High-Dose Neutrophil-Depleted Platelet-Rich Plasma Therapy for Knee Osteoarthritis: A Retrospective Study
Abstract
1. Introduction
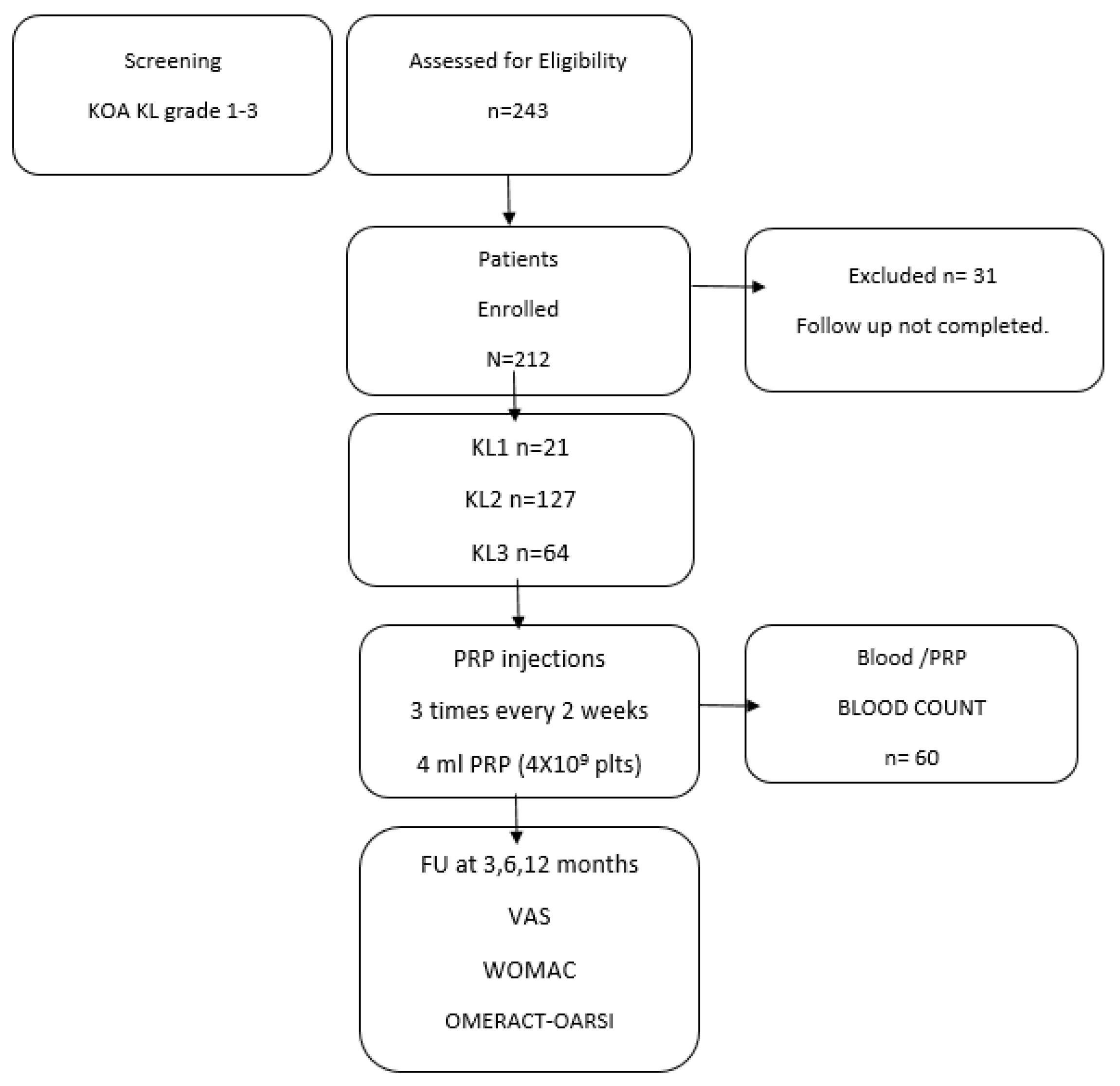
2. Materials and Methods
2.1. Study Design and Patients
- Age: >18 years;
- Hgb: >12 g/dL;
- PLT (minimum value): ≥120,000/µL;
- WBC < 10,000/mm3;
- No corticosteroid therapies for more than one month;
- No NSAIDs for at least one week.
Data Collection
2.2. PRP Preparation and Protocol
2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Biological Characteristics of Injected PRP
3.3. Clinical Outcome after PRP According to OMERCAT-OARSI, VAS, and WOMAC Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee Osteoarthritis: A Review of Pathogenesis and State-of-the-Art Non-Operative Therapeutic Considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; van Erp, A.; DeSimone, A.; Cohen, D.S.; Gardner, R.D. Platelet Rich Plasma in Orthopedic Surgical Medicine. Platelets 2021, 32, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Giannotti, L.; Di Chiara Stanca, B.; Spedicato, F.; Nitti, P.; Damiano, F.; Demitri, C.; Calabriso, N.; Carluccio, M.A.; Palermo, A.; Siculella, L.; et al. Progress in Regenerative Medicine: Exploring Autologous Platelet Concentrates and Their Clinical Applications. Genes 2023, 14, 1669. [Google Scholar] [CrossRef]
- Laver, L.; Filardo, G.; Sanchez, M.; Magalon, J.; Tischer, T.; Abat, F.; Bastos, R.; Cugat, R.; Iosifidis, M.; Kocaoglu, B.; et al. The Use of Injectable Orthobiologics for Knee Osteoarthritis: A European ESSKA-ORBIT Consensus. Part 1—Blood-derived Products (Platelet-rich Plasma). Knee Surg. Sports Traumatol. Arthrosc. 2024, 32, 783–797. [Google Scholar] [CrossRef]
- Gangadharan, S.B.; Satapathy, S.; Dixit, T.; Sukumaran, C.; Ravindran, S.; Parida, P.K. Platelet-Rich Plasma Treatment for Knee Osteoarthritis: A Systematic Investigation. Multidiscip. Rev. 2024, 6, 2023ss015. [Google Scholar] [CrossRef]
- Moldovan, F.; Gligor, A.; Moldovan, L.; Bataga, T. An Investigation for Future Practice of Elective Hip and Knee Arthroplasties during COVID-19 in Romania. Medicina 2023, 59, 314. [Google Scholar] [CrossRef] [PubMed]
- Gesheff, M.G.; Scalzitti, D.A.; Bains, S.S.; Dubin, J.; Delanois, R.E. Time to Total Knee Arthroplasty (TKA) Post Intra-Articular Injection. J. Clin. Med. 2024, 13, 3764. [Google Scholar] [CrossRef] [PubMed]
- Zahir, H.; Dehghani, B.; Yuan, X.; Chinenov, Y.; Kim, C.; Burge, A.; Bandhari, R.; Nemirov, D.; Fava, P.; Moley, P.; et al. In Vitro Responses to Platelet-Rich-Plasma Are Associated with Variable Clinical Outcomes in Patients with Knee Osteoarthritis. Sci. Rep. 2021, 11, 11493. [Google Scholar] [CrossRef]
- Gato-Calvo, L.; Magalhaes, J.; Ruiz-Romero, C.; Blanco, F.J.; Burguera, E.F. Platelet-Rich Plasma in Osteoarthritis Treatment: Review of Current Evidence. Ther. Adv. Chronic Dis. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Andia, I.; Maffulli, N. Platelet-Rich Plasma for Managing Pain and Inflammation in Osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; Mazzola, T.; Mautner, K.; Randelli, P.S.; Podesta, L. Modifying Orthobiological PRP Therapies Are Imperative for the Advancement of Treatment Outcomes in Musculoskeletal Pathologies. Biomedicines 2022, 10, 2933. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; Lana, J.F.; Onishi, K.; Buford, D.; Peng, J.; Mahmood, A.; Fonseca, L.F.; van Zundert, A.; Podesta, L. Angiogenesis and Tissue Repair Depend on Platelet Dosing and Bioformulation Strategies Following Orthobiological Platelet-Rich Plasma Procedures: A Narrative Review. Biomedicines 2023, 11, 1922. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, J.; Wang, Y.; He, J.; Chen, L.; Chu, J.; Wu, H. Platelet Rich Plasma in the Repair of Articular Cartilage Injury: A Narrative Review. Cartilage 2022, 13, 1–16. [Google Scholar] [CrossRef]
- Gupta, A.; Jeyaraman, M.; Potty, A.G. Leukocyte-Rich vs. Leukocyte-Poor Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis. Biomedicines 2023, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Boffa, A.; Andriolo, L.; Romandini, I.; Altamura, S.A.; Cenacchi, A.; Roverini, V.; Zaffagnini, S.; Filardo, G. Leukocyte-Rich versus Leukocyte-Poor Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: A Double-Blind Randomized Trial. Am. J. Sports Med. 2022, 50, 609–617. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Cao, S.; Han, Y.; Shao, J.; Fu, Q.; Wang, B.; Wu, J.; Xiang, D.; Liu, Z.; et al. Clinical Efficacy of Intra-Articular Injection with P-PRP Versus That of L-PRP in Treating Knee Cartilage Lesion: A Randomized Controlled Trial. Orthop. Surg. 2023, 15, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; Malanga, G.A.; Paul, R.V.; Rothenberg, J.B.; Stephens, N.; Mautner, K.R. Assessing Clinical Implications and Perspectives of the Pathophysiological Effects of Erythrocytes and Plasma Free Hemoglobin in Autologous Biologics for Use in Musculoskeletal Regenerative Medicine Therapies. A Review. Regen. Ther. 2019, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Gong, C.; Peng, X.; Liu, X.; Su, X.; Tao, X.; Li, Y.; Wen, Y.; Li, W. Efficacy and Safety of Platelet-Rich Plasma Injections for the Treatment of Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Med. 2023, 10, 1204144. [Google Scholar] [CrossRef]
- Carlsson, A.M. Assessment of Chronic Pain. I. Aspects of the Reliability and Validity of the Visual Analogue Scale. Pain 1983, 16, 87–101. [Google Scholar] [CrossRef]
- Pham, T.; van der Heijde, D.; Altman, R.D.; Anderson, J.J.; Bellamy, N.; Hochberg, M.; Simon, L.; Strand, V.; Woodworth, T.; Dougados, M. OMERACT-OARSI Initiative: Osteoarthritis Research Society International Set of Responder Criteria for Osteoarthritis Clinical Trials Revisited. Osteoarthr. Cartil. 2004, 12, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Magalon, J.; Chateau, A.L.; Bertrand, B.; Louis, M.L.; Silvestre, A.; Giraudo, L.; Veran, J.; Sabatier, F. DEPA Classification: A Proposal for Standardising PRP Use and a Retrospective Application of Available Devices. BMJ Open Sport Exerc. Med. 2016, 2, e000060. [Google Scholar] [CrossRef] [PubMed]
- Delong, J.M.; Russell, R.P.; Mazzocca, A.D. Platelet-Rich Plasma: The PAW Classification System. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Bansal, H.; Leon, J.; Pont, J.L.; Wilson, D.A.; Bansal, A.; Agarwal, D.; Preoteasa, I. Platelet-Rich Plasma (PRP) in Osteoarthritis (OA) Knee: Correct Dose Critical for Long Term Clinical Efficacy. Sci. Rep. 2021, 11, 3971. [Google Scholar] [CrossRef]
- Patel, S.; Gahlaut, S.; Thami, T.; Chouhan, D.K.; Jain, A.; Dhillon, M.S. Comparison of Conventional Dose Versus Superdose Platelet-Rich Plasma for Knee Osteoarthritis: A Prospective, Triple-Blind, Randomized Clinical Trial. Orthop. J. Sports Med. 2024, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Saita, Y.; Kobayashi, Y.; Nishio, H.; Wakayama, T.; Fukusato, S.; Uchino, S.; Momoi, Y.; Ikeda, H.; Kaneko, K. Predictors of Effectiveness of Platelet-Rich Plasma Therapy for Knee Osteoarthritis: A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 4514. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Yan, L.; Feng, Y.; Li, X.; Zhang, K.; Lv, Z.; Xu, C.; Zhao, S.; Liu, F.; Yang, X.; et al. Efficacy and Safety of Corticosteroids, Hyaluronic Acid, and PRP and Combination Therapy for Knee Osteoarthritis: A Systematic Review and Network Meta-Analysis. BMC Musculoskelet. Disord. 2023, 24, 926. [Google Scholar] [CrossRef] [PubMed]
- Ivander, G.; Anggono, Y. A Comparison of Intra-Articular Hyaluronic Acid and Platelet-Rich Plasma for Knee Osteoarthritis: A Systematic Review. Orthop. Rev. 2024, 16, 94236. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Driessen, A.; Quack, V.; Sippel, N.; Cooper, B.; Mansy, Y.E.; Tingart, M.; Eschweiler, J. Comparison between Intra-Articular Infiltrations of Placebo, Steroids, Hyaluronic and PRP for Knee Osteoarthritis: A Bayesian Network Meta-Analysis. Arch. Orthop. Trauma Surg. 2021, 141, 1473–1490. [Google Scholar] [CrossRef]
- Berrigan, W.A.; Bailowitz, Z.; Park, A.; Reddy, A.; Liu, R.; Lansdown, D. A Higher Platelet Dose May Yield Better Clinical Outcomes for PRP in the Treatment of Knee Osteoarthritis: A Systematic Review. Arthroscopy 2024, in press. [Google Scholar] [CrossRef]
- Prost, D.; Bardot, T.; Baud, A.; Calvo, A.; Aumont, S.; Collado, H.; Borne, J.; Rajon, O.; Ponsot, A.; Malaterre, A.; et al. Long Term Improvement of Knee Osteoarthritis after Injection of Single High/Very High Volume of Very Pure PRP: A Retrospective Analysis of Patients Optimally Managed in Dedicated Centers. Regen. Ther. 2024, 25, 203–212. [Google Scholar] [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.L.; Paterson, K.L.; Metcalf, B.R.; Duong, V.; Eyles, J.; Kasza, J.; Wang, Y.; Cicuttini, F.; Buchbinder, R.; Forbes, A.; et al. Effect of Intra-Articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients with Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. JAMA 2021, 326, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Johal, H.; Khan, M.; Yung, S.-H.P.; Dhillon, M.S.; Fu, F.H.; Bedi, A.; Bhandari, M. Impact of Platelet-Rich Plasma Use on Pain in Orthopaedic Surgery: A Systematic Review and Meta-Analysis. Sports Health 2019, 11, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Maffulli, N.; Jain, V.K. Red Blood Cells in Platelet-Rich Plasma: Avoid If at All Possible. Biomedicines 2023, 11, 2425. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.; Huber, S.C.; Purita, J.; Tambeli, C.H.; Santos, G.S.; Paulus, C.; Annichino-Bizzacchi, J.M. Leukocyte-Rich PRP versus Leukocyte-Poor PRP - The Role of Monocyte/Macrophage Function in the Healing Cascade. J Clin Orthop Trauma 2019, 10, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yin, W.; Zhang, Y.; Qi, X.; Chen, Y.; Xie, X.; Zhang, C. Comparative Evaluation of Leukocyte-and Platelet-Rich Plasma and Pure Platelet-Rich Plasma for Cartilage Regeneration. Sci. Rep. 2017, 7, 43301. [Google Scholar] [CrossRef]
- Peng, Y.; Guanglan, W.; Jia, S.; Zheng, C. Leukocyte-Rich and Leukocyte-Poor Platelet-Rich Plasma in Rotator Cuff Repair: A Meta-Analysis. Int. J. Sports Med. 2022, 43, 921–930. [Google Scholar] [CrossRef]
- Fedorova, N.V.; Ksenofontov, A.L.; Serebryakova, M.V.; Stadnichuk, V.I.; Gaponova, T.V.; Baratova, L.A.; Sud’ina, G.F.; Galkina, S.I. Neutrophils Release Metalloproteinases during Adhesion in the Presence of Insulin, but Cathepsin G in the Presence of Glucagon. Mediators Inflamm. 2018, 2018, 1574928. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, Y.B.; Ha, C.W.; Roh, Y.J.; Park, J.G. Adverse Reactions and Clinical Outcomes for Leukocyte-Poor Versus Leukocyte-Rich Platelet-Rich Plasma in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Orthop. J. Sports Med. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Belk, J.W.; Kraeutler, M.J.; Houck, D.A.; Goodrich, J.A.; Dragoo, J.L.; McCarty, E.C. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Sports Med. 2021, 49, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Gómez-Aristizábal, A.; Gandhi, R.; Marshall, W.; Mahomed, N.; Viswanathan, S. Ex Vivo Polarized Pro-Inflammatory vs. Homeostatic Monocytes/Macrophages Elicit Differential Responses within a Human Osteoarthritic Joint Explant Model. Osteoarthr. Cartil. 2019, 27, S379–S380. [Google Scholar] [CrossRef]
- Ogle, M.E.; Segar, C.E.; Sridhar, S.; Botchwey, E.A.; Coulter, W.H. Monocytes and Macrophages in Tissue Repair: Implications for Immunoregenerative Biomaterial Design. Exp. Biol. Med. 2016, 241, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Sánchez, N.; Alonso-Alonso, S.; Nagy, L. Regenerative Inflammation: When Immune Cells Help to Re-Build Tissues. FEBS J. 2024, 291, 1597–1614. [Google Scholar] [CrossRef] [PubMed]
- Groppa, E.; Colliva, A.; Vuerich, R.; Kocijan, T.; Zacchigna, S. Immune Cell Therapies to Improve Regeneration and Revascularization of Non-Healing Wounds. Int. J. Mol. Sci. 2020, 21, 5235. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.J.; Rosenthal, N. Preparing the Ground for Tissue Regeneration: From Mechanism to Therapy. Nat. Med. 2014, 20, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M.; Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting Tissue Regeneration by Modulating the Immune System. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Chisari, E.; Rehak, L.; Khan, W.S.; Maffulli, N. The Role of the Immune System in Tendon Healing: A Systematic Review. Br. Med. Bull. 2020, 133, 49–54. [Google Scholar] [CrossRef]
- Chisari, E.; Rehak, L.; Khan, W.S.; Maffulli, N. Tendon Healing in Presence of Chronic Low-Level Inflammation: A Systematic Review. Br. Med. Bull. 2019, 132, 97–116. [Google Scholar] [CrossRef]
- Rehak, L.; Giurato, L.; Meloni, M.; Panunzi, A.; Manti, G.M.; Uccioli, L. The Immune-Centric Revolution in the Diabetic Foot: Monocytes and Lymphocytes Role in Wound Healing and Tissue Regeneration—A Narrative Review. J. Clin. Med. 2022, 11, 889. [Google Scholar] [CrossRef]
- Hopper, N.M.; Wardale, J.; Rushton, N. Mononuclear Cells Enhance Cell Migration out of Human Articular Cartilage. J. Tissue Eng. Regen. Med. 2012, 6, 279. [Google Scholar]
- Hopper, N.; Wardale, J.; Brooks, R.; Power, J.; Rushton, N. Peripheral Blood Mononuclear Cells Enhance Cartilage Repair in In Vivo Osteochondral Defect Model. PLoS ONE 2015, 10, e0133937. [Google Scholar] [CrossRef]
- Hopper, N.; Wardale, J.; Howard, D.; Brooks, R.; Rushton, N.; Henson, F. Peripheral Blood Derived Mononuclear Cells Enhance the Migration and Chondrogenic Differentiation of Multipotent Mesenchymal Stromal Cells. Stem Cells Int. 2015, 2015, 323454. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Jiang, D.; Yang, M.; Tao, J.; Hu, X.; Yang, X.; Zeng, Y. Emerging Roles of Macrophage Polarization in Osteoarthritis: Mechanisms and Therapeutic Strategies. Orthop. Surg. 2024, 16, 532–550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fu, W. Peripheral Blood-Derived Stem Cells for the Treatment of Cartilage Injuries: A Systematic Review. Front. Bioeng. Biotechnol. 2022, 10, 956614. [Google Scholar] [CrossRef]
- Abdine, N.M.; Moustafa, K.A.; Bakery, R.H.E.; Sarhan, N.E.; Salah, E.F. Effect of Intra-Articular Injection of Peripheral Blood Mononuclear Cells Versus Platelet-Rich Plasma on Restoration of Collagen Fibers of the Articular Cartilage in a Rat Model of Knee Osteoarthritis. Egypt. J. Histol. 2023, 46, 1861–1869. [Google Scholar] [CrossRef]
- Bohaud, C.; Contreras-Lopez, R.; De La Cruz, J.; Terraza-Aguirre, C.; Wei, M.; Djouad, F.; Jorgensen, C. Pro-Regenerative Dialogue Between Macrophages and Mesenchymal Stem/Stromal Cells in Osteoarthritis. Front. Cell Dev. Biol. 2021, 9, 2367. [Google Scholar] [CrossRef]
- Kanda, K.; Asawa, Y.; Inaki, R.; Fujihara, Y.; Hoshi, K.; Hikita, A. Requirement of Direct Contact between Chondrocytes and Macrophages for the Maturation of Regenerative Cartilage. Sci. Rep. 2021, 11, 22476. [Google Scholar] [CrossRef]
- Li, M.; Yin, H.; Yan, Z.; Li, H.; Wu, J.; Wang, Y.; Wei, F.; Tian, G.; Ning, C.; Li, H.; et al. The Immune Microenvironment in Cartilage Injury and Repair. Acta Biomater. 2022, 140, 23–42. [Google Scholar] [CrossRef]
- Sprugel, K.H.; Mcpherson, J.M.; Clowes, A.W.; Ross, R. Effects of Growth Factors In Vivo I. Cell Ingrowth into Porous Subcutaneous Chambers. Am. J. Pathol. 1987, 129, 601. [Google Scholar] [PubMed]
- Roh, Y.H.; Kim, W.; Park, K.U.; Oh, J.H. Cytokine-Release Kinetics of Platelet-Rich Plasma According to Various Activation Protocols. Bone Jt. Res. 2016, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Woodall, J.; Vieira, A. Treatment of Tendon and Muscle Using Platelet-Rich Plasma. Clin. Sports Med. 2009, 28, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Tummala, P.; King, A.; Lee, B.; Kraus, M.; Tse, V.; Jacobs, C.R. Buffered Platelet-Rich Plasma Enhances Mesenchymal Stem Cell Proliferation and Chondrogenic Differentiation. Tissue Eng. Part C Methods 2009, 15, 431–435. [Google Scholar] [CrossRef]
- Han, B.; Woodell-May, J.; Ponticiello, M.; Yang, Z.; Nimni, M. The Effect of Thrombin Activation of Platelet-Rich Plasma on Demineralized Bone Matrix Osteoinductivity. J. Bone Jt. Surg. Am. 2009, 91, 1459–1470. [Google Scholar] [CrossRef]

| n = 212 | |
|---|---|
| Age (mean +/− SD) Range (years) | 56.09 +/− 9.23 19–89 |
| Gender Male n (%) Female n (%) | 42% 58% |
| BMI (kg/m2) Range BMI | 25.3 +/− 4.1 23–28.3 |
| Side | |
| Left/Right Knee (%) | 44%/56% |
| KL Grade | |
| Grade 1 n (%) | 21 out 212 (9.9%) |
| Grade 2 n (%) | 127 out 212 (59.9%) |
| Grade 3 n (%) | 64 out 212 (30.2%) |
| Basal Platelets (×103/µL) | 225 +/− 37 |
| VAS score | 6.49 +/− 1.15 |
| WOMAC score | 41.98 +/− 6.8 |
| Blood | PRP | |
|---|---|---|
| Blood volume/PRP volume * | 20 mL | 4.1 +/− 0.3 |
| Platelet conc. (103/µL) | 235 +/− 37 | 960 +/− 108 |
| Platelet dose (billions 109) | 4.7 +/− 0.79 | 3.9 +/− 0.2 |
| WBC (103/µL) | 10.3 +/− 2.3 | 3 +/− 0.22 |
| Granulocyte (103/µL) | 7.5 +/− 0.89 | 0.5 +/− 0.05 |
| Lymphocytes (103/µL) | 2.7 +/− 1.6 | 2.1 +/− 0.8 |
| Monocytes (103/µL) | 0.78 +/− 1.8 | 0.42 +/− 0.49 |
| PB-MNC (103/µL) | 3.56 +/− 1.2 | 2.38 +/− 0.98 |
| RBC (106/µL) | 5.40 +/− 2.9 | 0.053 +/− 0.01 |
| Platelet conc. fold | 4× | |
| Platelet recovery rate % | 82.1 +/− 0.4 | |
| % Mononuclear cells/WBC | 80% |
| Non-Responder | Responder | Patients | Responder Rate | |
|---|---|---|---|---|
| Overall | ||||
| 3 months | 61 | 151 | n = 212 | 71.2% |
| 6 months | 56 | 156 | n = 212 | 73.6% |
| 12 months | 66 | 147 | n = 212 | 69.3% |
| KL Grade 1 | ||||
| 3 months | 6 | 15 | n = 21 | 71.4% |
| 6 months | 5 | 16 | n = 21 | 76.2% |
| 12 months | 6 | 15 | n = 21 | 71.4% |
| KL Grade 2 | ||||
| 3 months | 36 | 91 | n = 127 | 71.7% |
| 6 months | 33 | 94 | n = 127 | 74.0% |
| 12 months | 39 | 88 | n = 127 | 69.3% |
| KL Grade 3 | ||||
| 3 months | 19 | 45 | n = 64 | 70.3% |
| 6 months | 18 | 46 | n = 64 | 71.9% |
| 12 months | 20 | 44 | n = 64 | 68.8% |
| Basal—Pretreatment | 3 m | 6 m | 12 m | |
|---|---|---|---|---|
| VAS pain | ||||
| Overall | 6.49 +/− 1.15 | 4.3 +/− 1.8 * | 2.69 +/− 0.98 * | 3.79 +/− 0.78 * |
| KL1 | 6.02 +/− 1.09 | 4.66 +/− 1.23 * | 2.81 +/− 0.6 * | 3.81 +/− 0.3 * |
| KL2 | 6.41 +/− 0.65 | 4.15 +/− 0.98 * | 2.6 +/− 0.53 * | 3.65 +/− 0.89 * |
| KL3 | 6.58 +/− 1.09 | 4.99 +/− 1.2 * | 2.4 +/− 0.99 * | 3.48 +/− 1.1 * |
| WOMAC | ||||
| Overall | 41.98 +/− 6.8 | 36.55 +/− 13.5 * | 34.9 +/− 11.8 * | 39.3 +/− 8.05 |
| KL1 | 40.65 +/− 11.9 | 36.99 +/− 9.5 * | 35.8 +/− 12.3 * | 38.2 +/− 9.99 |
| KL2 | 41.65 +/− 9.5 | 35.05 +/− 9.99 * | 34.1 +/− 14.0 * | 38.1 +/− 8.67 |
| KL3 | 40.65 +/− 3.9 | 36.18 +/− 4.89 * | 35.8 +/− 9.06 * | 40.13 +/− 12.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Matthaeis, A.; Bianchi, M.; Putzulu, R.; Maccauro, G. High-Dose Neutrophil-Depleted Platelet-Rich Plasma Therapy for Knee Osteoarthritis: A Retrospective Study. J. Clin. Med. 2024, 13, 4816. https://doi.org/10.3390/jcm13164816
De Matthaeis A, Bianchi M, Putzulu R, Maccauro G. High-Dose Neutrophil-Depleted Platelet-Rich Plasma Therapy for Knee Osteoarthritis: A Retrospective Study. Journal of Clinical Medicine. 2024; 13(16):4816. https://doi.org/10.3390/jcm13164816
Chicago/Turabian StyleDe Matthaeis, Andrea, Maria Bianchi, Rossana Putzulu, and Giulio Maccauro. 2024. "High-Dose Neutrophil-Depleted Platelet-Rich Plasma Therapy for Knee Osteoarthritis: A Retrospective Study" Journal of Clinical Medicine 13, no. 16: 4816. https://doi.org/10.3390/jcm13164816
APA StyleDe Matthaeis, A., Bianchi, M., Putzulu, R., & Maccauro, G. (2024). High-Dose Neutrophil-Depleted Platelet-Rich Plasma Therapy for Knee Osteoarthritis: A Retrospective Study. Journal of Clinical Medicine, 13(16), 4816. https://doi.org/10.3390/jcm13164816






