Magnetic Resonance Evaluation of Tissue Iron Deposition and Cardiac Function in Adult Regularly Transfused Thalassemia Intermedia Compared with Thalassemia Major Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Patients
2.2. Magnetic Resonance Imaging
2.3. Biochemical Assays
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of TI and TM Patients
3.2. Clinical Correlates of Tissue Iron Levels in TI and TM
3.3. Comparison of Tissue Iron Levels between TI and TM Patients
3.4. Clinical Correlates of Biventricular Function Parameters in TI and TM
3.5. Comparison of Biventricular Function Parameters between TI and TM Patients
3.6. Replacement Myocardial Fibrosis in TI and TM
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weatherall, D.J. The thalassaemias. BMJ 1997, 314, 1675–1678. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Galanello, R. Beta-thalassemia. Genet. Med. 2010, 12, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Mumtaz, S.; Shakir, H.A.; Khan, M.; Tahir, H.M.; Mumtaz, S.; Mughal, T.A.; Hassan, A.; Kazmi, S.A.R.; Sadia; et al. Current status of beta-thalassemia and its treatment strategies. Mol. Genet. Genom. Med. 2021, 9, e1788. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, N.F.; Nathan, D.G.; MacMillan, J.H.; Wayne, A.S.; Liu, P.P.; McGee, A.; Martin, M.; Koren, G.; Cohen, A.R. Survival in medically treated patients with homozygous beta-thalassemia. N. Engl. J. Med. 1994, 331, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Weatherall, D.J.; Clegg, J.B. The Thalassemia Syndromes; Blackwell Science: Oxford, UK, 2001. [Google Scholar]
- Porter, J.B.; Shah, F.T. Iron overload in thalassemia and related conditions: Therapeutic goals and assessment of response to chelation therapies. Hematol. Oncol. Clin. N. Am. 2010, 24, 1109–1130. [Google Scholar] [CrossRef] [PubMed]
- Di Maggio, R.; Maggio, A. The new era of chelation treatments: Effectiveness and safety of 10 different regimens for controlling iron overloading in thalassaemia major. Br. J. Haematol. 2017, 178, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.T.; Porter, J.B.; Sadasivam, N.; Kaya, B.; Moon, J.C.; Velangi, M.; Ako, E.; Pancham, S. Guidelines for the monitoring and management of iron overload in patients with haemoglobinopathies and rare anaemias. Br. J. Haematol. 2021, 196, 336–350. [Google Scholar] [CrossRef] [PubMed]
- Forni, G.L.; Kattamis, A.; Kuo, K.H.M.; Maggio, A.; Sheth, S.; Taher, A.T.; Viprakasit, V. Iron chelation therapy for children with transfusion-dependent β-thalassemia: How young is too young? Pediatr. Blood Cancer 2024, 71, e31035. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.A. Disorders of iron metabolism. N. Engl. J. Med. 2000, 342, 1293. [Google Scholar] [PubMed]
- Ozment, C.P.; Turi, J.L. Iron overload following red blood cell transfusion and its impact on disease severity. Biochim. Biophys. Acta 2009, 1790, 694–701. [Google Scholar] [CrossRef]
- Yiannikourides, A.; Latunde-Dada, G.O. A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders. Medicines 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Musallam, K.M.; Rivella, S.; Vichinsky, E.; Rachmilewitz, E.A. Non-transfusion-dependent thalassemias. Haematologica 2013, 98, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Shash, H. Non-Transfusion-Dependent Thalassemia: A Panoramic Review. Medicina 2022, 58, 1496. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.T.; Musallam, K.M.; El-Beshlawy, A.; Karimi, M.; Daar, S.; Belhoul, K.; Saned, M.S.; Graziadei, G.; Cappellini, M.D. Age-related complications in treatment-naive patients with thalassaemia intermedia. Br. J. Haematol. 2010, 150, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.T.; Musallam, K.M.; Karimi, M.; El-Beshlawy, A.; Belhoul, K.; Daar, S.; Saned, M.S.; El-Chafic, A.H.; Fasulo, M.R.; Cappellini, M.D. Overview on practices in thalassemia intermedia management aiming for lowering complication rates across a region of endemicity: The OPTIMAL CARE study. Blood 2010, 115, 1886–1892. [Google Scholar] [CrossRef]
- Haddad, A.; Tyan, P.; Radwan, A.; Mallat, N.; Taher, A. beta-Thalassemia Intermedia: A Bird’s-Eye View. Turk. J. Haematol. 2014, 31, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Ricchi, P.; Meloni, A.; Pistoia, L.; Spasiano, A.; Rita Gamberini, M.; Maggio, A.; Gerardi, C.; Messina, G.; Campisi, S.; Allo, M.; et al. Longitudinal follow-up of patients with thalassaemia intermedia who started transfusion therapy in adulthood: A cohort study. Br. J. Haematol. 2020, 191, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, J.; Tarhini, A.; Bou-Fakhredin, R.; Saliba, A.N.; Cappellini, M.D.; Taher, A.T. Non-Transfusion-Dependent Thalassemia: An Update on Complications and Management. Int. J. Mol. Sci. 2018, 19, 182. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.; Vichinsky, E.; Musallam, K.; Cappellini, M.D.; Viprakasit, V. Guidelines for the Management of Non Transfusion Dependent Thalassaemia (NTDT); Thalassaemia International Federation: Nicosia, Cyprus, 2013. [Google Scholar]
- Bou-Fakhredin, R.; Bazarbachi, A.H.; Chaya, B.; Sleiman, J.; Cappellini, M.D.; Taher, A.T. Iron Overload and Chelation Therapy in Non-Transfusion Dependent Thalassemia. Int. J. Mol. Sci. 2017, 18, 2778. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, G.; Wang, M.; Wei, X.; Pan, L.; Liu, J.; Lei, Y.; Peng; Long, L.; Lai, Y.; et al. Iron overload status in patients with non-transfusion-dependent thalassemia in China. Ther. Adv. Hematol. 2022, 13, 20406207221084639. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.C.; Ghugre, N. Magnetic resonance imaging assessment of excess iron in thalassemia, sickle cell disease and other iron overload diseases. Hemoglobin 2008, 32, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Pennell, D.J.; Udelson, J.E.; Arai, A.E.; Bozkurt, B.; Cohen, A.R.; Galanello, R.; Hoffman, T.M.; Kiernan, M.S.; Lerakis, S.; Piga, A.; et al. Cardiovascular function and treatment in beta-thalassemia major: A consensus statement from the American Heart Association. Circulation 2013, 128, 281–308. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.L. MRI for Iron Overload in Thalassemia. Hematol./Oncol. Clin. 2018, 32, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.C. Estimating tissue iron burden: Current status and future prospects. Br. J. Haematol. 2015, 170, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Holden, S.; Davis, B.; Prescott, E.; Charrier, C.C.; Bunce, N.H.; Firmin, D.N.; Wonke, B.; Porter, J.; Walker, J.M.; et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur. Heart J. 2001, 22, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Positano, V.; Ruffo, G.B.; Spasiano, A.; D’Ascola, D.G.; Peluso, A.; Keilberg, P.; Restaino, G.; Valeri, G.; Renne, S.; et al. Improvement of heart iron with preserved patterns of iron store by CMR-guided chelation therapy. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Au, W.Y.; Lam, W.W.; Chu, W.; Tam, S.; Wong, W.K.; Liang, R.; Ha, S.Y. A T2* magnetic resonance imaging study of pancreatic iron overload in thalassemia major. Haematologica 2008, 93, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Noetzli, L.J.; Papudesi, J.; Coates, T.D.; Wood, J.C. Pancreatic iron loading predicts cardiac iron loading in thalassemia major. Blood 2009, 114, 4021–4026. [Google Scholar] [CrossRef] [PubMed]
- Pepe, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Peluso, A.; Messina, G.; Spasiano, A.; Allo, M.; Bisconte, M.G.; Putti, M.C.; et al. The Close Link of Pancreatic Iron With Glucose Metabolism and With Cardiac Complications in Thalassemia Major: A Large, Multicenter Observational Study. Diabetes Care 2020, 43, 2830–2839. [Google Scholar] [CrossRef]
- Hashemieh, M. Assessment of Organ Specific Iron Overload in Transfusion-dependent Thalassemia by Magnetic Resonance Imaging Techniques. Iran. J. Blood Cancer 2019, 11, 39–46. [Google Scholar]
- Chapchap, E.C.; Silva, M.M.A.; de Assis, R.A.; Kerbauy, L.N.; Diniz, M.D.S.; Rosemberg, L.A.; Loggetto, S.R.; Araujo, A.D.S.; Fabron Junior, A.; Verissimo, M.P.A.; et al. Cardiac iron overload evaluation in thalassaemic patients using T2* magnetic resonance imaging following chelation therapy: A multicentre cross-sectional study. Hematol. Transfus. Cell Ther. 2023, 45, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Saadatifar, H.; Mard-Soltani, M.; Niayeshfar, A.; Shakerian, N.; Pouriamehr, S.; Alinezhad Dezfuli, D.; Khalili, S.; Saadatifar, S.; Mashhadi, S.M. Correlation between plasma biochemical parameters and cardio-hepatic iron deposition in thalassemia major patients. Scand. J. Clin. Lab. Investig. 2024, 83, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Musallam, K.M.; Barella, S.; Origa, R.; Ferrero, G.B.; Lisi, R.; Pasanisi, A.; Longo, F.; Gianesin, B.; Forni, G.L. Revisiting iron overload status and change thresholds as predictors of mortality in transfusion-dependent β-thalassemia: A 10-year cohort study. Ann. Hematol. 2024, 103, 2283–2297. [Google Scholar] [CrossRef] [PubMed]
- Abdi, S.; Taheri, N.; Haghighi, F.Z.; Khaki, M.; Najafi, H.; Komasi, M.M.H.; Hassani, B. The relationship of myocardial and liver T2* values with cardiac function and laboratory findings in transfusion-dependent thalassemia major patients: A retrospective cardiac MRI study. J. Cardiovasc. Thorac. Res. 2023, 15, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Wahidiyat, P.A.; Liauw, F.; Sekarsari, D.; Putriasih, S.A.; Berdoukas, V.; Pennell, D.J. Evaluation of cardiac and hepatic iron overload in thalassemia major patients with T2* magnetic resonance imaging. Hematology 2017, 22, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Khadivi Heris, H.; Nejati, B.; Rezazadeh, K.; Sate, H.; Dolatkhah, R.; Ghoreishi, Z.; Esfahani, A. Evaluation of iron overload by cardiac and liver T2* in β-thalassemia: Correlation with serum ferritin, heart function and liver enzymes. J. Cardiovasc. Thorac. Res. 2021, 13, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Pistoia, L.; Gamberini, M.R.; Ricchi, P.; Cecinati, V.; Sorrentino, F.; Cuccia, L.; Allo, M.; Righi, R.; Fina, P.; et al. The Link of Pancreatic Iron with Glucose Metabolism and Cardiac Iron in Thalassemia Intermedia: A Large, Multicenter Observational Study. J. Clin. Med. 2021, 10, 5561. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Pistoia, L.; Ricchi, P.; Longo, F.; Cecinati, V.; Sorrentino, F.; Cuccia, L.; Corigliano, E.; Rossi, V.; Righi, R.; et al. Multiparametric cardiac magnetic resonance in patients with thalassemia intermedia: New insights from the E-MIOT network. Radiol. Med. 2024, 129, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Varat, M.A.; Adolph, R.J.; Fowler, N.O. Cardiovascular effects of anemia. Am. Heart J. 1972, 83, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, A.; Vollbon, W.; Jellis, C.; Prior, D.; Wahi, S.; Marwick, T. Echocardiographic assessment of raised pulmonary vascular resistance: Application to diagnosis and follow-up of pulmonary hypertension. Heart 2010, 96, 2005–2009. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J., Jr.; Meshel, J.C.; Patterson, R.H. The cardiovascular manifestations of sickle cell disease. Arch. Intern. Med. 1974, 133, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Kremastinos, D.T.; Tsiapras, D.P.; Tsetsos, G.A.; Rentoukas, E.I.; Vretou, H.P.; Toutouzas, P.K. Left ventricular diastolic Doppler characteristics in beta-thalassemia major. Circulation 1993, 88, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Coates, T.; Wood, J.C. Atrial dysfunction as a marker of iron cardiotoxicity in thalassemia major. Haematologica 2008, 93, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.F.; Chan, G.C.; Ha, S.Y. Arterial stiffness and endothelial function in patients with beta-thalassemia major. Circulation 2002, 106, 2561–2566. [Google Scholar] [CrossRef]
- Cheung, Y.F.; Chan, G.C.; Ha, S.Y. Effect of deferasirox (ICL670) on arterial function in patients with beta-thalassaemia major. Br. J. Haematol. 2008, 141, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.C.; Enriquez, C.; Ghugre, N.; Otto-Duessel, M.; Aguilar, M.; Nelson, M.D.; Moats, R.; Coates, T.D. Physiology and pathophysiology of iron cardiomyopathy in thalassemia. Ann. N. Y. Acad. Sci. 2005, 1054, 386–395. [Google Scholar] [CrossRef]
- Pennell, D.J. Cardiovascular Magnetic Resonance. Circulation 2010, 121, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Merlo, M.; Gagno, G.; Baritussio, A.; Bauce, B.; Biagini, E.; Canepa, M.; Cipriani, A.; Castelletti, S.; Dellegrottaglie, S.; Guaricci, A.I.; et al. Clinical application of CMR in cardiomyopathies: Evolving concepts and techniques: A position paper of myocardial and pericardial diseases and cardiac magnetic resonance working groups of Italian society of cardiology. Heart Fail. Rev. 2023, 28, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Pitocco, F.; Di Giampietro, I.; De Vivo, A.E.; Schena, E.; Giurazza, F.; Sorrentino, F.; Zobel, B.B. Magnetic resonance comparison of left-right heart volumetric and functional parameters in thalassemia major and thalassemia intermedia patients. Biomed. Res. Int. 2015, 2015, 857642. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.; Gotsis, E.; Ladis, V.; Berdousis, E.; Verganelakis, D.; Toulas, P.; Cokkinos, D.V. Magnetic resonance evaluation of liver and myocardial iron deposition in thalassemia intermedia and b-thalassemia major. Int. J. Cardiovasc. Imaging 2008, 24, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, Y.; Zhao, S.; Lu, M. Detection of myocardial fibrosis: Where we stand. Front. Cardiovasc. Med. 2022, 9, 926378. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, M.; Pontone, G. Clinical implications of cardiac magnetic resonance imaging fibrosis. Eur. Heart J. Suppl. 2022, 24, I123–I126. [Google Scholar] [CrossRef] [PubMed]
- Pepe, A.; Meloni, A.; Rossi, G.; Midiri, M.; Missere, M.; Valeri, G.; Sorrentino, F.; D’Ascola, D.G.; Spasiano, A.; Filosa, A.; et al. Prediction of cardiac complications for thalassemia major in the widespread cardiac magnetic resonance era: A prospective multicentre study by a multi-parametric approach. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Pistoia, L.; Ricchi, P.; Maggio, A.; Cecinati, V.; Longo, F.; Sorrentino, F.; Borsellino, Z.; Salvo, A.; Rossi, V.; et al. Prognostic Role of Multiparametric Cardiac Magnetic Resonance in Neo Transfusion-Dependent Thalassemia. J. Clin. Med. 2024, 13, 1281. [Google Scholar] [CrossRef] [PubMed]
- Ramazzotti, A.; Pepe, A.; Positano, V.; Rossi, G.; De Marchi, D.; Brizi, M.G.; Luciani, A.; Midiri, M.; Sallustio, G.; Valeri, G.; et al. Multicenter validation of the magnetic resonance t2* technique for segmental and global quantification of myocardial iron. J. Magn. Reson. Imaging 2009, 30, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; De Marchi, D.; Pistoia, L.; Grassedonio, E.; Peritore, G.; Preziosi, P.; Restaino, G.; Righi, R.; Riva, A.; Renne, S.; et al. Multicenter validation of the magnetic resonance T2* technique for quantification of pancreatic iron. Eur. Radiol. 2019, 29, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Positano, V.; Salani, B.; Pepe, A.; Santarelli, M.F.; De Marchi, D.; Ramazzotti, A.; Favilli, B.; Cracolici, E.; Midiri, M.; Cianciulli, P.; et al. Improved T2* assessment in liver iron overload by magnetic resonance imaging. Magn. Reson. Imaging 2009, 27, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Restaino, G.; Meloni, A.; Positano, V.; Missere, M.; Rossi, G.; Calandriello, L.; Keilberg, P.; Mattioni, O.; Maggio, A.; Lombardi, M.; et al. Regional and global pancreatic T*(2) MRI for iron overload assessment in a large cohort of healthy subjects: Normal values and correlation with age and gender. Magn. Reson. Med. 2011, 65, 764–769. [Google Scholar] [CrossRef]
- Meloni, A.; Restaino, G.; Borsellino, Z.; Caruso, V.; Spasiano, A.; Zuccarelli, A.; Valeri, G.; Toia, P.; Salvatori, C.; Positano, V.; et al. Different patterns of myocardial iron distribution by whole-heart T2* magnetic resonance as risk markers for heart complications in thalassemia major. Int. J. Cardiol. 2014, 177, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Positano, V.; Pepe, A.; Santarelli, M.F.; Scattini, B.; De Marchi, D.; Ramazzotti, A.; Forni, G.; Borgna-Pignatti, C.; Lai, M.E.; Midiri, M.; et al. Standardized T2* map of normal human heart in vivo to correct T2* segmental artefacts. NMR Biomed. 2007, 20, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Luciani, A.; Positano, V.; De Marchi, D.; Valeri, G.; Restaino, G.; Cracolici, E.; Caruso, V.; Dell’amico, M.C.; Favilli, B.; et al. Single region of interest versus multislice T2* MRI approach for the quantification of hepatic iron overload. J. Magn. Reson. Imaging 2011, 33, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.C.; Enriquez, C.; Ghugre, N.; Tyzka, J.M.; Carson, S.; Nelson, M.D.; Coates, T.D. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 2005, 106, 1460–1465. [Google Scholar] [CrossRef] [PubMed]
- St Pierre, T.G.; Clark, P.R.; Chua-anusorn, W.; Fleming, A.J.; Jeffrey, G.P.; Olynyk, J.K.; Pootrakul, P.; Robins, E.; Lindeman, R. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 2005, 105, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; De Marchi, D.; Positano, V.; Neri, M.G.; Mangione, M.; Keilberg, P.; Lendini, M.; Cirotto, C.; Pepe, A. Accurate estimate of pancreatic T2* values: How to deal with fat infiltration. Abdom. Imaging 2015, 40, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar] [PubMed]
- Meloni, A.; Righi, R.; Missere, M.; Renne, S.; Schicchi, N.; Gamberini, M.R.; Cuccia, L.; Lisi, R.; Spasiano, A.; Roberti, M.G.; et al. Biventricular Reference Values by Body Surface Area, Age, and Gender in a Large Cohort of Well-Treated Thalassemia Major Patients without Heart Damage Using a Multiparametric CMR Approach. J. Magn. Reson. Imaging 2021, 53, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Pepe, A.; Positano, V.; Capra, M.; Maggio, A.; Lo Pinto, C.; Spasiano, A.; Forni, G.; Derchi, G.; Favilli, B.; Rossi, G.; et al. Myocardial scarring by delayed enhancement cardiovascular magnetic resonance in thalassaemia major. Heart 2009, 95, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- Marsella, M.; Borgna-Pignatti, C.; Meloni, A.; Caldarelli, V.; Dell’Amico, M.C.; Spasiano, A.; Pitrolo, L.; Cracolici, E.; Valeri, G.; Positano, V.; et al. Cardiac iron and cardiac disease in males and females with transfusion-dependent thalassemia major: A T2* magnetic resonance imaging study. Haematologica 2011, 96, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Wong, T.; Keel, S.; Pagano, M.; Chung, J.; Kamdar, A.; Rao, L.; Ikeda, A.; Puthenveetil, G.; Shah, S.; et al. The transfusion management of beta thalassemia in the United States. Transfusion 2021, 61, 3027–3039. [Google Scholar] [CrossRef]
- Gardenghi, S.; Marongiu, M.F.; Ramos, P.; Guy, E.; Breda, L.; Chadburn, A.; Liu, Y.; Amariglio, N.; Rechavi, G.; Rachmilewitz, E.A.; et al. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood 2007, 109, 5027–5035. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.-R.; Frazer, D.M.; Bowden, D.K.; Anderson, G.J. Transfusion suppresses erythropoiesis and increases hepcidin in adult patients with β-thalassemia major: A longitudinal study. Blood 2013, 122, 124–133. [Google Scholar] [CrossRef]
- Longo, F.; Piga, A. Does Hepcidin Tuning Have a Role among Emerging Treatments for Thalassemia? J. Clin. Med. 2022, 11, 5119. [Google Scholar] [CrossRef] [PubMed]
- Kattamis, A.; Papassotiriou, I.; Palaiologou, D.; Apostolakou, F.; Galani, A.; Ladis, V.; Sakellaropoulos, N.; Papanikolaou, G. The effects of erythropoetic activity and iron burden on hepcidin expression in patients with thalassemia major. Haematologica 2006, 91, 809–812. [Google Scholar] [PubMed]
- Origa, R.; Galanello, R.; Ganz, T.; Giagu, N.; Maccioni, L.; Faa, G.; Nemeth, E. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica 2007, 92, 583–588. [Google Scholar] [CrossRef]
- Meloni, A.; Positano, V.; Pepe, A.; Rossi, G.; Dell’Amico, M.; Salvatori, C.; Keilberg, P.; Filosa, A.; Sallustio, G.; Midiri, M.; et al. Preferential patterns of myocardial iron overload by multislice multiecho T*2 CMR in thalassemia major patients. Magn. Reson. Med. 2010, 64, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Ricchi, P.; Meloni, A.; Spasiano, A.; Neri, M.G.; Gamberini, M.R.; Cuccia, L.; Caruso, V.; Gerardi, C.; D’Ascola, D.G.; Rosso, R.; et al. Extramedullary hematopoiesis is associated with lower cardiac iron loading in chronically transfused thalassemia patients. Am. J. Hematol. 2015, 90, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Brissot, P.; Ropert, M.; Le Lan, C.; Loréal, O. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, Y.Z. Hepcidin-ferroportin axis in health and disease. Vitam. Horm. 2019, 110, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Noetzli, L.J.; Carson, S.M.; Nord, A.S.; Coates, T.D.; Wood, J.C. Longitudinal analysis of heart and liver iron in thalassemia major. Blood 2008, 112, 2973–2978. [Google Scholar] [CrossRef] [PubMed]
- Oudit, G.Y.; Sun, H.; Trivieri, M.G.; Koch, S.E.; Dawood, F.; Ackerley, C.; Yazdanpanah, M.; Wilson, G.J.; Schwartz, A.; Liu, P.P.; et al. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat. Med. 2003, 9, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Chattipakorn, N.; Kumfu, S.; Fucharoen, S.; Chattipakorn, S. Calcium channels and iron uptake into the heart. World J. Cardiol. 2011, 3, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.L.; Loggetto, S.R.; Veríssimo, M.P.; Fertrin, K.Y.; Baldanzi, G.R.; Fioravante, L.A.; Tan, D.M.; Higa, T.; Mashima, D.A.; Piga, A.; et al. A randomized trial of amlodipine in addition to standard chelation therapy in patients with thalassemia major. Blood 2016, 128, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Westwood, M.A.; Anderson, L.J.; Maceira, A.M.; Shah, F.T.; Prescott, E.; Porter, J.B.; Wonke, B.; Walker, J.M.; Pennell, D.J. Normalized left ventricular volumes and function in thalassemia major patients with normal myocardial iron. J. Magn. Reson. Imaging 2007, 25, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.P.; Alpendurada, F.; Deac, M.; Maceira, A.; Garbowski, M.; Kirk, P.; Walker, J.M.; Porter, J.B.; Shah, F.; Banya, W.; et al. Right ventricular volumes and function in thalassemia major patients in the absence of myocardial iron overload. J. Cardiovasc. Magn. Reson. 2010, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Kawel-Boehm, N.; Hetzel, S.J.; Ambale-Venkatesh, B.; Captur, G.; Francois, C.J.; Jerosch-Herold, M.; Salerno, M.; Teague, S.D.; Valsangiacomo-Buechel, E.; van der Geest, R.J.; et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Detterich, J.; Berdoukas, V.; Pepe, A.; Lombardi, M.; Coates, T.D.; Wood, J.C. Comparison of biventricular dimensions and function between pediatric sickle-cell disease and thalassemia major patients without cardiac iron. Am. J. Hematol. 2013, 88, 213–218. [Google Scholar] [CrossRef]
- Bing, R.; Dweck, M.R. Myocardial fibrosis: Why image, how to image and clinical implications. Heart 2019, 105, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Martini, N.; Positano, V.; De Luca, A.; Pistoia, L.; Sbragi, S.; Spasiano, A.; Casini, T.; Bitti, P.P.; Allò, M.; et al. Myocardial iron overload by cardiovascular magnetic resonance native segmental T1 mapping: A sensitive approach that correlates with cardiac complications. J. Cardiovasc. Magn. Reson. 2021, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Kraigher-Krainer, E.; Shah, A.M.; Gupta, D.K.; Santos, A.; Claggett, B.; Pieske, B.; Zile, M.R.; Voors, A.A.; Lefkowitz, M.P.; Packer, M.; et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2014, 63, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, W.; Zhao, S.; Lu, M. State-of-the-art myocardial strain by CMR feature tracking: Clinical applications and future perspectives. Eur. Radiol. 2022, 32, 5424–5435. [Google Scholar] [CrossRef] [PubMed]
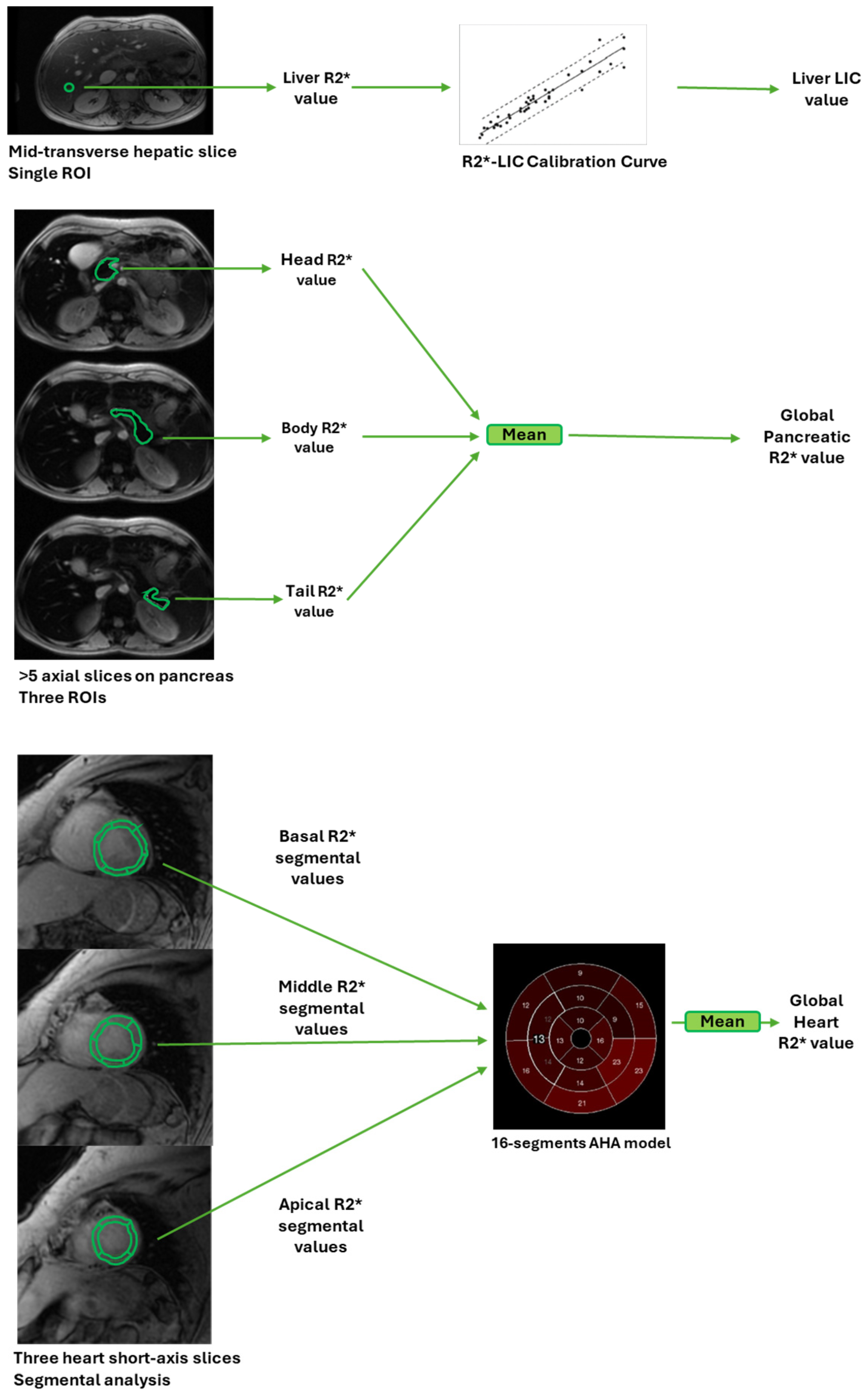
| TI Group (n = 135) | TM Group (n = 135) | p-Value | |
|---|---|---|---|
| Age (yrs) | 44.73 ± 12.16 | 43.35 ± 9.83 | 0.306 |
| Females, n (%) | 77 (57.0) | 77 (57.0) | 1.000 |
| Splenectomy, n (%) | 109 (80.7) | 104 (77.0) | 0.456 |
| Age at splenectomy (years) | 16.29 ± 10.61 | 13.44 ± 8.17 | 0.124 |
| Age at start of regular transfusions (years) | 19.19 ± 18.51 | 1.34 ± 1.51 | <0.0001 |
| Duration of regular transfusions (years) | 25.15 ± 15.26 | 41.37 ± 9.62 | <0.0001 |
| Patients in chelation therapy, n (%) | 130 (96.3) | 145 (100) | 0.060 |
| Age at start of chelation therapy (years) | 17.76 ± 16.19 | 6.41 ± 5.98 | <0.0001 |
| Mean pre-transfusion hemoglobin (g/dL) | 9.49 ± 0.58 | 9.72 ± 0.51 | 0.005 |
| Mean serum ferritin (ng/mL) | 861.82 ± 947.34 | 762.79 ± 736.82 | 0.584 |
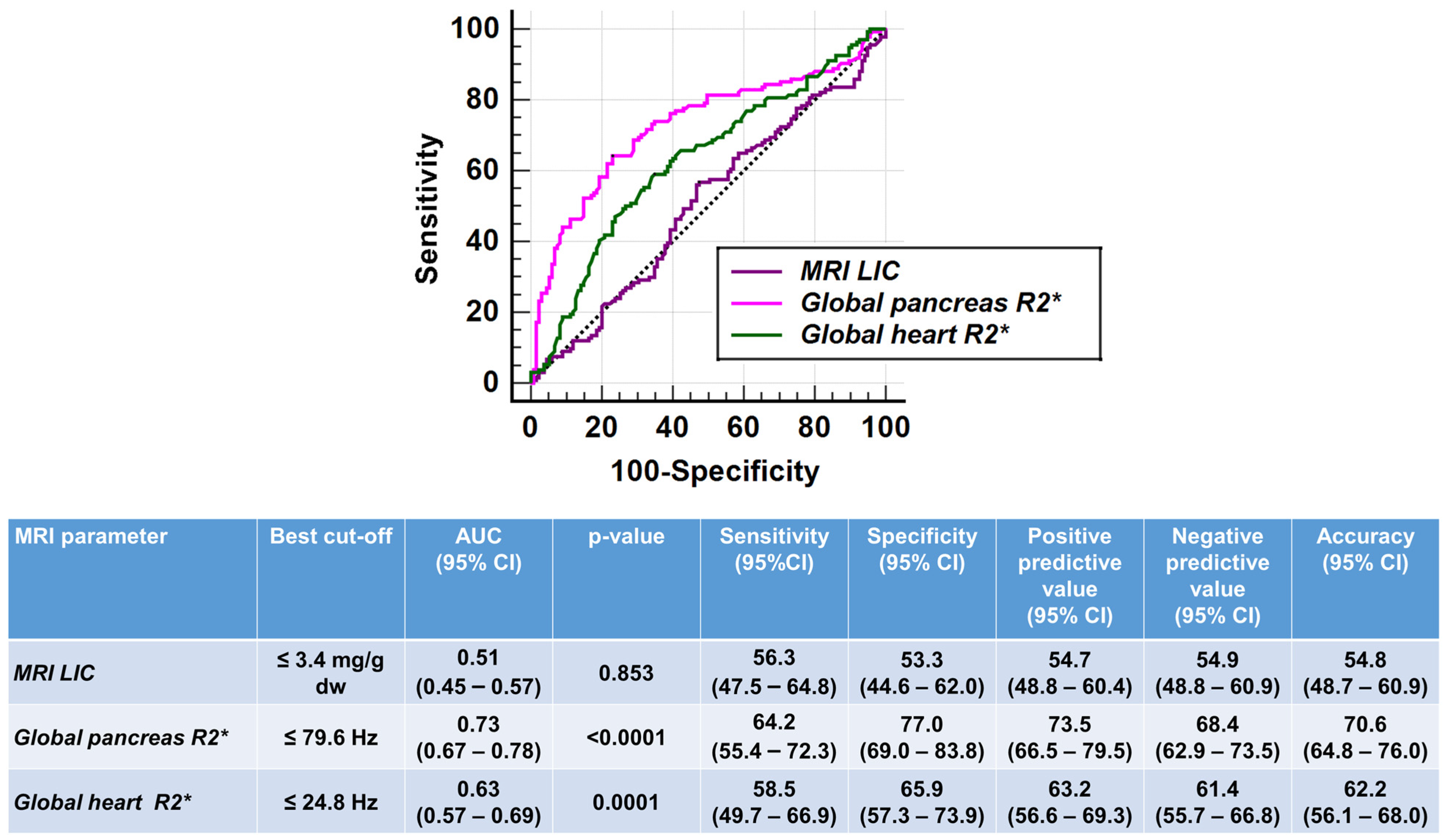
| MRI LIC (mg/g dw) | 6.54 ± 14.68 | 5.22 ± 6.62 | 0.853 |
| Hepatic iron overload, n (%) | 87 (64.4) | 90 (66.7) | 0.701 |
| Global pancreas R2* (Hz) | 93.47 ± 93.23 | 148.38 ± 107.73 | <0.0001 |
| Pancreatic iron overload, n (%) | 92 (68.1) | 127 (94.1) | <0.0001 |
| Global heart R2* (Hz) | 26.19 ± 7.17 | 30.38 ± 16.57 | <0.0001 |
| Significant myocardial iron overload, n (%) | 4 (3.0) | 7 (5.2) | 0.540 |
| n. of segments with R2* > 50 Hz | 0.44 ± 2.04 | 1.24 ± 3.73 | 0.010 |
| At least 1 segment with R2* > 50 Hz, n (%) | 13 (9.6) | 28 (20.7) | 0.011 |
| LV EDVI (mL/m2) | 86.77 ± 16.57 | 81.36 ± 16.84 | 0.003 |
| LV ESVI (mL/m2) | 33.17 ± 11.80 | 29.49 ± 10.29 | 0.006 |
| LV mass index (g/m2) | 57.88 ± 13.28 | 54.63 ± 13.27 | 0.049 |
| LV EF (%) | 62.89 ± 7.36 | 64.24 ± 7.10 | 0.129 |
| RV EDVI (mL/m2) | 83.45 ± 17.49 | 78.96 ± 17.05 | 0.046 |
| RV ESVI (mL/m2) | 31.27 ± 12.06 | 29.31 ± 10.34 | 0.253 |
| RV EF (%) | 63.41 ± 8.15 | 62.61 ± 9.21 | 0.546 |
| Wall motion abnormalities, n (%) | 11 (8.1) | 11 (8.1) | 1.000 |
| Replacement myocardial fibrosis, n (%) | 10/42 (23.8) | 11/39 (28.2) | 0.652 |
| TI Patients | TM Patients | |||||
|---|---|---|---|---|---|---|
| MRI LIC | Global Pancreas R2* | Global Heart R2* | MRI LIC | Global Pancreas R2* | Global Heart R2* | |
| Categorical variables | Difference of MRI iron overload parameter between two groups (absent vs. present) | |||||
| Female sex | 6.39 ± 19.54 vs. 6.65 ± 9.68 mg/g dw (p = 0.824) | 69.16 ± 76.26 vs. 111.47 ± 100.76 Hz (p = 0.011) | 25.44 ± 4.94 vs. 26.77 ± 8.46 Hz (p = 0.986) | 5.94 ± 7.29 vs. 4.68 ± 6.06 mg/g dw (p = 0.363) | 148.77 ± 100.35 vs. 148.08 ± 113.62 Hz (p = 0.922) | 28.97 ± 11.29 vs. 31.44 ± 19.64 Hz (p = 0.769) |
| Splenectomy | 7.36 ± 10.70 vs. 6.34 ± 15.52 mg/g dw (p = 0.056) | 62.21 ± 70.92 vs. 100.99 ± 96.21 Hz (p = 0.004) | 24.71 ± 2.92 vs. 26.55 ± 7.82 Hz (p = 0.491) | 6.31 ± 7.26 vs. 4.90 ± 6.42 mg/g dw (p = 0.104) | 121.87 ± 91.78 vs. 154.19 ± 111.78 Hz (p = 0.150) | 28.37 ± 13.16 vs. 30.98 ± 17.47 Hz (p = 0.384) |
| Continuous variables | Correlation (R, p-value) with MRI iron overload parameter | |||||
| Age | R = −0.154, p = 0.075 | R = −0.158, p = 0.069 | R = −0.134, p = 0.121 | R = −0.135, p = 0.118 | R = 0.086, p = 0.321 | R = 0.035, p = 0.690 |
| Duration of regular transfusions | R = −0.197, p = 0.085 | R = 0.253, p = 0.007 | R = −0.061, p = 0.518 | R= −0.144, p = 0.108 | R = 0.148, p = 0.100 | R = 0.057, p = 0.525 |
| Pre-transfusion hemoglobin | R = −0.126, p = 0.200 | R = 0.004, p = 0.972 | R = 0.068, p = 0.491 | R = −0.165, p = 0.075 | R = −0.121, p = 0.095 | R = −0.004, p = 0.970 |
| Mean serum ferritin | R = 0.707, p < 0.0001 | R = 0.148, p = 0.129 | R = 0.160, p = 0.102 | R = 0.650, p < 0.0001 | R = 0.085, p = 0.367 | R = 0.251, p = 0.007 |
| MRI LIC | R = 0.253 p = 0.003 | R = 0.212 p = 0.014 | R = 0.167, p = 0.042 | R = 0.274, p = 0.001 | ||
| Global pancreas R2* | R = 0.253 p = 0.003 | R = 0.306 p < 0.0001 | R = 0.167, p = 0.042 | R = 0.275, p = 0.001 | ||
| Global heart R2* | R = 0.212 p = 0.014 | R = 0.306 p < 0.0001 | R = 0.274, p = 0.001 | R = 0.275, p = 0.001 | ||
| LV EDVI | LV ESVI | LV Mass Index | LV EF | RV EDVI | RV ESVI | RV EF | |
|---|---|---|---|---|---|---|---|
| Categorical variables | Difference in biventricular parameters between two groups (absent vs. present) | ||||||
| Female sex | 95.52 ± 16.09 vs. 80.18 ± 13.69 mL/m2 (p < 0.0001) | 38.45 ± 11.08 vs. 29.19 ± 10.79 mL/m2 (p < 0.0001) | 65.91 ± 13.47 vs. 51.83 ± 9.42 g/m2 (p < 0.0001) | 60.34 ± 7.78 vs. 64.82 ± 6.44 % (p < 0.0001) | 93.97 ± 17.18 vs. 75.54 ± 13.05 mL/m2 (p < 0.0001) | 38.54 ± 12.47 vs. 25.79 ± 8.31 mL/m2 (p < 0.0001) | 60.10 ± 7.24 vs. 65.91 ± 7.95 % (p < 0.0001) |
| Splenectomy | 79.15 ± 13.75 vs. 88.59 ± 16.72 mL/m2 (p = 0.009) | 29.04 ± 9.37 vs. 34.16 ± 12.14 mL/m2 (p = 0.047) | 53.42 ± 15.71 vs. 58.95 ± 12.48 g/m2 (p = 0.144) | 63.46 ± 7.32 vs. 62.76 ± 7.39 % (p = 0.665) | 78.54 ± 17.64 vs. 84.63 ± 17.33 mL/m2 (p = 0.111) | 29.23 ± 12.35 vs. 31.75 ± 11.99 mL/m2 (p = 0.191 | 65.35 ± 8.27 vs. 62.95 ± 8.09 % (p = 0.337) |
| Continuous variables | Correlation (R, p-value) with biventricular function parameter | ||||||
| Age | R = −0.310, p < 0.0001 | R = −0.279, p = 0.001 | R = −0.191, p = 0.027 | R = 0.211, p = 0.014 | R = −0.250, p = 0.003 | R = −0.231, p = 0.007 | R = 0.178, p = 0.039 |
| Duration of regular transfusions | R = −0.211, p = 0.023 | R = −0.124, p = 0.187 | R = −0.031, p = 0.741 | R = −0.023, p = 0.806 | R = −0.111, p = 0.236 | R = −0.017, p = 0.854 | R = −0.115, p = 0.220 |
| Pre-transfusion hemoglobin | R = −0.026, p = 0.795 | R = 0.034, p = 0.728 | R = 0.036, p = 0.714 | R = −0.084, p = 0.391 | R = 0.015, p = 0.882 | R = 0.027, p = 0.780 | R = −0.103, p = 0.293 |
| Mean serum ferritin | R = 0.008, p = 0.936 | R = 0.051, p = 0.601 | R = 0.112, p = 0.253 | R = −0.116, p = 0.236 | R = −0.046, p = 0.636 | R = −0.025, p = 0.801 | R = 0.018, p = 0.857 |
| MRI LIC | R = 0.043, p = 0.617 | R = 0.027, p = 0.754 | R = 0.105, p = 0.225 | R = −0.023, p = 0.794 | R = 0.074, p = 0.394 | R = 0.061, p = 0.480 | R = −0.018, p = 0.834 |
| Global pancreas R2* | R = −0.081, p = 0.335 | R = −0.116, p = 0.183 | R = −0.079, p = 0.362 | R = 0.121, p = 0.165 | R = −0.040, p = 0.647 | R = −0.072, p = 0.410 | R = 0.065, p = 0.457 |
| Global heart R2* | R = −0.006, p = 0.948 | R = −0.042, p = 0.625 | R = −0.036, p = 0.679 | R = 0.090, p = 0.298 | R = −0.052, p = 0.550 | R = −0.098, p = 0.258 | R = 0.165, p = 0.055 |
| LV EDVI | LV ESVI | LV Mass Index | LV EF | RV EDVI | RV ESVI | RV EF | |
|---|---|---|---|---|---|---|---|
| Categorical variables | Difference in biventricular parameters between two groups (absent vs. present) | ||||||
| Female sex | 92.02 ± 16.66 vs. 73.34 ± 11.84 mL/m2 (p < 0.0001) | 35.55 ± 10.19 vs. 24.92 ± 7.71 mL/m2 (p < 0.0001) | 62.07 ± 12.92 vs. 49.03 ± 10.54 g/m2 (p < 0.0001) | 61.76 ± 5.94 vs. 66.10 ± 7.37 % (p < 0.0001) | 89.66 ± 15.09 vs. 70.91 ± 13.74 mL/m2 (p < 0.0001) | 34.76 ± 10.28 vs. 25.19 ± 8.32 mL/m2 (p < 0.0001) | 61.22 ± 7.46 vs. 63.66 ± 10.26 % (p = 0.012) |
| Splenectomy | 80.55 ± 16.86 vs. 81.61 ± 16.91 mL/m2 (p = 0.761) | 29.36 ± 9.90 vs. 29.53 ± 10.44 mL/m2 (p = 0.981) | 50.77 ± 13.19 vs. 55.78 ± 13.14 g/m2 (p = 0.102) | 63.84 ± 7.12 vs. 64.36 ± 7.13 % (p = 0.723) | 77.00 ± 17.52 vs. 79.55 ± 16.95 mL/m2 (p = 0.467) | 29.94 ± 11.55 vs. 29.13 ± 10.01 mL/m2 (p = 0.908) | 62.19 ± 8.60 vs. 62.74 ± 9.42 % (p = 0.773) |
| Continuous variables | Correlation (R, p-value) with biventricular function parameter | ||||||
| Age | R = −0.289, p = 0.001 | R = −0.298, p < 0.0001 | R = −0.044, p = 0.614 | R = 0.223, p = 0.009 | R = −0.363, p < 0.0001 | R = −0.336, p < 0.0001 | R = 0.177, p = 0.040 |
| Duration of regular transfusions | R = −0.320, p < 0.0001 | R = −0.342, p < 0.0001 | R = −0.048, p = 0.597 | R = 0.272, p = 0.002 | R = −0.384, p < 0.0001 | R = −0.357, p < 0.0001 | R = 0.185, p = 0.039 |
| Pre-transfusion hemoglobin | R = −0.181, p = 0.051 | R = −0.107, p = 0.250 | R = 0.035, p = 0.706 | R = 0.003, p = 0.977 | R = −0.105, p = 0.261 | R = −0.072, p = 0.439 | R = 0.030, p = 0.748 |
| Mean serum ferritin | R = 0.010, p = 0.917 | R = 0.105, p = 0.263 | R = 0.076, p = 0.415 | R = −0.202, p = 0.080 | R = −0.070, p = 0.455 | R = 0.091, p = 0.331 | R = −0.067, p = 0.476 |
| MRI LIC | R = 0.134, p = 0.121 | R = 0.173, p = 0.055 | R = 0.134, p = 0.120 | R = −0.200, p = 0.060 | R = 0.084, p = 0.333 | R = 0.109, p = 0.207 | R = −0.064, p = 0.458 |
| Global pancreas R2* | R = −0.067, p = 0.438 | R = −0.044, p = 0.615 | R = 0.032, p = 0.709 | R = 0.054, p = 0.536 | R = −0.064, p = 0.464 | R = −0.089, p = 0.306 | R = −0.017, p = 0.844 |
| Global heart R2* | R = −0.068, p = 0.432 | R = −0.090, p = 0.298 | R = −0.081, p = 0.352 | R = 0.101, p = 0.245 | R = −0.066, p = 0.445 | R = −0.164, p = 0.057 | R = 0.148, p = 0.086 |
| TI Patients | TM Patients | |||||
|---|---|---|---|---|---|---|
| No LGE (n = 32) | LGE (n = 10) | p-Value | No LGE (n = 28) | LGE (n = 11) | p-Value | |
| Age (yrs) | 43.71 ± 11.11 | 50.63 ± 7.54 | 0.035 | 41.62 ± 8.31 | 51.41 ± 8.27 | 0.002 |
| Females, n (%) | 17 (53.1) | 8 (80.0) | 0.162 | 15 (53.6) | 7 (63.6) | 0.725 |
| Splenectomy, n (%) | 25 (78.1) | 9 (90.0) | 0.655 | 7 (75.0) | 11 (100.0) | 0.159 |
| Duration of regular transfusions (years) | 26.29 ± 15.36 | 21.09 ± 12.24 | 0.372 | 39.88 ± 8.05 | 49.24 ± 8.26 | 0.003 |
| Patients in chelation therapy, n (%) | 31 (96.9) | 10 (100.0) | 0.572 | 28 (100.0) | 11 (100.0) | - |
| Mean pre-transfusion hemoglobin (g/dL) | 9.46 ± 0.57 | 9.56 ± 0.43 | 0.810 | 9.82 ± 0.54 | 9.82 ± 0.49 | 0.773 |
| Mean serum ferritin (ng/mL) | 971.33 ± 1130.75 | 625.25 ± 553.49 | 0.408 | 1134.68 ± 1028.42 | 837.45 ± 947.53 | 0.117 |
| MRI LIC (mg/g dw) | 6.29 ± 9.75 | 5.98 ± 8.73 | 0.555 | 5.38 ± 7.11 | 4.60 ± 3.58 | 0.685 |
| Global pancreas R2* (Hz) | 75.55 ± 80.61 | 133.15 ± 105.99 | 0.140 | 165.22 ± 146.02 | 199.63 ± 136.23 | 0.224 |
| Global heart R2* (Hz) | 25.70 ± 6.15 | 24.38 ± 1.83 | 0.779 | 31.58 ± 17.60 | 27.25 ± 6.27 | 0.827 |
| LV EDVI (mL/m2) | 85.28 ± 18.05 | 80.80 ± 11.24 | 0.465 | 83.00 ± 16.54 | 84.36 ± 20.78 | 0.950 |
| LV ESVI (mL/m2) | 34.06 ± 12.41 | 30.30 ± 10.32 | 0.425 | 29.25 ± 9.00 | 34.55 ± 13.41 | 0.217 |
| LV mass index (g/m2) | 61.19 ± 14.95 | 58.50 ± 6.65 | 0.658 | 58.00 ± 14.75 | 59.09 ± 11.21 | 0.876 |
| LV EF (%) | 62.22 ± 8.26 | 62.60 ± 8.09 | 0.899 | 64.71 ± 6.02 | 58.82 ± 9.09 | 0.055 |
| RV EDVI (mL/m2) | 84.41 ± 11.17 | 75.80 ± 11.08 | 0.136 | 82.64 ± 15.57 | 81.36 ± 24.73 | 0.743 |
| RV ESVI (mL/m2) | 32.59 ± 10.39 | 27.90 ± 11.59 | 0.147 | 31.29 ± 9.97 | 35.09 ± 14.40 | 0.673 |
| RV EF (%) | 61.91 ± 7.05 | 63.40 ± 9.79 | 0.598 | 62.14 ± 6.61 | 56.64 ± 8.20 | 0.057 |
| Wall motion abnormalities, n (%) | 1 (3.1) | 1 (10.0) | 0.424 | 0 (0.0) | 3 (27.3) | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meloni, A.; Pistoia, L.; Ricchi, P.; Longo, F.; Cecinati, V.; Sorrentino, F.; Borsellino, Z.; Bagnato, S.; Rossi, V.; Fina, P.; et al. Magnetic Resonance Evaluation of Tissue Iron Deposition and Cardiac Function in Adult Regularly Transfused Thalassemia Intermedia Compared with Thalassemia Major Patients. J. Clin. Med. 2024, 13, 4791. https://doi.org/10.3390/jcm13164791
Meloni A, Pistoia L, Ricchi P, Longo F, Cecinati V, Sorrentino F, Borsellino Z, Bagnato S, Rossi V, Fina P, et al. Magnetic Resonance Evaluation of Tissue Iron Deposition and Cardiac Function in Adult Regularly Transfused Thalassemia Intermedia Compared with Thalassemia Major Patients. Journal of Clinical Medicine. 2024; 13(16):4791. https://doi.org/10.3390/jcm13164791
Chicago/Turabian StyleMeloni, Antonella, Laura Pistoia, Paolo Ricchi, Filomena Longo, Valerio Cecinati, Francesco Sorrentino, Zelia Borsellino, Sergio Bagnato, Vincenza Rossi, Priscilla Fina, and et al. 2024. "Magnetic Resonance Evaluation of Tissue Iron Deposition and Cardiac Function in Adult Regularly Transfused Thalassemia Intermedia Compared with Thalassemia Major Patients" Journal of Clinical Medicine 13, no. 16: 4791. https://doi.org/10.3390/jcm13164791







