P.; Cook, W.J. Progressive renal insufficiency associated with amiodarone-induced phospholipidosis. Kidney Int. 2008, 74, 1354–1357. [Google Scholar] [CrossRef] [PubMed]

| Neurological | |
| 1. Peripheral Nervous system | |
| (A) Sensory | Acroparesthesias, pain crises/Fabry crises, varying degrees of sensory loss (small-fiber neuropathy/altered temperature perception: hypo/anhidrosis) |
| (B) Autonomic | Orthostatic hypotension and syncope due to vasomotor involvement |
| 2. Central Nervous System | Most common: Intracranial vasculopathy manifesting as small/large vessel strokes; Periventicular white matter lesions and verterbrobasilar dolichoectasias, and common imaging findings Less common: Neuropsychiatric involvement with cognitive dysfunction may be observed |
| Ocular | Cornea verticillata Retinal vasculopathy Premature cataracts |
| Auditory/Vestibular | Hearing loss Tinnitus Vertigo |
| Dermatological | Characteristic lesions: Angiokeratomas |
| Gastrointestinal | Dysregulation of gut motility due to enteric/autonomic neuropathy or GI smooth muscle involvement: leads to early satiety, vomiting/nausea, constipation or diarrhea and weight loss |
| Renal | Accelerated development of proteinuric chronic kidney disease: due to glomerular (both endothelial/podocytes), tubular and interstitial sclerosis |
| Technique | Role in Disease Management | Advantages of Technique | Disadvantages of Technique |
|---|---|---|---|
| Conventional echocardiography | Identification of cardiac involvement in asymptomatic patients such as LVH, reduced tissue Doppler velocity. Longitudinal monitoring of patients to response to ERT. | Rapid, easily accessible, inexpensive. Early detection of diastolic dysfunction can assist in pre-clinical diagnosis (prior to development of LVH). | Operator-dependent; non-specific. |
| Speckle tracking echocardiography | Allows for the early evaluation of cardiac involvement in patients with FD, thereby having implications for prompt ERT initiation. | Easy access and quick to perform. Act as a surrogate marker for LGE on cMRI. | Operator-dependent; non-specific. Lack of standardized measurements across different machines. |
| Cardiac MRI | Confirmatory testing after identification of LVH on echocardiography to allow for the precise quantification of LV mass (can also be used for longitudinal disease monitoring). Evaluation of low T1 intensity in suspected patients may allow for the early diagnosis of cardiac involvement. | High spatial resolution. Accurate and highly reproducible measurements of LV geometry. Standardized measurements, allowing for establishing reference values. Allows for disease characterization in cases of unexplained LVH. | Not readily accessible. Expensive. Limitations in those without compatible metallic implants. |
| Nuclear scintigraphy/PET | Assessment of microvascular dysfunction. Evaluation of areas of myocardial inflammation with hybrid imaging. | Noninvasive measurement of coronary blood flow. | Non-specific. Expensive. Not readily available. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, S.; Bhalla, J.S.; Erwin, A.L.; Jaber, W.; Wang, T.K.M. Contemporary Multimodality Imaging for Diagnosis and Management of Fabry Cardiomyopathy. J. Clin. Med. 2024, 13, 4771. https://doi.org/10.3390/jcm13164771
Kaur S, Bhalla JS, Erwin AL, Jaber W, Wang TKM. Contemporary Multimodality Imaging for Diagnosis and Management of Fabry Cardiomyopathy. Journal of Clinical Medicine. 2024; 13(16):4771. https://doi.org/10.3390/jcm13164771
Chicago/Turabian StyleKaur, Simrat, Jaideep Singh Bhalla, Angelika L. Erwin, Wael Jaber, and Tom Kai Ming Wang. 2024. "Contemporary Multimodality Imaging for Diagnosis and Management of Fabry Cardiomyopathy" Journal of Clinical Medicine 13, no. 16: 4771. https://doi.org/10.3390/jcm13164771






