Bacteria, Fungi, and Scalp Psoriasis: Understanding the Role of the Microbiome in Disease Severity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects and Sample Collection
2.2. Definition of PASI and Scalp PASI
2.3. DNA Extraction
2.4. Fungal Identification by Internal Transcribed Spacer (ITS) Sequencing Analysis
2.5. Bacterial Identification
2.6. Prediction of Functional Profiling
2.7. Statistical Analysis
3. Results
3.1. Demographics of the Patients with Scalp Psoriasis
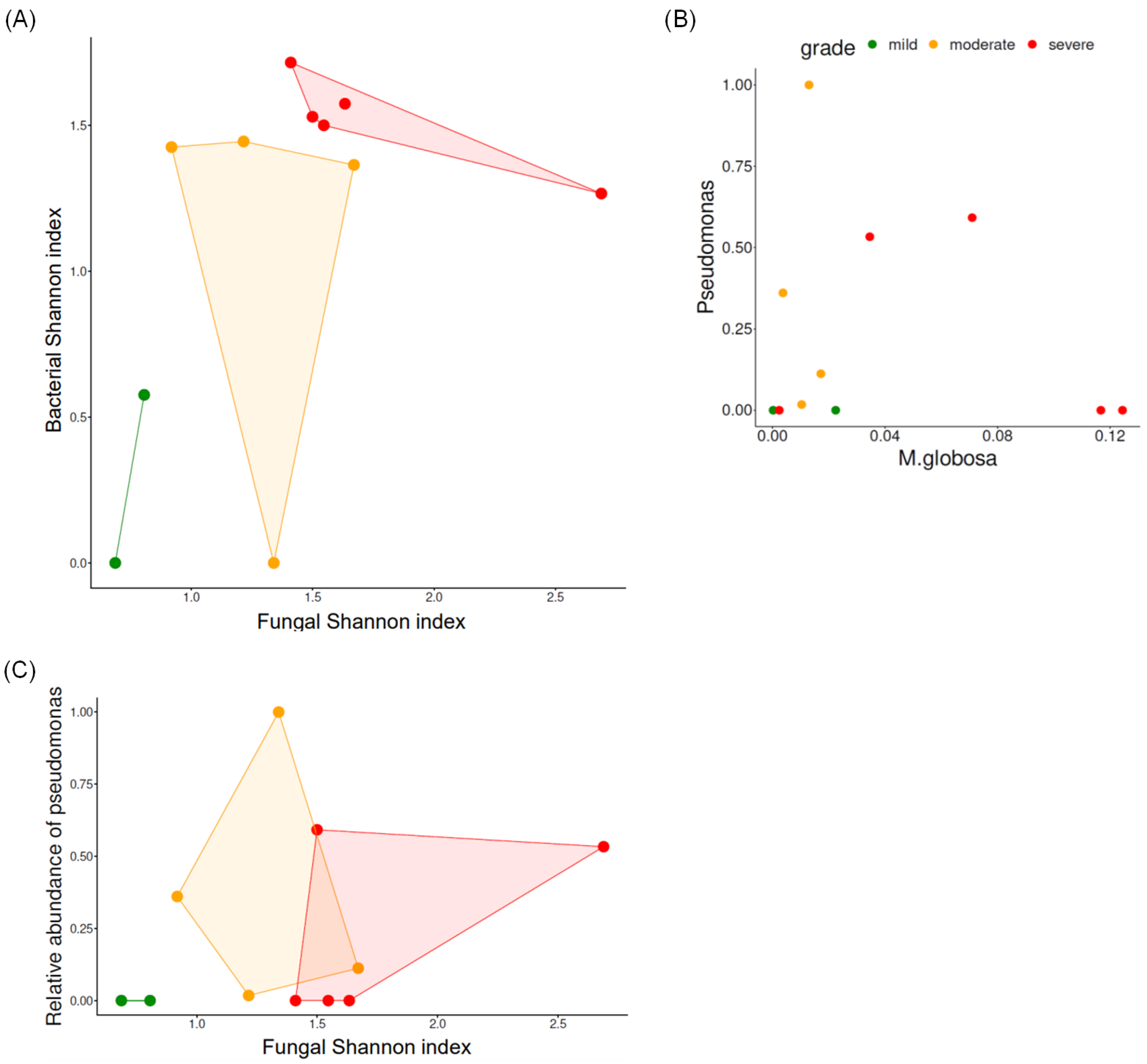
3.2. Biodiversity According to the Severity of Scalp Psoriasis
3.2.1. Fungal Biodiversity According to Scalp Psoriasis Severity
3.2.2. Bacterial Biodiversity According to Scalp Psoriasis Severity
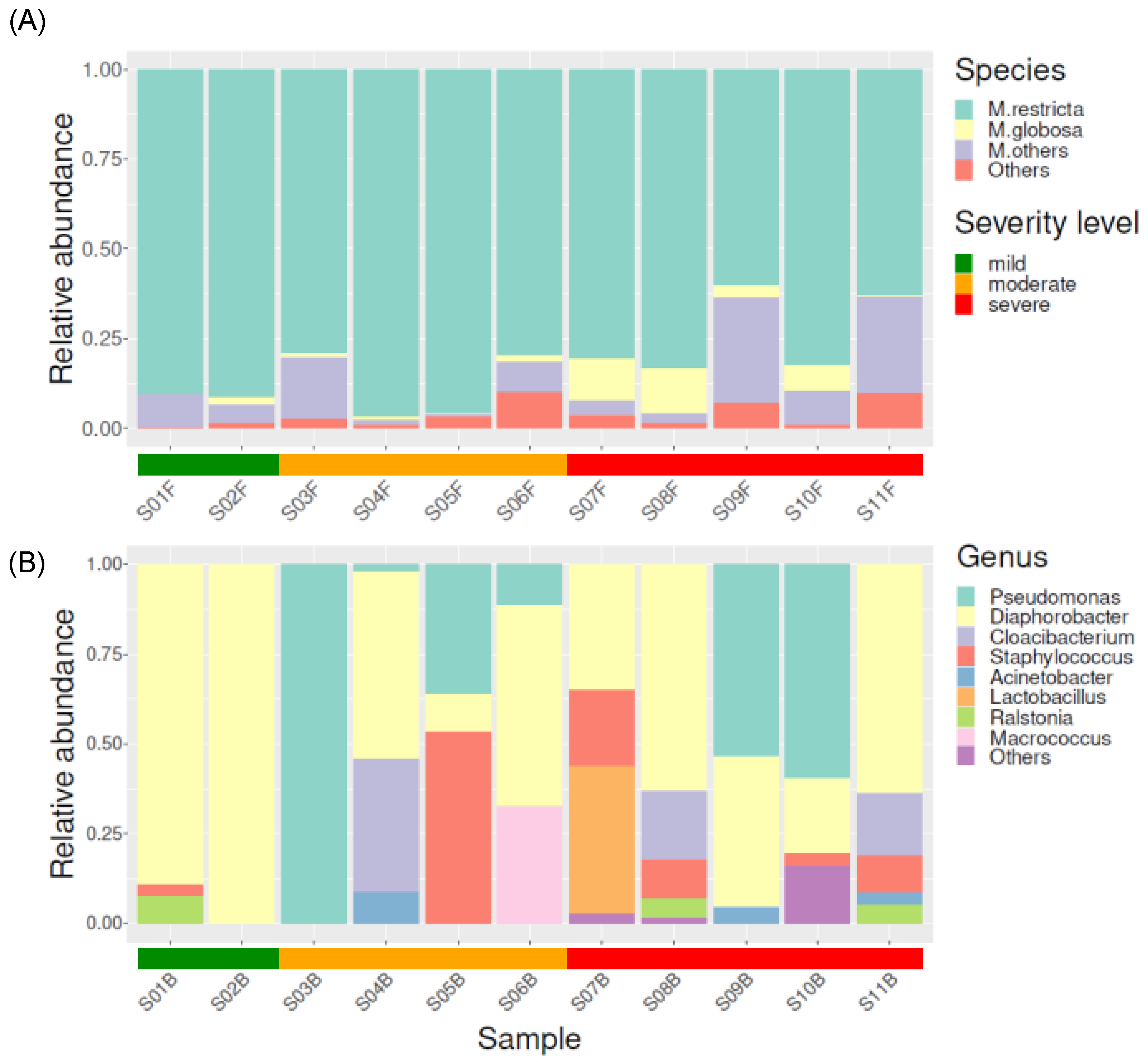
3.3. Taxonomical Compositions of Each Severity Group
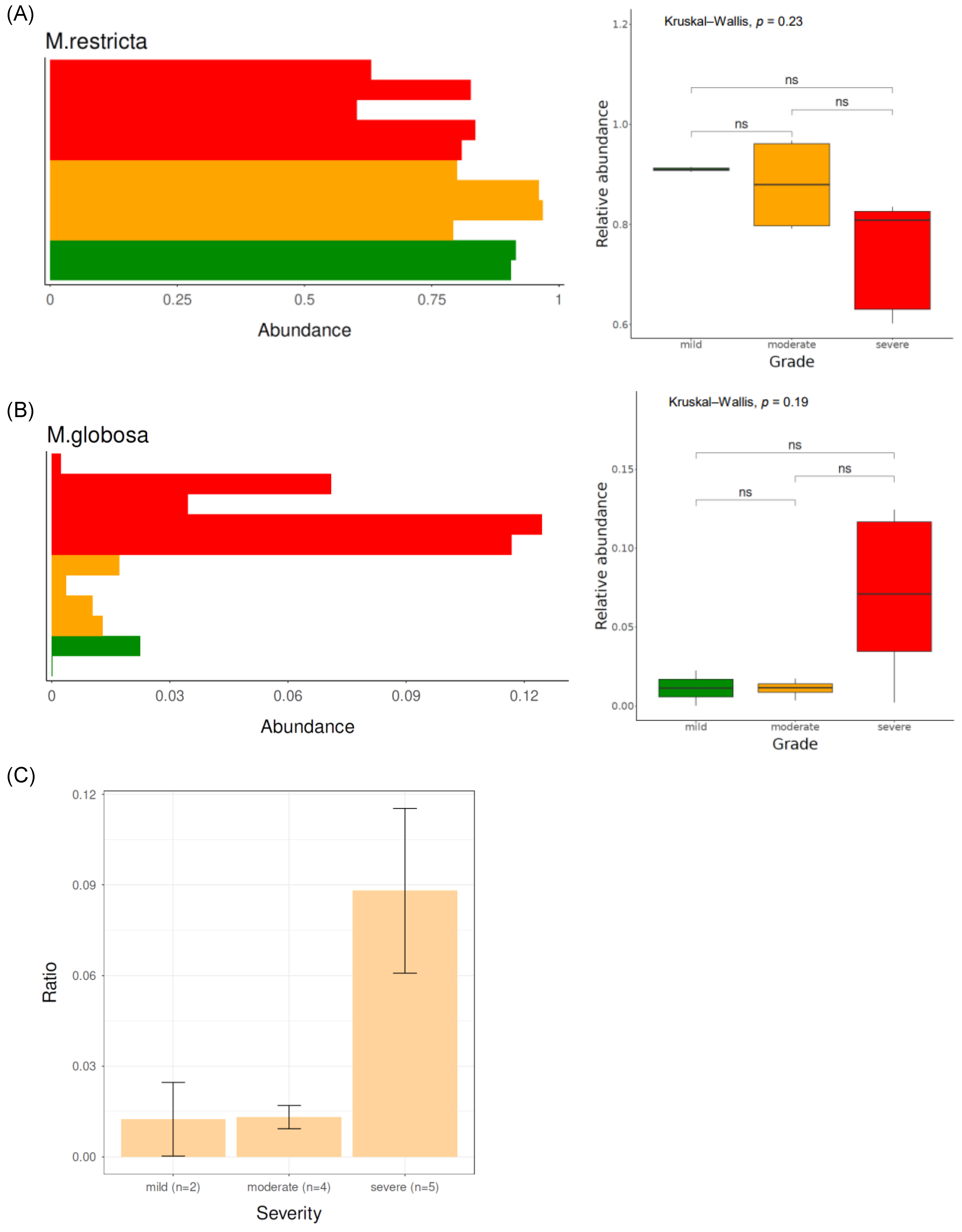
3.3.1. Fungal Taxonomical Compositions of Each Severity Group
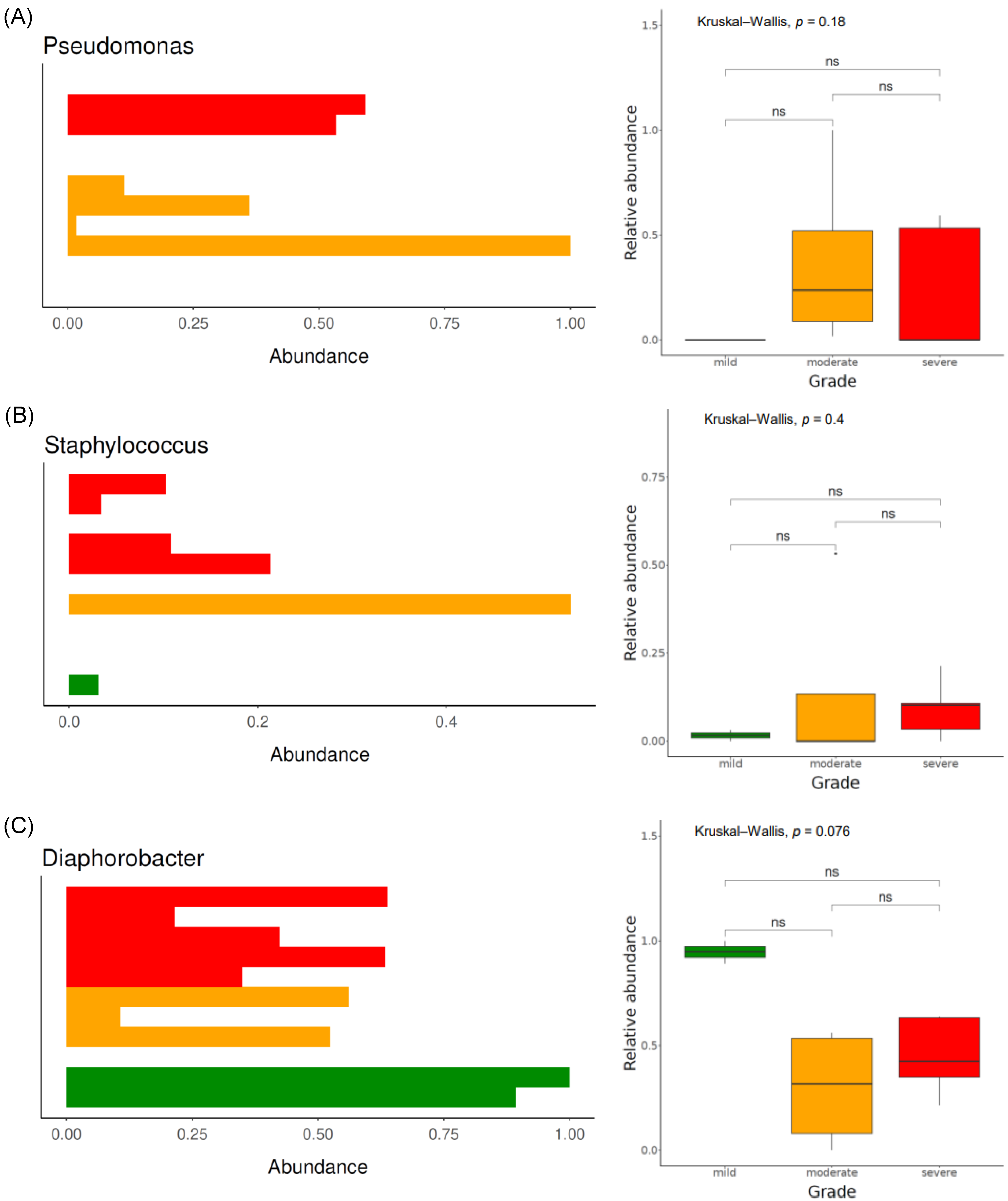
3.3.2. Bacterial Taxonomical Compositions of Each Severity Group
3.3.3. Functional Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stern, R.S.; Nijsten, T.; Feldman, S.R.; Margolis, D.J.; Rolstad, T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J. Investig. Dermatol. Symp. Proc. 2004, 9, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Vanderpuye-Orgle, J.; Zhao, Y.; Lu, J.; Shrestha, A.; Sexton, A.; Seabury, S.; Lebwohl, M. Evaluating the economic burden of psoriasis in the United States. J. Am. Acad. Dermatol. 2015, 72, 961–967.e5. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Debbaneh, M.G.; Liao, W. Genetic Epidemiology of Psoriasis. Curr. Dermatol. Rep. 2014, 3, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Moltrasio, C.; Romagnuolo, M.; Marzano, A.V. Epigenetic Mechanisms of Epidermal Differentiation. Int. J. Mol. Sci. 2022, 23, 4874. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.W.; Noah, P.W.; Skinner, R.B., Jr. Microorganisms and psoriasis. J. Natl. Med. Assoc. 1994, 86, 305. [Google Scholar] [PubMed]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Roth, R.R.; James, W.D. Microbiology of the skin: Resident flora, ecology, infection. J. Am. Acad. Dermatol. 1989, 20, 367–390. [Google Scholar] [CrossRef] [PubMed]
- Fahlen, A.; Engstrand, L.; Baker, B.S.; Powles, A.; Fry, L. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch. Dermatol. Res. 2012, 304, 15–22. [Google Scholar] [CrossRef]
- Wang, H.; Chan, M.W.M.; Chan, H.H.; Pang, H. Longitudinal Changes in Skin Microbiome Associated with Change in Skin Status in Patients with Psoriasis. Acta Derm. Venereol. 2020, 100, adv00329. [Google Scholar] [CrossRef]
- Chang, H.W.; Yan, D.; Singh, R.; Liu, J.; Lu, X.; Ucmak, D.; Lee, K.; Afifi, L.; Fadrosh, D.; Leech, J.; et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome 2018, 6, 154. [Google Scholar] [CrossRef]
- Baroni, A.; Paoletti, I.; Ruocco, E.; Agozzino, M.; Tufano, M.A.; Donnarumma, G. Possible role of Malassezia furfur in psoriasis: Modulation of TGF-β1, integrin, and HSP70 expression in human keratinocytes and in the skin of psoriasis-affected patients. J. Cutan. Pathol. 2004, 31, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J.; Chan, W.H.; Hinojosa, T.; Hsu, S.; Feldman, S.R. Mechanisms of microbial pathogenesis and the role of the skin microbiome in psoriasis: A review. Clin. Dermatol. 2019, 37, 160–166. [Google Scholar] [CrossRef]
- Lober, C.W.; Belew, P.W.; Rosenberg, E.W.; Bale, G. Patch tests with killed sonicated microflora in patients with psoriasis. Arch. Dermatol. 1982, 118, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Farr, P.; Marks, J.; Krause, L.; Shuster, S. Response of scalp psoriasis to oral ketoconazole. Lancet 1985, 326, 921–922. [Google Scholar] [CrossRef]
- Shuttleworth, D.; Galloway, D.; Boorman, G.; Donald, A. A double-blind, placebo-controlled study of the clinical efficacy of ciclopirox olamine (1.5%) shampoo for the control of scalp psoriasis. J. Dermatol. Treat. 1998, 9, 163–167. [Google Scholar] [CrossRef]
- Hjuler, K.F.; Iversen, L.; Rasmussen, M.K.; Kofoed, K.; Skov, L.; Zachariae, C. Localization of treatment-resistant areas in patients with psoriasis on biologics. Br. J. Dermatol. 2019, 181, 332–337. [Google Scholar] [CrossRef]
- Callewaert, C.; Nakatsuji, T.; Knight, R.; Kosciolek, T.; Vrbanac, A.; Kotol, P.; Ardeleanu, M.; Hultsch, T.; Guttman-Yassky, E.; Bissonnette, R.; et al. IL-4Ralpha Blockade by Dupilumab Decreases Staphylococcus aureus Colonization and Increases Microbial Diversity in Atopic Dermatitis. J. Investig. Dermatol. 2020, 140, 191–202.e7. [Google Scholar] [CrossRef]
- Koike, Y.; Kuwatsuka, S.; Nishimoto, K.; Motooka, D.; Murota, H. Skin Mycobiome of Psoriasis Patients is Retained during Treatment with TNF and IL-17 Inhibitors. Int. J. Mol. Sci. 2020, 21, 3892. [Google Scholar] [CrossRef]
- Ortonne, J.; Chimenti, S.; Luger, T.; Puig, L.; Reid, F.; Trüeb, R. Scalp psoriasis: European consensus on grading and treatment algorithm. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1435–1444. [Google Scholar] [CrossRef]
- Papp, K.; Berth-Jones, J.; Kragballe, K.; Wozel, G.; De La Brassinne, M. Scalp psoriasis: A review of current topical treatment options. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1151–1160. [Google Scholar] [CrossRef]
- Fredriksson, T.; Pettersson, U. Severe psoriasis–oral therapy with a new retinoid. Dermatology 1978, 157, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, A.; Esposito, M.; Carboni, I.; Schipani, C.; Chimenti, S. Clobetasol propionate foam 0.05% as a novel topical formulation for plaque-type and scalp psoriasis. J. Dermatol. Treat. 2007, 18, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Kim, H.; Koo, H.-Y.-R.; You, J.; Yu, D.-S.; Lee, Y.-B.; Lee, M. Severe Scalp Psoriasis Microbiome Has Increased Biodiversity and Relative Abundance of Pseudomonas Compared to Mild Scalp Psoriasis. J. Clin. Med. 2022, 11, 7133. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.K.; Di Rienzi, S.C.; Poole, A.C.; Koren, O.; Walters, W.A.; Caporaso, J.G.; Knight, R.; Ley, R.E. Conducting a microbiome study. Cell 2014, 158, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Barb, J.J.; Oler, A.J.; Kim, H.-S.; Chalmers, N.; Wallen, G.R.; Cashion, A.; Munson, P.J.; Ames, N.J. Development of an Analysis Pipeline Characterizing Multiple Hypervariable Regions of 16S rRNA Using Mock Samples. PLoS ONE 2016, 11, e0148047. [Google Scholar] [CrossRef]
- Benhadou, F.; Mintoff, D.; Schnebert, B.; Thio, H.B. Psoriasis and Microbiota: A Systematic Review. Diseases 2018, 6, 47. [Google Scholar] [CrossRef]
- Takemoto, A.; Cho, O.; Morohoshi, Y.; Sugita, T.; Muto, M. Molecular characterization of the skin fungal microbiome in patients with psoriasis. J. Dermatol. 2015, 42, 166–170. [Google Scholar] [CrossRef]
- Gupta, A.K.; Batra, R.; Bluhm, R.; Boekhout, T.; Dawson, T.L., Jr. Skin diseases associated with Malassezia species. J. Am. Acad. Dermatol. 2004, 51, 785–798. [Google Scholar] [CrossRef]
- Prohic, A.; Jovovic Sadikovic, T.; Krupalija-Fazlic, M.; Kuskunovic-Vlahovljak, S. Malassezia species in healthy skin and in dermatological conditions. Int. J. Dermatol. 2016, 55, 494–504. [Google Scholar] [CrossRef]
- Takahata, Y.; Sugita, T.; Hiruma, M.; Muto, M. Quantitative analysis of Malassezia in the scale of patients with psoriasis using a real-time polymerase chain reaction assay. Br. J. Dermatol. 2007, 157, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Amaya, M.; Tajima, M.; Okubo, Y.; Sugita, T.; Nishikawa, A.; Tsuboi, R. Molecular analysis of Malassezia microflora in the lesional skin of psoriasis patients. J. Dermatol. 2007, 34, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Moyano, E.; Crespo-Erchiga, V.; Martinez-Pilar, L.; Godoy Diaz, D.; Martinez-Garcia, S.; Lova Navarro, M.; Vera Casano, A. Do Malassezia species play a role in exacerbation of scalp psoriasis? J. Mycol. Med. 2014, 24, 87–92. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Honnavar, P.; Chakrabarti, A.; Dogra, S.; Singh, P.; Handa, S. Association of Malassezia species with psoriatic lesions. Mycoses 2014, 57, 483–488. [Google Scholar] [CrossRef]
- Juntachai, W.; Oura, T.; Murayama, S.Y.; Kajiwara, S. The lipolytic enzymes activities of Malassezia species. Med. Mycol. 2009, 47, 477–484. [Google Scholar] [CrossRef]
- DeAngelis, Y.M.; Saunders, C.W.; Johnstone, K.R.; Reeder, N.L.; Coleman, C.G.; Kaczvinsky, J.R., Jr.; Gale, C.; Walter, R.; Mekel, M.; Lacey, M.P.; et al. Isolation and expression of a Malassezia globosa lipase gene, LIP1. J. Investig. Dermatol. 2007, 127, 2138–2146. [Google Scholar] [CrossRef]
- Dawson, T.L., Jr. Malassezia globosa and restricta: Breakthrough understanding of the etiology and treatment of dandruff and seborrheic dermatitis through whole-genome analysis. J. Investig. Dermatol. Symp. Proc. 2007, 12, 15–19. [Google Scholar] [CrossRef]
- Ro, B.I.; Dawson, T.L. The role of sebaceous gland activity and scalp microfloral metabolism in the etiology of seborrheic dermatitis and dandruff. J. Investig. Dermatol. Symp. Proc. 2005, 10, 194–197. [Google Scholar] [CrossRef]
- Donato-Trancoso, A.; Correa Atella, G.; Romana-Souza, B. Dietary olive oil intake aggravates psoriatic skin inflammation in mice via Nrf2 activation and polyunsaturated fatty acid imbalance. Int. Immunopharmacol. 2022, 108, 108851. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Fleming, C.; Yan, J. New insights of T cells in the pathogenesis of psoriasis. Cell Mol. Immunol. 2012, 9, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, J.E.; Chan, T.C.; Krueger, J.G. Psoriasis pathogenesis and the development of novel targeted immune therapies. J. Allergy Clin. Immunol. 2017, 140, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Kashem, S.W.; Kaplan, D.H. Skin Immunity to Candida albicans. Trends Immunol. 2016, 37, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Setoyama, A.; Sakuragi, Y.; Saito-Sasaki, N.; Yoshioka, H.; Nakamura, M. The Role of IL-17-Producing Cells in Cutaneous Fungal Infections. Int. J. Mol. Sci. 2021, 22, 5794. [Google Scholar] [CrossRef] [PubMed]
- Lanternier, F.; Pathan, S.; Vincent, Q.B.; Liu, L.; Cypowyj, S.; Prando, C.; Migaud, M.; Taibi, L.; Ammar-Khodja, A.; Stambouli, O.B.; et al. Deep dermatophytosis and inherited CARD9 deficiency. N. Engl. J. Med. 2013, 369, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Sparber, F.; De Gregorio, C.; Steckholzer, S.; Ferreira, F.M.; Dolowschiak, T.; Ruchti, F.; Kirchner, F.R.; Mertens, S.; Prinz, I.; Joller, N.; et al. The Skin Commensal Yeast Malassezia Triggers a Type 17 Response that Coordinates Anti-fungal Immunity and Exacerbates Skin Inflammation. Cell Host Microbe 2019, 25, 389–403.e6. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Fujimura, T.; Tanita, K.; Chunbing, L.; Matsushita, S.; Fujisawa, Y.; Otsuka, A.; Yamamoto, Y.; Hidaka, T.; Aiba, S. Malassezia-derived aryl hydrocarbon receptor ligands enhance the CCL20/Th17/soluble CD163 pathogenic axis in extra-mammary Paget’s disease. Exp. Dermatol. 2019, 28, 933–939. [Google Scholar] [CrossRef]
- Sanad, E.M.K.; Nazmy, N.N.; Abd-El Hamid El Sayed, R.; Hamed, A.M. Interleukin-17A gene single nucleotide polymorphism and its relation to fungal growth in psoriatic patients: A preliminary study. J. Cosmet. Dermatol. 2022, 21, 3059–3067. [Google Scholar] [CrossRef]

| Total (n = 11) | Mild Scalp Psoriasis (n = 2) | Moderate Scalp Psoriasis (n = 4) | Severe Scalp Psoriasis (n = 5) | |
|---|---|---|---|---|
| Age, years (mean ± SD *) | 41.36 ± 19.77 | 39.25 ± 23.44 | 41.6 ± 20.82 | 42.13 ± 19.61 |
| Gender (Male/Female) | 9:2 | 2:0 | 4:0 | 3:2 |
| Scalp PASI (mean ± SD *) | 1.87 ± 0.93 | 0.55 ± 0.35 | 1.4 ± 0.28 | 2.62 ± 0.54 |
| PASI (mean ± SD *) | 7.53 ± 6.29 | 1.95 ± 0.07 | 8.13 ± 8.35 | 9 ± 5.47 |
| Current treatment | ||||
| Biologics | 7 | 2 | 3 | 2 |
| Cyclosporine | 3 | 0 | 1 | 2 |
| Topical steroid/calcipotriol | 1 | 0 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-Y.; Kim, H.; Min, K.-H.; Song, W.-H.; Yu, D.-S.; Lee, M.; Lee, Y.-B. Bacteria, Fungi, and Scalp Psoriasis: Understanding the Role of the Microbiome in Disease Severity. J. Clin. Med. 2024, 13, 4846. https://doi.org/10.3390/jcm13164846
Choi J-Y, Kim H, Min K-H, Song W-H, Yu D-S, Lee M, Lee Y-B. Bacteria, Fungi, and Scalp Psoriasis: Understanding the Role of the Microbiome in Disease Severity. Journal of Clinical Medicine. 2024; 13(16):4846. https://doi.org/10.3390/jcm13164846
Chicago/Turabian StyleChoi, Jin-Young, Hyunseong Kim, Kyung-Hyun Min, Woo-Hyun Song, Dong-Soo Yu, Minho Lee, and Young-Bok Lee. 2024. "Bacteria, Fungi, and Scalp Psoriasis: Understanding the Role of the Microbiome in Disease Severity" Journal of Clinical Medicine 13, no. 16: 4846. https://doi.org/10.3390/jcm13164846





