Abstract
The prevalence of nonalcoholic fatty liver disease (NAFLD) is likely to be approaching 38% of the world’s population. It is predicted to become worse and is the main cause of morbidity and mortality due to hepatic pathologies. It is particularly worrying that NAFLD is increasingly diagnosed in children and is closely related, among other conditions, to insulin resistance and metabolic syndrome. Against this background is the concern that the awareness of patients with NAFLD is low; in one study, almost 96% of adult patients with NAFLD in the USA were not aware of their disease. Thus, studies on the therapeutic tools used to treat NAFLD are extremely important. One promising treatment is a well-formulated ketogenic diet (KD). The aim of this paper is to present a review of the available publications and the current state of knowledge of the effect of the KD on NAFLD. This paper includes characteristics of the key factors (from the point of view of NAFLD regression), on which ketogenic diet exerts its effects, i.e., reduction in insulin resistance and body weight, elimination of fructose and monosaccharides, limitation of the total carbohydrate intake, anti-inflammatory ketosis state, or modulation of gut microbiome and metabolome. In the context of the evidence for the effectiveness of the KD in the regression of NAFLD, this paper also suggests the important role of taking responsibility for one’s own health through increasing self-monitoring and self-education.
1. Introduction
A dramatic increase in the incidence of nonalcoholic fatty liver disease (NAFLD) presents a problem for public health worldwide. NAFLD is a worsening pandemic, which today is not only the most frequent liver disease but also the main cause of morbidity and mortality due to hepatic pathologies. It is estimated that it may affect from 32.4% to 38% of the world’s population, with a significantly higher incidence observed in men [1,2]. Once regarded as a domain of alcohol-addicted individuals, fatty liver degeneration is today also a scourge for patients with metabolic diseases [3,4,5,6]. The relationship between NAFLD and metabolic disorders is so significant that an international expert panel (from 135 countries worldwide) has recently suggested an update of the terminology and a change in the nomenclature of that disease to metabolic (dysfunction)-associated fatty liver disease (MAFLD) [7]. It is particularly worrying that NAFLD is increasingly diagnosed even in children and is closely related, among other conditions, to insulin resistance and metabolic syndrome [8]. The incidence of NAFLD and its intensity are significantly higher in individuals with metabolic risk factors and they increase in parallel with increasing grade of obesity and type 2 diabetes mellitus (75% of patients with type 2 diabetes also have NAFLD). It is expected that by 2030 the incidence of NAFLD-induced liver cirrhosis will double (in relation to 2016) [2,9]. Another paper suggests that the incidence of NAFLD alone may triple by 2030 [10]. This constitutes a real challenge for contemporary medicine and for the authorities designing healthcare systems worldwide.
NAFLD is a metabolic disease in which an excessive accumulation of fatty tissue occurs in the liver (the presence of steatosis in >5% hepatocytes), which is associated with insulin resistance. The term NAFLD was introduced in 1986 [11] and the disease includes two main stages, i.e., nonalcoholic fatty liver (NAFL) (in which the inflammatory condition and liver injury are slight) and nonalcoholic steatohepatitis (NASH) (in which a significant inflammatory condition and liver injury are present) [12]. Intracellular accumulation of fatty acids (from hepatic de novo lipogenesis, visceral adipose tissue lipolysis, excessively high energy content in the diet, and other factors) in the course of MAFLD leads to lipotoxicity (which can lead to cell damage, cell death, and damage to cellular organelles). This in turn leads to a chronic inflammatory state (among others, as a result of the influence of toxic lipids on cell damage by modifying the functions of mitochondria or the endoplasmic reticulum, directly influencing the modification of intracellular signaling pathways, or interacting with specific proinflammatory kinases in the cell). Fatty acid accumulation can also more directly enhance the production of reactive oxygen species (oxidative stress). An additional pathway involves mitochondrial dysfunction mediated by β-oxidation and peroxisomes, which activates a hepatic inflammatory response. In this way, and through a number of other mechanisms (e.g., through disruptions in the functioning of the gut microbiome), MAFLD progresses to MASH [13,14], although the exact mechanisms of progression are most likely not yet fully elucidated. Due to the complexity of the pathogenesis and of the clinical course of the disease, MAFLD is characterized by non homogeneity and diversity of clinical phenotypes. Biopsy is the gold standard of MAFLD diagnosis, although the disease is commonly detected, among other methods, by ultrasonography (USG) or computed tomography (CT). Importantly, despite the fact that the disease is so widespread, most patients remain undiagnosed and no MAFLD treatment methods have been approved [15,16,17].
MAFLD is an increasing problem, not only in healthcare, but also in terms of its economic aspects. The costs associated with MAFLD differ depending on the methodology, database, country, and specific phenotype of the disease, so a full estimation of all related costs (including indirect ones) is not possible. However, all sources agree that these costs are huge, compared to individuals without the disease [18]. It is worth noting that some cases of MAFLD progress to MASH, which is another stage of hepatic steatosis that carries a significant risk of cirrhosis. According to Younossi et al., the predicted cost of healthcare in patients with nonalcoholic steatohepatitis (NASH) (currently metabolic dysfunction-associated steatohepatitis (MASH) [19] in the USA alone in the years 2020–2039 (as the total cumulative cost) is as high as USD 1662.36 billion (USD 1208.47 billion in obese patients with MASH and USD 453.88 billion in nonobese individuals with MASH); this corresponds to an increase in the cost per patient from USD 3573 in 2020 to USD 6601 in 2039 [20]. Importantly, MASH is the most rapidly increasing indication for liver transplantation in the United States; therefore, the huge costs associated with the procedure should be taken into account [21]. The estimated cost of a single transplantation can even exceed USD 1 million when peritransplant costs are taken into consideration [22,23]. For that reason, this article should primarily serve as an appeal to the authorities requesting that they take these data into account and update their knowledge of the possibilities of regression of many chronic diseases, exemplified by fatty liver degeneration, as the key method of improving the overburdened and underfinanced healthcare systems worldwide. Concentrating on prophylaxis, education, and innovative therapeutic methods, including the use of artificial intelligence and modern applications, may play a significant role in reducing those enormous costs and, more importantly, in improving patients’ quality of life.
Alarming data suggest that the awareness of patients with MAFLD is low. The results of the study by Alqahtani et al. have demonstrated that almost 96% of adult patients with MAFLD in the USA were not aware of their disease [24]. It turned out that even among patients in whom MAFLD was diagnosed during a check-up, a majority of them (55.3%) were not provided with any education or guidelines concerning the treatment, and only 40.2% of patients diagnosed with MAFLD visited clinical departments [25].
A ketogenic diet (KD) may be a new therapeutic option in the treatment of MAFLD. It has already been used in the treatment of epilepsy (as an official indication) for over 100 years, frequently being the last resort when pharmacotherapy has failed (drug-resistant epilepsy). The ketogenic diet has been used for the treatment of intractable epilepsy since 1921 [26]. The mechanism by which it controls seizures is likely rooted in its ability to induce nutritional ketosis, thereby safely mimicking the fasted metabolic state while avoiding the risks of starvation and malnutrition. This intervention has been demonstrated to effectively control seizures in those with drug-resistant epilepsy. While on a KD, the insulin levels are low enough to allow an increased production of ketone bodies (i.e., β-hydroxybutyrate, acetoacetate, and acetone) to take place, leading to a state of ketosis in which the body utilizes fatty acids instead of glucose as the main source of energy [27]. The ketogenic diet is a high-fat, moderate-protein, and low-carbohydrate diet. The total daily distribution of macronutrients is typically 5–10% carbohydrates (and no more than 50 g), 60 to 80–90% fat (depending on the individuals therapeutic need), and 10–30% protein. These ranges allow a degree of flexibility for individual cases, and when used for different purposes. For example, treatment of intractable epilepsy usually requires a much higher proportion of fat while keeping carbohydrate consumption extremely low. The maximum amount of protein that can safely be consumed without compromising the efficacy of the diet remains somewhat unclear. Whilst these percentages provide a rough guide to formulate individual meal plans, it is worth emphasizing that the percentage of each macronutrient strictly depends on the total energy content of the diet per day, which varies slightly for each person. In contrast, standard dietary approaches typically suggest obtaining up to 35% of energy from fat (including up to 10% from saturated fats), 45–75% from carbohydrates (up to 10% from sugars), and 10–35% from protein. In addition to the percentage of macronutrients, important (and often neglected) is the quality of formulation of the diet. For example, diet soda and pepperoni sausage would constitute a ketogenic diet, but no one could claim this would be healthy. Depending on the purpose of a KD, the proportion of macrocomponents can be modified by increasing the percentage of protein in the diet while decreasing the percentage of fat (e.g., the modified Atkins diet). In view of the nature of the ketogenic diet, considering it as a therapeutic option for MAFLD may be justified, as evidenced, among others, by the mechanisms underlying KD and findings from other research [28,29,30,31,32,33,34,35]. Indeed, the ketogenic diet has the potential to have a beneficial effect on NAFLD, if only through the fact of reducing inflammation (which is a significant advantage), reducing body weight (and it is often used for this purpose), and improving glycemia and insulin sensitivity, as supported by its low-carbohydrate (excluding sources of simple sugars and marginalizing fructose) nature. These, and other mechanisms, will be considered in this manuscript.
This article is an analysis of the literature dealing with the effect of ketogenic diet in MAFLD (including a number of mechanisms of action) and it focuses on the potential role of KD in the regression of the disease. We include recent scientific knowledge, at the same time building a bridge between science and real-life situations, pointing to the role a diet change can play in the struggle against disease, not only in health-related but also economic aspects. The aim of this article is not only to present the current state of knowledge of the effect of KD on MAFLD, but also to highlight the importance of making informed health decisions and to indicate the role of self-care and self-education in the prevention and treatment of MAFLD.
2. Potential Therapeutic Mechanisms of Ketogenic Diet in MAFLD
2.1. Reduction in Insulin Resistance
It is known that insulin resistance is one of the main factors underlying MAFLD development, which has been confirmed in numerous publications [36,37,38]. Insulin resistance occurs as a result of prolonged, elevated blood glucose levels and fatty liver itself. Insulin resistance impairs glucose disposal and leads to hyperinsulinemia in a vicious cycle. Then, fatty liver degeneration starts to occur through increased de novo synthesis of fatty acids in the liver, inflow of fatty acids from the adipose tissue (absence of lipolysis inhibition), and increased production of proinflammatory adipokines and cytokines in the adipose tissue. All this is actually stimulated by hyperinsulinemia and leads to the progressive deposition of triglycerides (TG) in the liver parenchyma and MAFLD development [38,39,40,41,42].
One of the benefits of the ketogenic diet is the reduction in insulin and glucose concentrations in the blood, thus improving sensitivity of cells to insulin action [43]. A number of publications demonstrate that KD decreases concentrations of insulin, glucose, and glycated hemoglobin (HbA1c) through numerous mechanisms of action [44,45,46]. The first of them is body weight reduction (owing to the nature of the ketogenic diet, achieving a calorie deficit is extremely easy and intuitive, with no need to count calories). This, in turn, is associated with an improvement in insulin sensitivity (including as a result of the mechanism of increasing the level of adiponectin (the concentration of which is lower in obese people and increases during the reduction in fat tissue), which has anti-inflammatory effects and improves insulin sensitivity), reduction in glycaemia and insulin concentration values, and, consequently, with an improvement in MAFLD [44,47,48]. It is one of the key mechanisms, although not the only one, since low-carb diets have been found to improve the sensitivity to insulin, even in the absence of major weight loss [49,50,51,52,53]. The second mechanism of action is associated with the absence of monosaccharides (and significant reduction in the total amount of carbohydrates to <50 g daily) in the ketogenic diet, which directly prevents excessive fluctuations of glucose levels and insulin spikes [34,54,55,56]. The stabilization of glycaemia and insulin concentration contributes to an improvement in the MAFLD condition. The third mechanism consists of the fact that a ketogenic diet almost entirely eliminates fructose (present, among others, in fruit juices, high-fructose syrups, or fruits), which can disturb insulin signaling in the liver (including as a result of mechanisms such as promoting de novo lipogenesis (DNL), inducing endoplasmic reticulum (ER) stress, impairing fatty acid oxidation (FAO), and inducing hepatitis). An elimination of that component would, thus, exert a favorable effect on insulin sensitivity [57]. Detailed information regarding fructose is described in Section 2.3. Another possible mechanism includes the effect of ketone bodies themselves, and of the state of ketosis, on insulin sensitivity and MAFLD [29]. It is also known that an increase in their concentration, as an energy source alternative to glucose, is associated with a reduced utilization of glucose (and, consequently, a lower amount of insulin needed). The state of ketosis can also improve insulin sensitivity due to a reduction in oxidative stress, inflammatory condition, and improvement in mitochondrial efficiency [31,58,59].
2.2. Body Weight Reduction
Although also found in individuals with normal body weight, nonalcoholic fatty liver disease develops more frequently in patients with excessive body weight. A recent meta-analysis revealed that the prevalence of MAFLD worldwide in patients with overweight accounts for 69.99% (on average, NAFL (currently MAFL—metabolic associated fatty liver) 42.49% and NASH (MASH) 33.50%), while in obese patients that percentage is 75.27% (on average, NAFL (MAFL) 43.05% and MASH 33.67%) [60]. Other data suggest that in cases of extreme obesity, even 90% of patients can simultaneously have MAFLD [61]. Reduction in body weight by only >5% results in a detectable alleviation of fatty liver degeneration, while ≥10% provides the best effects including, among others, regression of fibrosis and disappearance of MASH [62,63].
A ketogenic diet works well for reduction in body weight [64], and it may be the most effective therapeutic option for many patients. Compared to standard diets, a KD provides a stronger feeling of satiety [65]; therefore, weight loss can be achieved without significant hunger, mood changes, and irritation (which occur when on standard weight-reducing diets) [66,67]. Importantly, the aforementioned mechanisms, together with sensitization to insulin (described in Section 2.1), create a calorie deficit in a natural way that can be achieved easily, without focusing on counting calories. In their systematic review based on data analysis, Gibson et al. demonstrated that the state of ketosis significantly suppresses appetite, while ketogenic diet prevents appetite increase, even in spite of body weight loss (negative calorie balance) [68]. This happens even with a great body weight loss, e.g., reduction by 17% in relation to the initial value, and the sensation of hunger significantly increases only after regression of the state of ketosis [69]. Roekenes and Martins revealed in their publication that all available evidence suggests that KD inhibits the secretion of ghrelin (a “hunger hormone” released in response to body weight loss on standard diets) in spite of body weight loss [70]. It was demonstrated in one study that a 10-week-long ketogenic diet applied in patients with type 2 diabetes mellitus not only led to a significant improvement in glycaemia values and dose reduction or complete withdrawal of antidiabetic drugs in 56.8% of patients, but also to body weight loss (by 7.2% on average). Importantly, despite a negative calorie balance, in the 10th week of the study the patients reported a less intense sensation of hunger than at its beginning [71]. Even if body weight reduction is at a similar level compared to a high-carb diet (a similar calorie deficit), a KD can additionally improve metabolic parameters while being safe to use [47,72,73,74].
2.3. Elimination of Fructose
In the context of MAFLD, fructose seems to be a particularly undesirable sugar. In view of its nature, it should be treated separately from other monosaccharides, and carbohydrates in general. According to Lustig, it can be described as “alcohol without the buzz” since it is metabolized similarly to ethanol and, thus, to some extent, it can also cause similar toxic health effects [75]. Most people realize that alcohol damages the liver. It is worth noting, however, that consumption of excessive amounts of processed products rich in sugar or HFCS (high-fructose corn syrup) can also have a detrimental effect on the liver. This is also true of excessive consumption of fructose from fruit juices, which are not infrequently used as a substitute for water, for hydration, and often given to children by their parents [76,77,78]. It has been postulated that excessive fructose intake may be a major cause of MAFLD development [79]. The association between consumption of fructose-containing food and increased incidence of MAFLD was confirmed by a meta-analysis in 2023 [80]. Fructose (especially in excess) is both an inducer and a substrate of hepatic de novo lipogenesis, a process that contributes to the development of steatosis in the liver (additionally causing insulin resistance and dyslipidemia). Also, it increases ectopic lipid accumulation in the liver, cellular stress, and liver inflammation, just to list some of the negative mechanisms of fructose action [81]. Fructose also has the potential to contribute to leptin (the satiety hormone) resistance, which can ultimately lead to disorders of the satiety center [82,83].
Fructose may be natural (mainly from fruit and fruit juices) and industrial, in the form of HFCS (high-fructose corn syrup) and in the disaccharide sucrose, found in lots of processed foods and sweetened beverages (which are the worst source of fructose). Leaving aside the obvious negative effect of industrial fructose, it turns out that potentially healthy 100% fruit juices may also carry a number of risks. The fructose content of such juices is up to ten times that of whole fruit and can be higher than that of sugar-sweetened beverages (67 g of fructose in 1 liter of 100% apple juice) [79,84,85,86]. Although whole fruits are better than industrial sources of fructose and fruit juices (due to their content of bioactive phytonutrients, such as phytosterols, catechins, proanthocyanidins, or resveratrol), the presence of sugars (mainly fructose) may offset the beneficial properties of these components, tipping the scales against fruit consumption in MAFLD [87]. One randomized controlled study compared the effect of consuming more than four servings of fruit per day vs. fewer than two servings of fruit per day for six months in patients with MAFLD. The group consuming more fruit was characterized, among others, by greater hepatic steatosis, dyslipidemia, worse glycaemia, and elevated levels of enzymes, such as alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transpeptidase (GGTP), or alkaline phosphatase (ALP) [88]. That study demonstrated that even potentially healthy fruits combined with caloric excess can be detrimental to patients with fatty liver degeneration. With this in mind, excessive fruit intake may not be a good option for patients with MAFLD (who are often also overweight/obese), and they may be added to the diet only after hepatic steatosis is reversed.
Fructose in the ketogenic diet is eliminated or at least limited to marginal levels, a direct result of the low-carb (and sugar-eliminating) nature of this diet. Considering this on the one hand, and the negative effects of fructose on fatty liver degeneration on the other, it can be concluded that individuals on a ketogenic diet are not exposed to one of the main negative factors exacerbating MAFLD, i.e., fructose excess, as it is naturally eliminated from their diet.
2.4. Elimination of Monosaccharides
It is known that consumption of monosaccharides, especially in excess, has a proinflammatory effect on the entire body. It is postulated that the presence of sugar and processed food (often rich in monosaccharides) in the diet may be a key factor leading to inflammation and its exacerbation [89,90,91,92]. On the other hand, it is known that MAFLD, and MASH in particular, has a proinflammatory background [12,93]. This is particularly relevant given how significantly a proinflammatory diet contributes to an increased risk of MAFLD, even independent of other factors, as shown in a 2023 publication [94]. Consumption of monosaccharides (due to their simple chemical structure) significantly increases serum glucose and insulin levels, causing negative health effects [54]. Repeated releases of insulin and glucose as a result can contribute to the development of insulin resistance, one of the main negative factors exacerbating fatty liver, as described in Section 2.1. On the other hand, insulin resistance itself exacerbates inflammation, if only by increasing the production of proinflammatory adipokines and cytokines in adipose tissue or the accumulation of proinflammatory macrophages, which creates a kind of a vicious circle [41,95,96].
By eliminating simple sugars, the ketogenic diet has the effect of reducing inflammation, on the one hand, and lowering and stabilizing glucose levels, on the other, reducing the risk of developing insulin resistance (thus, MAFLD). The elimination of one of the key proinflammatory factors, i.e., monosaccharides, is natural in individuals on KD, as this is due to the nature of their diet. KD, thus, shows an advantage over standard diets, which allow up to 5% of energy from monosaccharides [34,97].
2.5. Limitation of Carbohydrates
Complex carbohydrates characterized by a low glycemic index (GI) are considered better (than monosaccharides) because of their lower glucose and insulin release, and diets with a low GI and glycemic load (GL) are considered anti-inflammatory. Even so, complex carbohydrates are broken down to glucose in the body anyway, a process that takes longer than the readily available (as an energy source) monosaccharides [54,98]. In both cases, however, there is a significant increase in glucose and insulin levels. The authors of a 2022 publication suggest that complex carbohydrates may have an even greater effect on postprandial insulin surge than sucrose [99]. In addition, a meta-analysis demonstrated that replacing monosaccharides (glucose, fructose) and sucrose with starch had no effect on HbA1c concentration [100]. Another meta-analysis found that each 10% reduction in total carbohydrates has a lowering effect on fasting blood glucose (by an average of 0.34 mmol/L) and HbA1c (by an average of 0.20 HbA1c%). Importantly, even after 12 months, the downward trend in HbA1c was still evident [101]. People with MAFLD are recommended to follow the Mediterranean dietary (MD) model, in which the majority of energy comes from complex carbohydrates [102]. This is the main difference between KD and MD. Common features of both diets include low processing (in line with the original premise), avoiding simple sugars, high vegetable intake (recommended in both MD and KD), and consuming larger amounts of fats such as olive oil, fatty fish, avocados, and nuts (although it should be noted that even more fat is consumed on KD). KD usually allows for the consumption of a larger amount of total meat as well as red meat alone, compared to MD. It has been postulated that the Mediterranean diet (MD) may have a preventive effect on MAFLD, as shown in some studies [103,104,105,106]. On the other hand, the 2023 study compared glucose and insulin surge at nine time points (at baseline and after 10, 20, 30, 45, 60, 90, 120, and 180 min) after a standard Mediterranean diet meal and after a ketogenic diet meal of the same calorie content. It turned out that the glucose and insulin levels as well as insulin secretion rates were many times lower after the ketogenic meal compared to the MD meal. From an initial insulin value of 40 ± 4 pmol/L, there was an increase after eating the Mediterranean diet meal to maximum values of 497 ± 101 pmol/L after 20 min, while the maximum insulin level after the ketogenic meal was only 88 ± 12 pmol/L after 30 min (compared to an initial value of 44 ± 5 pmol/L), a huge difference between the two groups [107]. Continuous glucose monitoring (CGM) devices, which allow for real-time feedback, may be helpful as a motivator for long-term lifestyle changes, as increasingly discussed in publications [108,109,110]. The stability of glycaemia achieved using a low-carb or ketogenic diet can be an extremely important incentive, so that achieving a certain health outcome can be much easier.
In addition to its effect on carbohydrate metabolism, lowering total dietary carbohydrates may have an anti-inflammatory effect. Karimi et al. demonstrated that the total carbohydrate intake is associated with an increased risk of inflammation, while no increased risk of inflammation has been observed that would be associated with total dietary fat [111]. The authors suggest reconsidering global guidelines that rely on high-carbohydrate dietary models. The anti-inflammatory effect of carbohydrate restriction was also confirmed in a randomized controlled study in which the authors concluded that a low-carb diet reduced inflammation, which was not observed in the case of low-fat diet [112]. This is also supported by the results of other studies [113,114,115]. One study, moreover, found that a higher carbohydrate intake was associated with fatty liver degeneration in patients with type 2 diabetes aged ≤ 50 years [116].
In consideration of the foregoing, on the one hand, there is a potential anti-inflammatory effect of total carbohydrate restriction. On the other hand, given the high post-meal glycaemia and insulin values of the Mediterranean diet, the ketogenic diet is significantly better in controlling these parameters, which directly translates into a lower likelihood of occurrence of the main risk factor for MAFLD, i.e., insulin resistance. In view of this, even the most low-glycemic carbohydrate-based diets will still be characterized by a higher GI than the ketogenic diet, where the low GI is due to the nature of the diet and is achieved automatically when using this dietary model.
2.6. Anti-Inflammatory State of Ketosis
The inflammatory background of nonalcoholic fatty liver degeneration, particularly MASH, makes it reasonable to consider the inflammatory process as one of the main factors exacerbating the course of this disease [12,117,118]. It is known now that activation of the NLRP3 inflammasome, which is significantly increased in MAFLD patients, is strongly associated with the development of this disease. That protein complex plays a key role in the inflammatory process and is a kind of command center for proinflammatory cytokines. This means that its activation (as a result of unfavorable factors) is associated with an increase in inflammatory markers and often causes chronic inflammatory condition [34,119,120]. Currently, pharmacological treatment specifically targeting the NLRP3 inflammasome is suggested as one of the treatments for MAFLD, which includes as many as four categories of agents, i.e., NLRP3 inhibitors, inhibitors of inflammasome components, pharmacological chemotherapeutics, and botanical drugs [119]. However, the safety of their use remains questionable.
The ketogenic diet, through the state of ketosis, exerts a natural strong anti-inflammatory effect, which can even be considered as one of the main advantages of this dietary model [121,122,123,124,125]. This effect can be observed by determining C-reactive protein (CRP) levels [126,127]. Alleviation of the inflammatory condition by KD can even be seen when using an even more sensitive indicator of inflammation, hs-CRP (decrease from 3.45 ± 3.69 mg/dL to 1.80 ± 2.32 mg/dL after 30 days), as shown in a 2023 study [128] and reported in other publications [129,130]. KD also shows the ability to reduce proinflammatory interleukins (including IL-1β, IL-2, IL-4, IL-6, IL-18) and tumor necrosis factor alpha (TNFα), it inhibits the activity of cyclo-oxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), and reduces the level of inflammation-related transcription nuclear factor kappa B (NFκB) (which plays a key role in the transcription of coding genes inflammation-related proteins) [131,132,133]. It also appears to have the property of reducing (just like fasting) the activation of NLRP3 inflammasome, which means that it works through the same mechanism as some pharmacological drugs, but in a natural way, without negative health consequences. The main ketone body (β-hydroxybutyrate) BHB, produced as a result of the KD, has an inhibitory effect on NLRP3 inflammasome (via multiple mechanisms) [134]. The reliance on ketone bodies is so important that even just administering them in exogenous form reduced the values of the inflammation indicators, i.e., IL-1β, IL-6, IFN-γ, MCP-1, and RANTES [135]. BHB inhibits the NLRP3 inflammasome, preventing K(+) efflux and reducing adaptor-apoptosis-associated-speck-like protein (ASC) oligomerization and formation of specks (which are large ASC protein complexes following inflammasome activation). KD (via BHB) attenuates activation of caspase-1 and secretion of IL-1β in the models of diseases mediated by NLRP3 [133,136]. There are a number of potential mechanisms for KD’s effect on reducing NLRP3 inflammasome activation. The authors of a 2023 publication point to possible effects through, among others, intracellular signaling, reactive oxygen species (ROS), endoplasmic reticulum (ER), stress, autophagy, mitochondrial metabolism, post-translational modifications, and receptor-mediated action [134]. Thus, the multifaceted effect of the ketogenic diet on one of the major negative factors associated with MAFLD (inflammatory condition) makes it another potential therapeutic mechanism of KD in people with nonalcoholic fatty liver degeneration.
Oxidative stress exacerbates inflammation, in addition to its direct impact on MAFLD, leading to advancement of the disease process into its subsequent, more dangerous stages. The literature also suggests that several other mechanisms may be at play, including the effects of oxidative stress on 1. lipid metabolism, 2. damage to intracellular structures including several organelles involved in key metabolic pathways (mitochondrial and endoplasmic reticulum dysfunction are some examples), and 3. directly causing lipotoxic liver damage, which may ultimately contribute to the development and progression of MAFLD [137]. Given the favorable impact that the KD has on these key mechanisms, including reduced inflammation, reduced oxidative stress, and enhanced mitochondrial function, the KD is likely to play a beneficial role in preventing the progression of this disease. The mechanisms underpinning the role of the KD on liver function were described in a review by Paola and Cerullo. The authors discussed a number of factors, including 1. improved mitochondrial function (stimulating mitochondrial formation, mitochondrial dynamics and bioenergetic pathways) and 2. activating several key factors that alleviate oxidative stress, as possible routes through which the KD contributes to reduced inflammation and improved overall liver function [59].
2.7. Modulation of Intestinal Microbiome and Metabolome
Research is increasingly focusing on the role of intestinal microbiome and metabolome in the course of MAFLD. It has been found that dysbiosis of gut microbiome is associated with the etiopathogenesis of this disease from the early stages to the development of MASH and liver cirrhosis. The relationship is confirmed by the fact that the use of probiotics, prebiotics, synbiotics, postbiotics, and parabiotics alleviates the dysbiosis and improves liver function parameters [138,139]. Associated with the course of MAFLD is a change in the composition of the gut microbiome, frequently an increase in the abundance of harmful microorganisms at the expense of beneficial ones. The result is the creation of a proinflammatory intestinal environment, disruption of the integrity of the intestinal barrier, and greater exposure of the liver to harmful agents (including dietary ones), which directly exacerbates the course of MAFLD [140]. The authors of the publication explicitly list which bacterial phyla, families, and genera are most often associated with the disease (and to some extent also with its further stage (MASH)). At the phylum level, it is an increase in Proteobacteria, at the family level—an increase in Enterobacteriaceae and a decrease in Rikenellaceae and Ruminococcaceae, and at the genus level, an increase in Escherichia, Bacteroides, Dorea, and Peptoniphilus and a decrease in Faecalibacterium, Coprococcus, Anaerosporobacter, and Eubacterium [141,142,143,144,145,146,147]. There is even a link between liver cirrhosis and the presence of Prevotella, Veillonella, and Streptococcus strains in the oral cavity, which are rare in healthy individuals [148]. One publication has even shown that the signature of intestinal microbiome can predict the development of MAFLD, using obese women as an example [149]. In view of the foregoing, the microbiome may be a kind of biomarker of disease severity, as evidenced by publications suggesting a causal role of the microbiome in the progression of the disease [150]. An important role of the gut–liver axis is postulated, whose dysregulation affects the pathogenesis of MAFLD through disruption of intestinal integrity, endotoxemia, inflammatory condition, changes in bile acids profiles, and bacterial metabolites [151]. The occurrence of this axis and liver function make the liver particularly vulnerable to toxins from intestinal absorption, as it is the first line of defense against bacterial metabolites. Lipopolysaccharide (LPS), as one of the main proinflammatory products of the intestinal microbiota, can induce lipopolysaccharidaemia and, thus, adversely affect MAFLD [152,153]. It is also worth remembering that increased dietary fructose contributes increased intestinal fructose (as it is absorbed much slower than glucose), which leads to bacterial fermentation and the development of pathogenic bacteria, causing dysbiosis [154]. In addition to dysbiosis, it can also disrupt the integrity of the intestinal barrier (loss of tight junction proteins in the small intestine), thus contributing to MAFLD development [155]. A negative effect, also through the gut microbiome, is generally exerted by a high-sugar diet [156].
The ketogenic diet shows a strong effect on modulating the intestinal microbiome and metabolome. This mechanism is even being investigated as a major therapeutic effect in epilepsy, as the therapeutic effect of KD shows a correlation with changes in the intestinal microbiome [157,158]. This nutritional model may affect the composition, function, and diversity of the gut microbiome [159,160]. KD-specific modulation of the microbiome is associated with a reduction in intestinal proinflammatory Th17 cells [161]. On the other hand, it has been demonstrated in an animal model that a KD-specific metabolic state can alter the inflammatory response associated with acute endotoxemia. The high-carb group had higher levels of IL-6 and TNF-α, as well as five times higher expression of hepatic NFκB compared to the ketogenic group [162]. Moreover, it appears that a ketogenic diet, contrary to appearances, can increase the amount of all short-chain fatty acids (SCFAs) in the intestine (including the main one, sodium butyrate), as shown in a six-month-long study [163]. At the same time, it is also known that sodium butyrate, by regulating the intestinal microbiota, can alleviate fatty liver degeneration caused by fructose [164]. It has further been shown that patients with end-stage MAFLD, or liver cirrhosis, had lower levels of fecal SCFAs (mainly butyrate and acetate) [165]. Microbial metabolites, SCFAs in particular, are one of the subjects of studies on the course of MAFLD [166], and their blood levels (isobutyrate and methylbutyrate) show a favorable, significant, and negatively correlated relationship with the severity of MAFLD [167].
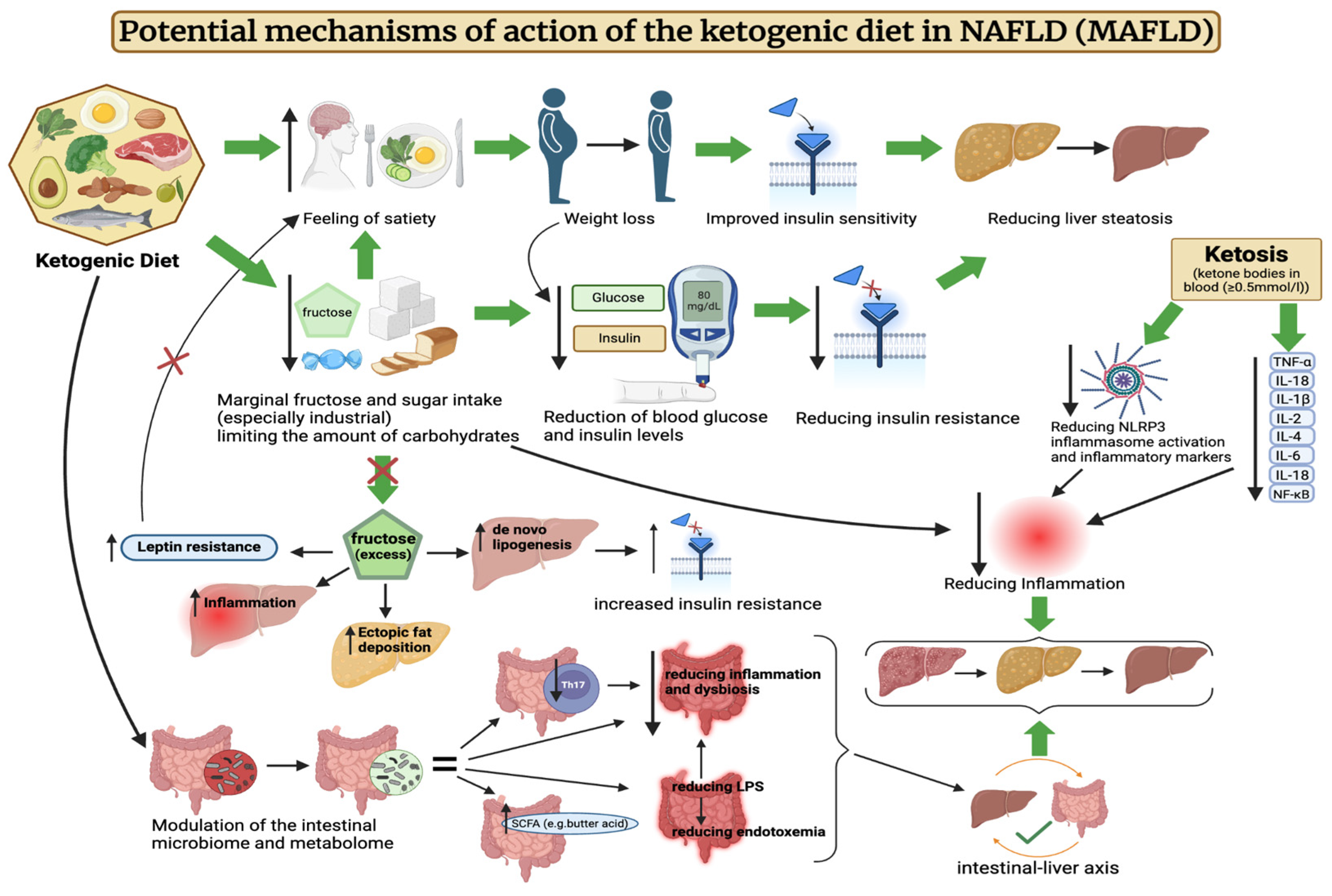
Potential mechanisms of action of the ketogenic diet in MAFLD are illustrated in Figure 1.
Figure 1.
Potential mechanisms of action of the ketogenic diet in NAFLD (MAFLD). The above figure was created with BioRender.com, accessed on 8 August 2024. Agreement number: CR275R2WWY.
3. Effect of Ketogenic Diet on MAFLD in Humans
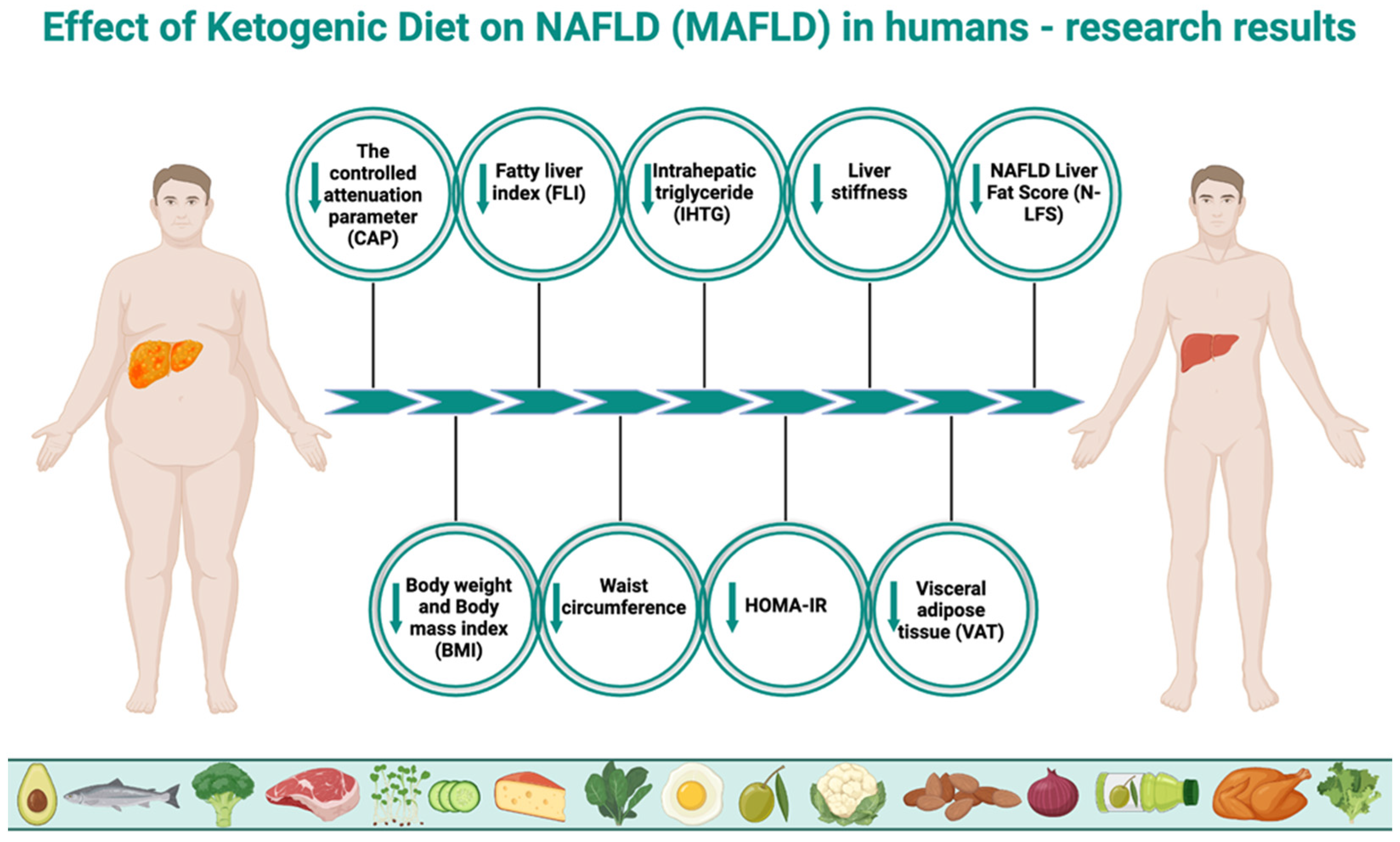
The effect of the ketogenic diet on nonalcoholic fatty liver disease is a growing focus of research. Although it is an area that requires more scientific exploration, the results of existing studies are promising. Optimistic conclusions have been drawn by the authors of a 2023 study, which assessed, among other parameters, the efficacy and safety of a very-low-calorie ketogenic diet (VLCKD) for the treatment of MAFLD in 33 patients with excessive body weight (BMI > 25, aged 18–64, who were not taking medications). After 8 weeks on VLCKD, a significant improvement in fatty liver degeneration was observed. The controlled attenuation parameter (CAP) decreased from 266.61 ± 67.96 to 223 ± 64.19, and fatty liver index (FLI) from 62.82 ± 27.46 to 44.09 ± 31.24. In addition, other MAFLD-related indices also changed favorably. Simultaneous reductions were observed, among other parameters, in insulin resistance measured by the homeostatic model assessment for insulin resistance (HOMA-IR) (from 3.11 ± 1.74 to 1.95 ± 0.97), insulin levels (from 12.97 ± 7.19 μU/mL to 8.93 ± 4.29 μU/mL), body mass index (BMI) (from 33.84 ± 6.55 to 30.89 ± 6.38), mass of body fat (MBF) (from 38.47 ± 12.59 kg to 30.98 ± 12.39 kg), waist circumference (from 106.67 ± 15.51 cm to 98.64 ± 16.21 cm), total cholesterol concentration (from 213.49 ± 42.25 mg/dL to 178.09 ± 28.14 mg/dL), LDL (from 140.85 ± 41.07 mg/dL to 113.27 ± 26.94 mg/dL), and triglycerides (from 112.82 ± 58.9 mg/dL to 86.42 ± 42.37 mg/dL). The authors clearly concluded that treatment of MAFLD using VLCKD is both effective and safe [168]. Another, similar study from 2023 also showed the benefits of this dietary model on a larger group of patients (58 women and 29 men, aged 18–64, also not taking medications). The aim of the authors was to evaluate the effect of VLCKD on, among others, white blood cells (WBCs), platelets (PLTs), and hs-CRP concentration (as inflammatory condition parameters) and its simultaneous influence on liver steatosis and liver fibrosis in patients with excessive body mass but with no comorbidities. After eight weeks, a reduction was observed in CAP from 287 (255; 325) to 230 (188; 278), liver stiffness parameters (kPA) from 5.50 (4.30; 6.50) to 5.30 (4.00; 6.50), BMI (kg/m2) from 35.59 (6.31) to 32.59 (6.06), waist circumference from 112.15 (16.17) cm to 103.98 (15.71) cm, and mass of body fat (MBF) from 40.17 (13.14) kg to 33.41 (11.91) kg, as well as total cholesterol, LDL, HDL, triglycerides, and hepatic enzymes (ALT, AST, γ-GT). Moreover, there had been a significant reduction in WBC and PLT levels in patients with steatosis (CAP ≥ 215 dB/m). Other parameters, including insulin and glucose concentrations, systolic and diastolic pressures, and the HOMA-IR index, had also been significantly lower after using VLCKD. The authors concluded that VLCKD reduces systemic and liver inflammation, positively contributing to the health of this organ [169]. The authors of a randomized controlled study (the groups consisted of participants who were overweight or obese, grade 1, aged 21–65) compared the effects of a ketogenic diet with placebo (KD + PL) (n = 13), a ketogenic diet with a ketone body supplement (LD + KS) (n = 12), and a low-fat diet (LFD) (n = 12), all with a similar caloric intake. Contrary to frequent allegations that high consumption of saturated fat would increase liver fat, however, the results showed a reduction in liver steatosis. In each group, there was a significant decrease in liver fat without major differences. For KD + PL it was −32%, for KD + KS it was −42%, and for LFD it was −52%. The absolute reduction in liver fat percentage was almost identical, accounting for −2.0% in the KD + PL group, −1.9% in the KD + KS group, and −2.1% in the LFD group. In addition, in the MAFLD subgroup, the ketogenic diet performed better in reducing insulin resistance, as the HOMA-IR index decreased from an average of 4.56 ± 0.90 to 1.48 ± 0.54, while in the LFD group it only decreased from 3.45 ± 1.06 to 2.46 ± 0.64. Similarly, glucose levels decreased from an average of 97.3 ± 6.2 to 84.7 ± 2.81 in the KD group, while they increased from 84.8 ± 6.6 to 86.2 ± 3.34 in the LFD group. The authors pointed out that a six-week hypocaloric diet rich in saturated fat with low carbohydrate intake does not adversely affect liver health, and even slightly improves liver parameters [170]. Results of another randomized controlled study showed that VLCKD (n = 20) was more effective than a standard low-calorie (LC) (n = 19) diet in reducing body weight, visceral adipose tissue, and liver fat (participants ≥ 18 years of age, BMI > 30 kg/m2, recruited among patients referred for weight loss treatment). In the VLCKD group, a greater loss of body weight (−9.59 ± 2.87% vs. −1.87 ± 2.4% in the LC group), visceral adipose tissue (VAT) (−32.0 cm2 vs. −12.58 cm2 in the LC group), and liver fat (−4.77 vs. 0.79% in the LC group) was observed. The authors suggest that VLCKD may be an effective alternative treatment for MAFLD. However, a limitation of that study is the fact that both groups differed significantly in caloric intake. In the case of VLCKD, it was just 600–800 kcal, while in LC it was 1400–1800 kcal [171]. Interesting results were obtained by Luukkonen et al., who decided to investigate the mechanism underlying the regression of liver steatosis and insulin resistance by a ketogenic diet, despite an increase in circulating nonesterified fatty acids (NEFA), among 10 participants (5 women and 5 men) aged 58.2 ± 2.8 years with a BMI of 31.6 ± 2.0 kg/m2. It was found that in spite of a 35% increase in NEFA level, KD reduced intrahepatic triglycerides (IHTGs) concentration by 31%, with a 58% reduction in liver insulin resistance and a 3% reduction in body weight in just six days. The authors indicated that the obtained results could have been affected by an increased net hydrolysis of IHTG (which was attributed to reduced liver insulin resistance) and partitioning of the resulting fatty acids towards ketogenesis (+232%) due to reduced insulin levels (−53%) and hepatic citrate synthase flux (−38%), which is associated with an increased redox status in hepatic mitochondria (+167%) and decreased plasma leptin (−45%) and triiodothyronine (T3) (−21%) concentrations. This study has demonstrated previously undescribed mitochondrial adaptations associated with the reversal of MAFLD through KD, related to the state of ketosis rather than IHTG synthesis. The authors highlight hepatic mitochondrial fluxes and redox state as potential targets for treatment of this disease [31]. Authors of another study set out to assess the effect of VLCKD (45 days) with transition to a hypocaloric low-carb diet (LCD) (for another 45 days) and to identify the predictive factors of MAFLD, among 65 obese patients (BMI > 30 kg/m2) aged 18–60, with stable body weight in the last 3 months. After 45 and 90 days, a number of parameters improved, including reductions in body weight, HbA1c, fibroblast growth factor 21 (FGF21), insulin resistance measured by HOMA-IR, hepatic steatosis index (HIS), and triglycerides, as shown in Table 1. HOMA-IR index (positive; R = 0.414) and FGF21 (negative; R = 0.364) have been demonstrated to be independent predictive factors of HSI reduction. The authors suggest a possible role of human FGF21 in alleviating MAFLD as a result of the diets used [172]. The aim of another study was to determine the effect of gender differences on body weight loss and alleviation of MAFLD following VLCKD in obese patients. The study included 42 women and 25 men with severe obesity (BMI ≥ 35 kg/m2 and ≥40 kg/m2), aged 18–64, with diseases comorbid with obesity (including metabolic disorders, cardiovascular diseases, respiratory diseases, etc.) Body weight loss was greater in men, as was the reduction in γ-glutamyl transferase (γ-GT) levels. The authors concluded that, in this regard, men benefit more from VLCKD compared to women, especially premenopausal women, with the differences disappearing after menopause. Moreover, a greater reduction in HbA1c and HOMA-IR values was observed in men compared with those in women. In view of that, a conclusion can be drawn that the effectiveness of VLCKD in severe obesity is affected by gender differences and, in the case of women, premenstrual status [173]. Interesting results were presented in another publication, the authors of which compared the effects of a one-year-long continuous care intervention (CCI) using a ketogenic diet (262 patients) to a one-year-long usual care (UC) based on the recommendations of the American Diabetes Association (ADA) (87 patients) in patients with type 2 diabetes and their impact on MAFLD and hepatic fibrosis outcomes. Patients were aged 21–65 years, with BMI > 25 kg/m2 and diagnosed with type 2 diabetes, excluding patients with significant alcohol consumption, any other cause of liver disease (or secondary causes of MAFLD), and decompensated cirrhosis. Importantly, patients on the ketogenic diet consumed <30 mg of carbohydrate daily, 1.5 g of protein per 1 kg of normal body mass, and were allowed to consume fat until satiety. This was, therefore, not a restrictive diet, as patients chose products according to their own preferences, but their ketosis status was monitored. After one year, patients in the CCI group had a reduction in MAFLD liver fat score (N-LFS) from 3.26 ± 0.21 to 1.30 ± 0.19, while in the UC group there was an increase in N-LFS, from 3.25 ± 0.38 to 3.71 ± 0.35. Body weight loss of ≥5% occurred in 79% of subjects in the CCI group, while only in 19% of subjects in the UC group. MAFLD fibrosis score (NFS) decreased in the CCI group from −0.43 ± 0.08 to −1.14 ± 0.09, and increased in the UC group from −0.62 ± 0.17 to −0.35 ± 0.18. The authors concluded that one year of remotely supported CCI improves MAFLD and liver fibrosis parameters in patients with type 2 diabetes [174]. The most important results in the context of the present study are shown in Table 1. Effects of ketogenic diet on MAFLD in humans are illustrated in Figure 2.

Table 1.
Effects of ketogenic diet on MAFLD parameters in humans.
Figure 2.
Effect of ketogenic diet on NAFLD (MAFLD) in humans. The above figure was created with BioRender.com, accessed on 8 August 2024. Agreement number: KZ275R2EJK.
4. Role of Education and Self-Treatment in the Process of MAFLD Reversal
4.1. A Healthcare System Problem
Healthcare systems are currently failing to manage such the widespread pandemic of MAFLD. Consider its constantly increasing incidence and pessimistic prognosis (even tripling in prevalence by 2030) [1,2,10]. As shown by Lee et al., a majority of patients incidentally diagnosed with MAFLD at their health check-up did not receive any disease management advice, and only 40.2% of those diagnosed visited the clinic [25]. In view of this, the issue is downplayed not only among patients but also among doctors. The issue of physicians’ lack of adequate preparation has been addressed in a number of publications, concluding that physicians underestimate the significance of the problem and lack sufficient knowledge regarding the management and assessment of MAFLD [175,176,177,178,179].
4.2. Key Role of Self-Education and Self-Treatment
Given, on the one hand, the above-described inefficiencies in the healthcare system in respect of MAFLD and, on the other hand, the lack of awareness (in up to 95% of individuals) and downplaying the disease by patients [24,25], the role of self-education may be crucial. A study by Stine et al. found that lack of education was one of the key barriers to implementing physical activity in MAFLD patients, which demonstrates how important that factor is [180]. Self-education of patients will reduce complications of the disease and increase the chances of reversing fatty liver degeneration and, on the other hand, educated healthy individuals will reduce the risk of MAFLD development through making informed decisions. Adequate education about MAFLD and other metabolic diseases is an essential tool for taking responsibility for one’s own health and initiating self-treatment, without relying solely on medical care (which may often be insufficient). Self-efficacy has been shown to play a key role in self-treatment, which has shown a significant correlation with self-monitoring outcomes and is the main determinant of MAFLD self-treatment [181]. The significant role of education was confirmed by the study by Vilar-Gomez et al., referred to in Section 3, which demonstrated the superiority of education and remote care (with respect to a ketogenic diet) over standard physician dietary care according to the ADA recommendations in improving liver steatosis in patients with type 2 diabetes within one year [174].
4.3. Self-Monitoring and Self-Education Tools
Patients can educate themselves in any way they wish, be it standard education by medical staff, reading books, e-books, guides, searching for information on the internet, or on various web portals, and also using other technologies [182,183]. It is important that patients do not rely on medical care only, but that they are aware of their disease, able to monitor their condition, and conduct the process of MAFLD self-treatment. The possible tools may include the continuous glucose monitoring (CGM) technology, which is the use of glucose sensors that show current blood glucose concentration without the need for constant finger-pricking, making it easier to stabilize glucose and ultimately reduce HbA1c levels [184]. It allows patients to increase their self-awareness and better understand their body response, thus making self-monitoring more effective. This is of great importance because chronically elevated serum glucose levels are associated with MAFLD, creating a vicious circle [185]. Individuals switching from their usual diet to a ketogenic diet usually experience a rapid stabilization of glycaemia, which may pay off in increased motivation and willingness to continue a healthy lifestyle [108,109,110]. The same is true of the promising continuous ketone monitoring (CKM) technology [186], which can be used as a tool to monitor the state of ketosis during a ketogenic diet, thus increasing self-awareness concerning one’s own body’s response. Other methods of self-education and increasing the control over one’s own body may include all kinds of mobile applications of all types. They can help minimize self-identified barriers to lifestyle change, such as lack of education, exercise resources, time, and costs [180,187,188]. One randomized controlled study showed that a mobile application performed better (compared to standard care) in weight loss and improvement in metabolic parameters in patients diagnosed with MAFLD [189]. Another study demonstrated that a six-month-long lifestyle intervention based on a mobile application was feasible and acceptable in patients with MAFLD. A majority of participants confirmed that it was easy to use and motivating for an active lifestyle [190]. There are also applications for strictly ketogenic diets, which have been shown in studies to be effective in reducing body weight, among other things [191,192].
With this in mind, self-education and self-monitoring have an invaluable impact on the prevention and treatment of MAFLD as well as other metabolic diseases. The patient should be aware of his/her disease and lifestyle; only then can the process of prevention or treatment proceed optimally. Relying only on professional care in such a heavily overburdened medical system, without taking responsibility for one’s own health and without changing lifestyle, is likely to be ineffective and may even be irresponsible.
5. Potential Side Effects of Using a Ketogenic Diet
It is worth clarifying at this point whether any side effects from the ketogenic diet are possible. The authors of one publication decided to investigate this. It was shown that, especially in the first few days after starting, symptoms such as nausea, polyurea, dizziness, halitosis, sluggishness, constipation, palpitations, and muscle pain can occur. Significantly, the same participants were overwhelmingly satisfied with the effects of the ketogenic diet, with the authors concluding by noting that KD gave a very positive overall experience [193]. However, these symptoms are the body’s physiological response to the change in fuel source from glucose to ketone bodies and are referred to as “keto flu” and subside within the first few days/weeks. Other authors point to similar symptoms reported in the early stages of KD use and these included headache, lethargy, fatigue, nausea, dizziness, decreased energy, gastrointestinal discomfort, changes in heartbeat, and feeling faint [194]. It is worth noting that these are symptoms reported by participants (on online forums) using the ketogenic diet alone. This means that they most likely did not carry it out properly, which could have been avoided. Therefore, these cannot be called side effects of the ketogenic diet, but symptoms of a poorly conducted ketogenic diet (and even in this case they are not serious and go away on their own).
6. Perspectives
The increasing dissemination of knowledge related to MAFLD offers hope for better management of this disease, both among healthcare professionals and patients (through self-education and self-monitoring). This could lead to a reduction in the number of new MAFLD cases and improved treatment of those currently affected. In the context of KD’s impact on MAFLD, there are several key areas that require further research. Primarily, studies should be long-term and focus on comparing KD with other diets of similar caloric content (or at least ad libitum), particularly examining normocaloric approaches (which would eliminate the weight loss factor). Efforts should also be concentrated on investigating the metabolic pathways through which KD affects MAFLD, as well as exploring KD’s impact on the microbiome in the context of MAFLD development, treatment, and prevention.
7. Summary
The ketogenic diet, by its very nature, may be one of the more effective known therapeutic options for nonalcoholic fatty liver disease. This is because it affects a number of key factors from the perspective of MAFLD regression.
One of the main recommendations in MAFLD therapy is to achieve a calorie deficit in order to reduce body weight. It has been found that KD can reduce calorie intake extremely easily without having to track the calories, by maintaining a high level of satiety and, thus, reducing the desire to snack. KD also impacts another factor, crucial from the point of view of MAFLD regression, i.e., insulin resistance. On the one hand, this is caused by monosaccharides and total carbohydrates intake reduction, which results in lower glucose and insulin spikes (and lower HbA1c levels) and, on the other hand, body weight loss itself (achievable easily on KD) reduces insulin resistance. Limiting the supply of those compounds (especially monosaccharides) also reduces inflammation, which is closely associated with MAFLD (particularly with MASH). Inflammation is also reduced by the state of ketosis itself, which is associated, among others, with the inhibition of NLRP3 inflammasome activation. It has been found that β-hydroxybutyrate (the main ketone body) acts by a similar mechanism as some medications, but without any adverse effects, thus providing a double benefit. The problem of another MAFLD-exacerbating factor, i.e., fructose excess, is absent while on the ketogenic diet. This is due to the marginalization or outright elimination of all forms of mono-, di-, and polysaccharides, including fructose. In view of this, any negative consequences for the liver resulting from the consumption of fructose (even more so, the industrial fructose) simply do not occur in KD users. Furthermore, KD specifically improves the intestinal microbiome and metabolome, disorders of which are correlated with MAFLD.
The available studies in humans have shown beneficial effects of KD on MAFLD regression; however, these are mostly low-calorie diets, which gives an argument to opponents of KD that the observed effects are solely due to weight loss. In view of the foregoing, constructing well-designed studies should be one of the main research aims regarding MAFLD in the coming years. Gender-associated impact of KD on MAFLD is also worth analyzing. In the face of inefficiencies in the healthcare system, patients (in parallel with medical care) should take responsibility for their health and not rely solely on medical care. The role of self-education and self-management is underestimated, and they might relieve the burden on the current system, while increasing patient control and management of their health. It is also worth placing more emphasis on training doctors how to treat MAFLD properly.
In conclusion, it can be said that a ketogenic diet may be an effective tool for MAFLD regression (particularly promising when combined with self-education and self-monitoring). However, there is a lack of well-designed studies in this area; therefore, exploration of this scientific field should be a focus of research in the coming years. Perhaps this could lead to the introduction of KD in the treatment of MAFLD on a broader scale, resulting in improved health of millions of people and significant savings for healthcare systems worldwide.
Author Contributions
Conceptualization: D.D., Ł.R. and M.R.; writing—original draft preparation: D.D.; writing—review and editing: Ł.R., D.U., D.Ł., M.R., A.T., M.H., S.K., A.D., Ż.G. and A.I.; review of the literature: D.D., M.H., Ł.R. and A.T.; supervision: Ł.R., D.U. and A.T.; visualization: D.D., Ł.R. and D.U.; funding acquisition: Ł.R. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
The publication was financed by Rodzen Brothers Foundation, 64-234 Wieleń, Poland.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Ekstedt, M.; Wong, G.L.; Hagström, H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Díaz, L.A.; Arab, J.P.; Louvet, A.; Bataller, R.; Arrese, M. The intersection between alcohol-related liver disease and nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 764–783. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.M.; Ghadieh, H.E.; Sekar, R.; Carraro, R.; Noriega, L.G.; Paes, A.M.A. Editorial: Metabolism in nonalcoholic fatty liver disease. Front. Physiol. 2023, 14, 1275319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uehara, T.; Wakui, H.; Tamura, K. Metabolic dysfunction-associated fatty liver disease reflects a significantly higher risk of hypertension than non-alcoholic fatty liver disease. Hypertens. Res. 2023, 46, 1165–1167. [Google Scholar] [CrossRef] [PubMed]
- Zohara, Z.; Adelekun, A.; Seffah, K.D.; Salib, K.; Dardari, L.; Taha, M.; Dahat, P.; Toriola, S.; Satnarine, T.; Franchini, A.P.A. The Prospect of Non-Alcoholic Fatty Liver Disease in Adult Patients with Metabolic Syndrome: A Systematic Review. Cureus 2023, 15, e41959. [Google Scholar] [CrossRef] [PubMed]
- Fouad, Y. Metabolic-associated fatty liver disease: New nomenclature and approach with hot debate. World J. Hepatol. 2023, 15, 123–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, K.; Kim, H.S.; Chae, H.W. Nonalcoholic fatty liver disease and insulin resistance in children. Clin. Exp. Pediatr. 2023, 66, 512. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R.; Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 32–42. [Google Scholar] [CrossRef]
- Sepulveda-Villegas, M.; Roman, S.; Rivera-Iniguez, I.; Ojeda-Granados, C.; Gonzalez-Aldaco, K.; Torres-Reyes, L.A.; Jose-Abrego, A.; Panduro, A. High prevalence of nonalcoholic steatohepatitis and abnormal liver stiffness in a young and obese Mexican population. PLoS ONE 2019, 14, e0208926. [Google Scholar] [CrossRef]
- Hepatology, T.L.G. The Lancet Gastroenterology Hepatology Redefining non-alcoholic fatty liver disease: What’s in a name? Lancet Gastroenterol. Hepatol. 2020, 5, 419. [Google Scholar]
- Available online: https://www.niddk.nih.gov/health-information/liver-disease/nafld-nash/definition-facts (accessed on 24 November 2023).
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression from NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Mavilia, M. Mechanisms of progression in Non-alcoholic fatty liver disease. Gastroenterol. Hepatol. Endosc. 2018, 3. [Google Scholar] [CrossRef]
- Han, S.K.; Baik, S.K.; Kim, M.Y. Non-alcoholic fatty liver disease: Definition and subtypes. Clin. Mol. Hepatol. 2023, 29, S5–S16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsai, E.; Lee, T.P. Diagnosis and Evaluation of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Including Noninvasive Biomarkers and Transient Elastography. Clin. Liver Dis. 2018, 22, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Spiers, J.; Brindley, J.H.; Li, W.; Alazawi, W. What’s new in non-alcoholic fatty liver disease? Frontline Gastroenterol. 2022, 13, e102–e108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allen, A.M.; Lazarus, J.V.; Younossi, Z.M. Healthcare and socioeconomic costs of NAFLD: A global framework to navigate the uncertainties. J. Hepatol. 2023, 79, 209–217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rinella, M.E.; Sookoian, S. From NAFLD to MASLD: Updated naming and diagnosis criteria for fatty liver disease. J. Lipid Res. 2024, 65, 100485. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Younossi, Z.M.; Paik, J.M.; Henry, L.; Yang, J.; Fernandes, G.; Stepanova, M.; Nader, F. The Growing Economic and Clinical Burden of Nonalcoholic Steatohepatitis (NASH) in the United States. J. Clin. Exp. Hepatol. 2023, 13, 454–467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Younossi, Z.M.; Stepanova, M.; Ong, J.; Trimble, G.; AlQahtani, S.; Younossi, I.; Ahmed, A.; Racila, A.; Henry, L. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin. Gastroenterol. Hepatol. 2021, 19, 580–589.e5. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Aby, E.S.; Rosenblatt, R. Liver transplant at all costs. Liver Transpl. 2023, 29, 568–569. [Google Scholar] [CrossRef] [PubMed]
- Turri, J.A.O.; Decimoni, T.C.; Ferreira, L.A.; Diniz, M.A.; Haddad, L.B.P.; Campolina, A.G. Higher MELD score increases the overall cost on the waiting list for liver transplantation: A micro-costing analysis based study. Arq. Gastroenterol. 2017, 54, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.A.; Paik, J.M.; Biswas, R.; Arshad, T.; Henry, L.; Younossi, Z.M. Poor Awareness of Liver Disease among Adults with NAFLD in the United States. Hepatol. Commun. 2021, 5, 1833–1847. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.H.; Jung, J.H.; Park, H.; Oh, J.H.; Ahn, S.B.; Yoon, E.L.; Jun, D.W. A survey on the awareness, current management, and barriers for non-alcoholic fatty liver disease among the general Korean population. Sci. Rep. 2023, 13, 15205. [Google Scholar] [CrossRef] [PubMed]
- Wilder, R.M. The effects of ketonemia on the course of epilepsy. Mayo Clin. Bull. 1921, 2, 307. [Google Scholar]
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef] [PubMed]
- Torres-Peña, J.D.; Arenas-de Larriva, A.P.; Alcala-Diaz, J.F.; Lopez-Miranda, J.; Delgado-Lista, J. Different Dietary Approaches, Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease: A Literature Review. Nutrients 2023, 15, 1483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Pondel, N.; Liśkiewicz, D.; Liśkiewicz, A. Dieta ketogeniczna–mechanizm działania i perspektywy zastosowania w terapii: Dane z badań klinicznych. Postępy Biochem. 2020, 66, 270–286. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Dufour, S.; Lyu, K.; Zhang, X.M.; Hakkarainen, A.; Lehtimäki, T.E.; Cline, G.W.; Petersen, K.F.; Shulman, G.I.; Yki-Järvinen, H. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2020, 117, 7347–7354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watanabe, M.; Tozzi, R.; Risi, R.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G.; Lubrano, C.; Gnessi, L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes. Rev. 2020, 21, e13024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic diet for human diseases: The underlying mechanisms and potential for clinical implementations. Signal Transduct Target Ther. 2022, 7, 11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilson, J.; Lowery, R. The Ketogenic Bible; Victory Belt Publishing Inc.: Las Vegas, NV, USA, 2017; ISBN 13 978-1-628601-04-6. [Google Scholar]
- Venn, B.J. Macronutrients and Human Health for the 21st Century. Nutrients 2020, 12, 2363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, P.; Cai, X.; Yu, X.; Gong, L. Markers of insulin resistance associated with non-alcoholic fatty liver disease in non-diabetic population. Sci. Rep. 2023, 13, 20470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grander, C.; Grabherr, F.; Tilg, H. Non-alcoholic fatty liver disease: Pathophysiological concepts and treatment options. Cardiovasc. Res. 2023, 119, 1787–1798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niranjan, S.; Phillips, B.E.; Giannoukakis, N. Uncoupling hepatic insulin resistance-hepatic inflammation to improve insulin sensitivity and to prevent impaired metabolism-associated fatty liver disease in type 2 diabetes. Front. Endocrinol. 2023, 14, 1193373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maciejewska, D.; Stachowska, E. Non-alcoholic fatty liver disease (NAFLD). Adv. Hyg. Exp. Med. 2018, 72, 659–670. [Google Scholar]
- Bugianesi, E.; Moscatiello, S.; Ciaravella, M.F.; Marchesini, G. Insulin resistance in nonalcoholic fatty liver disease. Curr. Pharm. Des. 2010, 16, 1941–1951. [Google Scholar] [CrossRef]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef]
- Mendez-Sanchez, N.; Cruz-Ramon, V.C.; Ramirez-Perez, O.L.; Hwang, J.P.; Barranco-Fragoso, B.; Cordova-Gallardo, J. New aspects of lipotoxicity in nonalcoholic steatohepatitis. Int. J. Mol. Sci. 2018, 19, 2034. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Phinney, S.D.; Forsythe, C.E.; Quann, E.E.; Wood, R.J.; Puglisi, M.J.; Kraemer, W.J.; Bibus, D.M.; Fernandez, M.L.; Feinman, R.D. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009, 44, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bianco, A.; Moro, T.; Mota, J.F.; Coelho-Ravagnani, C.F. The Effects of Ketogenic Diet on Insulin Sensitivity and Weight Loss, Which Came First: The Chicken or the Egg? Nutrients 2023, 15, 3120. [Google Scholar] [CrossRef]
- Dyńka, D.; Kowalcze, K.; Ambrozkiewicz, F.; Paziewska, A. Effect of the Ketogenic Diet on the Prophylaxis and Treatment of Diabetes Mellitus: A Review of the Meta-Analyses and Clinical Trials. Nutrients 2023, 15, 500. [Google Scholar] [CrossRef]
- Charlot, A.; Zoll, J. Beneficial Effects of the Ketogenic Diet in Metabolic Syndrome: A Systematic Review. Diabetology 2022, 3, 292–309. [Google Scholar] [CrossRef]
- Aronica, L.; Landry, M.J.; Rigdon, J.; Gardner, C.D. Weight, insulin resistance, blood lipids, and diet quality changes associated with ketogenic and ultra low-fat dietary patterns: A secondary analysis of the DIETFITS randomized clinical trial. Front. Nutr. 2023, 10, 1220020, Erratum in Front. Nutr. 2023, 10, 1275498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Lehrke, M.; Hendler, R.E.; Shulman, G.I. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005, 54, 603–608. [Google Scholar] [CrossRef]
- Hite, A.H.; Berkowitz, V.G.; Berkowitz, K. Low-carbohydrate diet review: Shifting the paradigm. Nutr. Clin. Pract. 2011, 26, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.C.; Nuttall, F.Q. Control of blood glucose in type 2 diabetes without weight loss by modification of diet composition. Nutr. Metab. 2006, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Badman, M.K.; Kennedy, A.R.; Adams, A.C.; Pissios, P.; Maratos-Flier, E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1197–E1204. [Google Scholar] [CrossRef] [PubMed]
- Feinman, R.D.; Volek, J.S. Low carbohydrate diets improve atherogenic dyslipidemia even in the absence of weight loss. Nutr. Metab. (Lond.) 2006, 3, 24. [Google Scholar] [CrossRef]
- Hyde, P.N.; Sapper, T.N.; Crabtree, C.D.; LaFountain, R.A.; Bowling, M.L.; Buga, A.; Fell, B.; McSwiney, F.T.; Dickerson, R.M.; Miller, V.J.; et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight 2019, 4, e128308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://www.hsph.harvard.edu/nutritionsource/carbohydrates/carbohydrates-and-blood-sugar/ (accessed on 2 December 2023).
- Dyńka, D.; Paziewska, A.; Kowalcze, K. Keto Menu–Effect of Ketogenic Menu and Intermittent Fasting on the Biochemical Markers and Body Composition in a Physically Active Man—A Controlled Case Study. Foods 2023, 12, 3219. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, J.; Yang, S.; Gao, M.; Cao, L.; Li, X.; Hong, D.; Tian, S.; Sun, C. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: A systematic review and meta-analysis. Nutr. Diabetes 2020, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Stanhope, K.L.; Boucher, J.; Divanovic, S.; Lanaspa, M.A.; Johnson, R.J.; Kahn, C.R. Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab. Sci. 2020, 57, 308–322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jani, S.; Da Eira, D.; Stefanovic, M.; Ceddia, R.B. The ketogenic diet prevents steatosis and insulin resistance by reducing lipogenesis, diacylglycerol accumulation and protein kinase C activity in male rat liver. J. Physiol. 2022, 600, 4137–4151. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Cerullo, G. Investigating the Link between Ketogenic Diet, NAFLD, Mitochondria, and Oxidative Stress: A Narrative Review. Antioxidants 2023, 12, 1065. [Google Scholar] [CrossRef]
- Quek, J.; Chan, K.E.; Wong, Z.Y.; Tan, C.; Tan, B.; Lim, W.H.; Tan, D.J.H.; Tang, A.S.P.; Tay, P.; Xiao, J.; et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Sung, K.C.; Ryu, S.; Lee, J.Y.; Kim, J.Y.; Wild, S.H.; Byrne, C.D. Effect of exercise on the development of new fatty liver and the resolution of existing fatty liver. J. Hepatol. 2016, 65, 791–797. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015, 149, 367–378. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, M.; Liang, J.; He, G.; Chen, N. Ketogenic Diet Benefits to Weight Loss, Glycemic Control, and Lipid Profiles in Overweight Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trails. Int. J. Environ. Res. Public Health 2022, 19, 10429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://www.virtahealth.com/blog/ketosis-appetite-hunger (accessed on 3 December 2023).
- Malhotra, A.; Noakes, T.; Phinney, S. It is time to bust the myth of physical inactivity and obesity: You cannot outrun a bad diet. Br. J. Sports Med. 2015, 49, 967–968. [Google Scholar] [CrossRef] [PubMed]
- Doucet, E.; Imbeault, P.; St-Pierre, S.; Almeras, N.; Mauriege, P.; Richard, D.; Tremblay, A. Appetite after weight loss by energy restriction and a low-fat diet-exercise follow-up. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 906–914. [Google Scholar] [CrossRef]
- Gibson, A.A.; Seimon, R.V.; Lee, C.M.; Ayre, J.; Franklin, J.; Markovic, T.P.; Caterson, I.D.; Sainsbury, A. Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes. Rev. 2015, 16, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Nymo, S.; Coutinho, S.R.; Jørgensen, J.; Rehfeld, J.F.; Truby, H.; Kulseng, B.; Martins, C. Timeline of changes in appetite during weight loss with a ketogenic diet. Int. J. Obes. (Lond.) 2017, 41, 1224–1231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roekenes, J.; Martins, C. Ketogenic diets and appetite regulation. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 359–363. [Google Scholar] [CrossRef]
- McKenzie, A.L.; Hallberg, S.J.; Creighton, B.C.; Volk, B.M.; Link, T.M.; Abner, M.K.; Glon, R.M.; McCarter, J.P.; Volek, J.S.; Phinney, S.D. A Novel Intervention Including Individualized Nutritional Recommendations Reduces Hemoglobin A1c Level, Medication Use, and Weight in Type 2 Diabetes. JMIR Diabetes 2017, 2, e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, Y.J.; Jeon, S.M.; Shin, S. Impact of a Ketogenic Diet on Metabolic Parameters in Patients with Obesity or Overweight and with or without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 2005. [Google Scholar] [CrossRef] [PubMed]
- Castellana, M.; Conte, E.; Cignarelli, A.; Perrini, S.; Giustina, A.; Giovanella, L.; Giorgino, F.; Trimboli, P. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 5–16. [Google Scholar] [CrossRef]
- Bueno, N.; De Melo, I.; De Oliveira, S.; Da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef]
- Lustig, R.H. Fructose: It’s “alcohol without the buzz”. Adv. Nutr. 2013, 4, 226–235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, Y.; Yu, B.; Wang, Y.; Wang, B.; Tan, X.; Lu, Y.; Zhang, K.; Wang, N. Associations of Sugar-Sweetened Beverages, Artificially Sweetened Beverages, and Pure Fruit Juice with Nonalcoholic Fatty Liver Disease: Cross-sectional and Longitudinal Study. Endocr. Pract. 2023, 29, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Heyman, M.B.; Abrams, S.A. Section on Gastroenterology, Hepatology, and Nutrition; Committee on Nutrition. Fruit Juice in Infants, Children, and Adolescents: Current Recommendations. Pediatrics 2017, 139, e20170967. [Google Scholar] [CrossRef] [PubMed]
- Boulton, J.; Hashem, K.M.; Jenner, K.H.; Lloyd-Williams, F.; Bromley, H.; Capewell, S. How much sugar is hidden in drinks marketed to children? A survey of fruit juices, juice drinks and smoothies. BMJ Open 2016, 6, e010330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, S.; Li, C.; Ji, G.; Zhang, L. The Contribution of Dietary Fructose to Non-alcoholic Fatty Liver Disease. Front. Pharmacol. 2021, 12, 783393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, W.; Zhai, D.; Zhang, T.; Mudoti, N.G.; Chang, Q.; Liu, Y.; Zhao, Y.; Ding, Y.; Xia, Y. Meta-analysis of the association between major foods with added fructose and non-alcoholic fatty liver disease. Food Funct. 2023, 14, 5551–5561. [Google Scholar] [CrossRef]
- Jegatheesan, P.; De Bandt, J.P. Fructose and NAFLD: The Multifaceted Aspects of Fructose Metabolism. Nutrients 2017, 9, 230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, R.J.; Lanaspa, M.A.; Sanchez-Lozada, L.G.; Tolan, D.; Nakagawa, T.; Ishimoto, T.; Andres-Hernando, A.; Rodriguez-Iturbe, B.; Stenvinkel, P. The fructose survival hypothesis for obesity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shapiro, A.; Mu, W.; Roncal, C.; Cheng, K.Y.; Johnson, R.J.; Scarpace, P.J. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1370–R1375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tseng, T.-S.; Lin, W.-T.; Ting, P.-S.; Huang, C.-K.; Chen, P.-H.; Gonzalez, G.V.; Lin, H.-Y. Sugar-Sweetened Beverages and Artificially Sweetened Beverages Consumption and the Risk of Nonalcoholic Fatty Liver (NAFLD) and Nonalcoholic Steatohepatitis (NASH). Nutrients 2023, 15, 3997. [Google Scholar] [CrossRef]
- Walker, R.W.; Dumke, K.A.; Goran, M.I. Fructose content in popular beverages made with and without high-fructose corn syrup. Nutrition 2014, 30, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Guler, B.; Ertuglu, L.A.; Dagel, T.; Afsar, B.; Incir, S.; Baygul, A.; Covic, A.; Andres-Hernando, A.; Sánchez-Lozada, L.G.; et al. The Speed of Ingestion of a Sugary Beverage Has an Effect on the Acute Metabolic Response to Fructose. Nutrients 2021, 13, 1916. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pathak, M.P.; Pathak, K.; Saikia, R.; Gogoi, U.; Patowary, P.; Chattopadhyay, P.; Das, A. Therapeutic potential of bioactive phytoconstituents found in fruits in the treatment of non-alcoholic fatty liver disease: A comprehensive review. Heliyon 2023, 9, e15347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alami, F.; Alizadeh, M.; Shateri, K. The effect of a fruit-rich diet on liver biomarkers, insulin resistance, and lipid profile in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Scand. J. Gastroenterol. 2022, 57, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nan, F.; Liang, H.; Shu, P.; Fan, X.; Song, X.; Hou, Y.; Zhang, D. Excessive intake of sugar: An accomplice of inflammation. Front. Immunol. 2022, 13, 988481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gillespie, K.M.; Kemps, E.; White, M.J.; Bartlett, S.E. The Impact of Free Sugar on Human Health-A Narrative Review. Nutrients 2023, 15, 889. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Della Corte, K.W.; Perrar, I.; Penczynski, K.J.; Schwingshackl, L.; Herder, C.; Buyken, A.E. Effect of Dietary Sugar Intake on Biomarkers of Subclinical Inflammation: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2018, 10, 606. [Google Scholar] [CrossRef]
- O’Connor, L.; Imamura, F.; Brage, S.; Griffin, S.J.; Wareham, N.J.; Forouhi, N.G. Intakes and sources of dietary sugars and their association with metabolic and inflammatory markers. Clin. Nutr. 2018, 37, 1313–1322. [Google Scholar] [CrossRef]
- Lee, Y.A.; Friedman, S.L. Inflammatory and fibrotic mechanisms in NAFLD-Implications for new treatment strategies. J. Intern. Med. 2022, 291, 11–31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petermann-Rocha, F.; Wirth, M.D.; Boonpor, J.; Parra-Soto, S.; Zhou, Z.; Mathers, J.C.; Livingstone, K.; Forrest, E.; Pell, J.P.; Ho, F.K.; et al. Associations between an inflammatory diet index and severe non-alcoholic fatty liver disease: A prospective study of 171,544 UK Biobank participants. BMC Med. 2023, 21, 123. [Google Scholar] [CrossRef]
- Shimobayashi, M.; Albert, V.; Woelnerhanssen, B.; Frei, I.C.; Weissenberger, D.; Meyer-Gerspach, A.C.; Clement, N.; Moes, S.; Colombi, M.; Meier, J.A.; et al. Insulin resistance causes inflammation in adipose tissue. J. Clin. Investig. 2018, 128, 1538–1550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rehman, K.; Akash, M.S.H. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J. Biomed. Sci. 2016, 23, 87. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. Why 5%? An Explanation of SACN’s Recommendations about Sugars and Health. PHE Publications Gateway Number 2015193. 2015. Available online: https://www.gov.uk/government/publications/sacns-sugars-and-health-recommendations-why-5 (accessed on 9 December 2023).
- Buyken, A.E.; Goletzke, J.; Joslowski, G.; Felbick, A.; Cheng, G.; Herder, C.; Brand-Miller, J.C. Association between carbohydrate quality and inflammatory markers: Systematic review of observational and interventional studies. Am. J. Clin. Nutr. 2014, 99, 813–833. [Google Scholar] [CrossRef] [PubMed]
- Veit, M.; van Asten, R.; Olie, A.; Prinz, P. The role of dietary sugars, overweight, and obesity in type 2 diabetes mellitus: A narrative review. Eur. J. Clin. Nutr. 2022, 76, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Neuenschwander, M.; Hoffmann, G.; Buyken, A.E.; Schlesinger, S. Dietary sugars and cardiometabolic risk factors: A network meta-analysis on isocaloric substitution interventions. Am. J. Clin. Nutr. 2020, 111, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Zeraattalab-Motlagh, S.; Jabbarzadeh, B.; Hosseini, Y.; Jibril, A.T.; Shahinfar, H.; Mirrafiei, A.; Hosseini, F.; Bidar, S.S. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: A systematic review and dose-response meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2022, 116, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Anania, C.; Perla, F.M.; Olivero, F.; Pacifico, L.; Chiesa, C. Mediterranean diet and nonalcoholic fatty liver disease. World J. Gastroenterol. 2018, 24, 2083–2094. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miryan, M.; Darbandi, M.; Moradi, M.; Najafi, F.; Soleimani, D.; Pasdar, Y. Relationship between the Mediterranean diet and risk of hepatic fibrosis in patients with non-alcoholic fatty liver disease: A cross-sectional analysis of the RaNCD cohort. Front. Nutr. 2023, 10, 1062008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giraldi, L.; Miele, L.; Aleksovska, K.; Manca, F.; Leoncini, E.; Biolato, M.; Arzani, D.; Pirro, M.A.; Marrone, G.; Cefalo, C.; et al. Mediterranean diet and the prevention of non-alcoholic fatty liver disease: Results from a case-control study. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7391–7398. [Google Scholar] [CrossRef] [PubMed]
- Montemayor, S.; Mascaró, C.M.; Ugarriza, L.; Casares, M.; Llompart, I.; Abete, I.; Zulet, M.Á.; Martínez, J.A.; Tur, J.A.; Bouzas, C. Adherence to Mediterranean Diet and NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients 2022, 14, 3186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of green-Mediterranean diet on intrahepatic fat: The DIRECT PLUS randomised controlled trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Battezzati, A.; Foppiani, A.; Leone, A.; De Amicis, R.; Spadafranca, A.; Mari, A.; Bertoli, S. Acute Insulin Secretory Effects of a Classic Ketogenic Meal in Healthy Subjects: A Randomized Cross-Over Study. Nutrients 2023, 15, 1119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vallis, M.; Ryan, H.; Berard, L.; Cosson, E.; Kristensen, F.B.; Levrat-Guillen, F.; Naiditch, N.; Rabasa-Lhoret, R.; Polonsky, W. How Continuous Glucose Monitoring Can Motivate Self-management: Can Motivation Follow Behaviour? Can. J. Diabetes 2023, 47, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.M. Continuous Glucose Monitoring in Practice. J. Fam. Pract. 2023, 72 (Suppl. 6), S13–S18. [Google Scholar] [CrossRef] [PubMed]
- Martens, T.W. Continuous glucose monitoring in primary care-are we there? Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Yarizadeh, H.; Setayesh, L.; Sajjadi, S.F.; Ghodoosi, N.; Khorraminezhad, L.; Mirzaei, K. High carbohydrate intakes may predict more inflammatory status than high fat intakes in pre-menopause women with overweight or obesity: A cross-sectional study. BMC Res. Notes 2021, 14, 279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forsythe, C.E.; Phinney, S.D.; Fernandez, M.L.; Quann, E.E.; Wood, R.J.; Bibus, D.M.; Kraemer, W.J.; Feinman, R.D.; Volek, J.S. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 2008, 43, 65–77. [Google Scholar] [CrossRef]
- Antunes, M.M.; Godoy, G.; de Almeida-Souza, C.B.; da Rocha, B.A.; da Silva-Santi, L.G.; Masi, L.N.; Carbonera, F.; Visentainer, J.V.; Curi, R.; Bazotte, R.B. A high-carbohydrate diet induces greater inflammation than a high-fat diet in mouse skeletal muscle. Braz. J. Med. Biol. Res. 2020, 53, e9039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moradi-binabaj, M.; Khorasanchi, Z.; Karbasi, S.; Ferns, G.A.; Bahrami, A. Investigating the relationship between alow carbohydrate diet score andinflammatory and oxidative stressbiomarkers in female students. Endocrinol. Res. Pract. 2023, 27, 121–126. [Google Scholar] [CrossRef]
- Raygan, F.; Bahmani, F.; Kouchaki, E.; Aghadavod, E.; Sharifi, S.; Akbari, E.; Heidari, A.; Asemi, Z. Comparative effects of carbohydrate versus fat restriction on metabolic profiles, biomarkers of inflammation and oxidative stress in overweight patients with Type 2 diabetic and coronary heart disease: A randomized clinical trial. ARYA Atheroscler. 2016, 12, 266–273. [Google Scholar] [PubMed] [PubMed Central]
- Alfadda, N.A.; Aljuraiban, G.S.; Awwad, H.M.; Khaleel, M.S.; Almaghamsi, A.M.; Sherbeeni, S.M.; Alqutub, A.N.; Aldosary, A.S.; Alfadda, A.A. Higher carbohydrate intake in relation to non-alcoholic fatty liver disease in patients with type 2 diabetes. Front. Nutr. 2022, 9, 996004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raeman, R. Inflammation: The Straw That Broke the NAFLD Liver! Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1273–1274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gehrke, N.; Schattenberg, J.M. Metabolic Inflammation-A Role for Hepatic Inflammatory Pathways as Drivers of Comorbidities in Nonalcoholic Fatty Liver Disease? Gastroenterology 2020, 158, 1929–1947.e6. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Hong, W.; Lu, S.; Li, Y.; Guan, Y.; Weng, X.; Feng, Z. The NLRP3 Inflammasome in Non-Alcoholic Fatty Liver Disease and Steatohepatitis: Therapeutic Targets and Treatment. Front. Pharmacol. 2022, 13, 780496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, X.; Lin, X.; Zhang, P.; Liu, Y.; Ling, W.; Guo, H. Upregulated NLRP3 inflammasome activation is attenuated by anthocyanins in patients with nonalcoholic fatty liver disease: A case-control and an intervention study. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101843. [Google Scholar] [CrossRef] [PubMed]
- Alkhorayef, N.; Almutery, F.T.; Rasheed, Z.; Althwab, S.A.; Aljohani, A.S.M.; Alhawday, Y.A.N.; Salem, T.; Alharbi, A.M.; Wahaq, A.A.A.B.; Alharbi, F.S.; et al. Regulatory effects of ketogenic diet on the inflammatory response in obese Saudi women. J. Taibah Univ. Med. Sci. 2023, 18, 1101–1107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dupuis, N.; Curatolo, N.; Benoist, J.F.; Auvin, S. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia 2015, 56, e95–e98. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Wang, J.; Li, R.; Huang, Z.; Wang, L. Ketogenic diet ameliorates inflammation by inhibiting the NLRP3 inflammasome in osteoarthritis. Arthritis Res. Ther. 2022, 24, 113. [Google Scholar] [CrossRef]
- Pinto, A.; Bonucci, A.; Maggi, E.; Corsi, M.; Businaro, R. Anti-Oxidant and Anti-Inflammatory Activity of Ketogenic Diet: New Perspectives for Neuroprotection in Alzheimer’s Disease. Antioxidants 2018, 7, 63. [Google Scholar] [CrossRef]
- Srivastava, S.; Pawar, V.A.; Tyagi, A.; Sharma, K.P.; Kumar, V.; Shukla, S.K. Immune Modulatory Effects of Ketogenic Diet in Different Disease Conditions. Immuno 2023, 3, 1–15. [Google Scholar] [CrossRef]
- Rondanelli, M.; Perna, S.; Ilyas, Z.; Peroni, G.; Bazire, P.; Sajuox, I.; Maugeri, R.; Nichetti, M.; Gasparri, C. Effect of very low-calorie ketogenic diet in combination with omega-3 on inflammation, satiety hormones, body composition, and metabolic markers. A pilot study in class I obese subjects. Endocrine 2022, 75, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.J.; Volek, J.S.; Davis, S.R.; Dell’Ova, C.; Fernandez, M.L. Effects of a carbohydrate-restricted diet on emerging plasma markers for cardiovascular disease. Nutr. Metab. 2006, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Verde, L.; Di Lorenzo, C.; Savastano, S.; Colao, A.; Muscogiuri, G. Can the ketogenic diet improve our dreams? Effect of very low-calorie ketogenic diet (VLCKD) on sleep quality. J. Transl. Med. 2023, 21, 479. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; de Alteriis, G.; Muscogiuri, G.; Vetrani, C.; Verde, L.; Camajani, E.; Aprano, S.; Colao, A.; Savastano, S. Impact of a VeryLow-Calorie Ketogenic Diet (VLCKD) on Changes in Handgrip Strength in Women with Obesity. Nutrients 2022, 14, 4213. [Google Scholar] [CrossRef] [PubMed]
- Ruth, M.R.; Port, A.M.; Shah, M.; Bourland, A.C.; Istfan, N.W.; Nelson, K.P.; Gokce, N.; Apovian, C.M. Consuming a hypocaloric high fat low carbohydrate diet for 12 weeks lowers C-reactive protein, and raises serum adiponectin and high density lipoprotein-cholesterol in obese subjects. Metabolism 2013, 62, 1779–1787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Włodarek, D. Role of Ketogenic Diets in Neurodegenerative Diseases (Alzheimer’s Disease and Parkinson’s Disease). Nutrients 2019, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, M.; Rho, J.M.; Mattson, M.P. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res. Rev. 2009, 59, 293–315. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Neudorf, H.; Little, J.P. Impact of fasting & ketogenic interventions on the NLRP3 inflammasome: A narrative review. Biomed. J. 2023, 47, 100677. [Google Scholar] [CrossRef] [PubMed]
- Poff, A.; Kesl, S.; Koutnik, A.; Ward, N.; Ari, C.; Deblasi, J.; D’Agostino, D. Characterizing the metabolic effects of exogenous ketone supplementation—An alternative or adjuvant to the ketogenic diet. FASEB J. 2017, 31, 970–977. [Google Scholar] [CrossRef]
- Stutz, A.; Horvath, G.L.; Monks, B.G.; Latz, E. ASC speck formation as a readout for inflammasome activation. Methods Mol. Biol. 2013, 1040, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Smirne, C.; Croce, E.; Di Benedetto, D.; Cantaluppi, V.; Comi, C.; Sainaghi, P.P.; Minisini, R.; Grossini, E.; Pirisi, M. Oxidative Stress in Non-Alcoholic Fatty Liver Disease. Livers 2022, 2, 30–76. [Google Scholar] [CrossRef]
- Pezzino, S.; Sofia, M.; Mazzone, C.; Castorina, S.; Puleo, S.; Barchitta, M.; Agodi, A.; Gallo, L.; La Greca, G.; Latteri, S. Gut Microbiome in the Progression of NAFLD, NASH and Cirrhosis, and Its Connection with Biotics: A Bibliometric Study Using Dimensions Scientific Research Database. Biology 2023, 12, 662. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Min, B.H.; Ganesan, R.; Gebru, Y.A.; Sharma, S.P.; Park, E.; Won, S.M.; Jeong, J.J.; Lee, S.B.; Cha, M.G.; et al. Gut Microbiome in Non-Alcoholic Fatty Liver Disease: From Mechanisms to Therapeutic Role. Biomedicines 2022, 10, 550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Hrncir, T.; Hrncirova, L.; Kverka, M.; Hromadka, R.; Machova, V.; Trckova, E.; Kostovcikova, K.; Kralickova, P.; Krejsek, J.; Tlaskalova-Hogenova, H. Gut Microbiota and NAFLD: Pathogenetic Mechanisms, Microbiota Signatures, and Therapeutic Interventions. Microorganisms 2021, 9, 957. [Google Scholar] [CrossRef]
- Hoyles, L.; Fernández-Real, J.M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Zheng, R.D.; Sun, X.Q.; Ding, W.J.; Wang, X.Y.; Fan, J.G. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017, 25, 1054–1062. [Google Scholar] [CrossRef]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef]
- Raman, M.; Ahmed, I.; Gillevet, P.M.; Probert, C.S.; Ratcliffe, N.M.; Smith, S.; Greenwood, R.; Sikaroodi, M.; Lam, V.; Crotty, P.; et al. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2013, 11, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, B. Gut microbiota and nonalcoholic fatty liver disease. Kosin Med. J. 2023, 38, 169–175. [Google Scholar] [CrossRef]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Loomba, R. Gut microbiome, microbial metabolites and the development of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 719–720. [Google Scholar] [CrossRef] [PubMed]
- Sharpton, S.R.; Ajmera, V.; Loomba, R. Emerging Role of the Gut Microbiome in Nonalcoholic Fatty Liver Disease: From Composition to Function. Clin. Gastroenterol. Hepatol. 2019, 17, 296–306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pezzino, S.; Sofia, M.; Faletra, G.; Mazzone, C.; Litrico, G.; La Greca, G.; Latteri, S. Gut–LiverAxis and Non-Alcoholic Fatty LiverDisease: A Vicious Circle ofDysfunctions Orchestrated by theGut Microbiome. Biology 2022, 11, 1622. [Google Scholar] [CrossRef] [PubMed]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholicfatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmicreticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, D.; Stachowska, E. Niealkoholowe stłuszczeniewątroby (NAFLD)-epidemia XXI wieku. Postępy Hig. Med. Doświadczalnej 2018, 72, 659–670. [Google Scholar] [CrossRef]
- Staltner, R.; Burger, K.; Baumann, A.; Bergheim, I. Fructose: A modulator of intestinal barrier function and hepatic health? Eur. J. Nutr. 2023, 62, 3113–3124. [Google Scholar] [CrossRef]
- Fajstova, A.; Galanova, N.; Coufal, S.; Malkova, J.; Kostovcik, M.; Cermakova, M.; Pelantova, H.; Kuzma, M.; Sediva, B.; Hudcovic, T.; et al. Diet Rich in Simple Sugars Promotes Pro-Inflammatory Response via Gut Microbiota Alteration and TLR4 Signaling. Cells 2020, 9, 2701. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Özcan, E.; Lum, G.R.; Hsiao, E.Y. Interactions between the gut microbiome and ketogenic diet in refractory epilepsy. Int. Rev. Neurobiol. 2022, 167, 217–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, L.; Li, H.; Chen, G.; Yang, L.; Wang, D.; Han, H.; Zheng, G.; Wang, X.; Liang, J.; et al. Effects of ketogenic diet on the classification and functional composition of intestinal flora in children with mitochondrial epilepsy. Front. Neurol. 2023, 14, 1237255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santangelo, A.; Corsello, A.; Spolidoro, G.C.I.; Trovato, C.M.; Agostoni, C.; Orsini, A.; Milani, G.P.; Peroni, D.G. The Influence of Ketogenic Diet on Gut Microbiota: Potential Benefits, Risks and Indications. Nutrients 2023, 15, 3680. [Google Scholar] [CrossRef] [PubMed]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nandivada, P.; Fell, G.L.; Pan, A.H.; Nose, V.; Ling, P.R.; Bistrian, B.R.; Puder, M. Eucaloric Ketogenic Diet Reduces Hypoglycemia and Inflammation in Mice with Endotoxemia. Lipids 2016, 51, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Meroni, E.; Casiraghi, M.C.; Tagliabue, A.; De Giorgis, V.; Erba, D. One Month of Classic Therapeutic Ketogenic Diet Decreases Short Chain Fatty Acids Production in Epileptic Patients. Front. Nutr. 2021, 8, 613100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Q.; Wu, L.; Zhang, A.; Wu, C.; Cai, L.; Xiao, Y.; Ni, Y. Sodium butyrate alleviates fructose-induced non-alcoholic fatty liver disease by remodeling gut microbiota to promote γ-amino butyric acid production. Food Sci. Hum. Wellness 2024, 13, 961–971. [Google Scholar] [CrossRef]
- Cao, X.; Zolnikova, O.; Maslennikov, R.; Reshetova, M.; Poluektova, E.; Bogacheva, A.; Zharkova, M.; Ivashkin, V. Low Short-Chain-Fatty-Acid-Producing Activity of the Gut Microbiota Is Associated with Hypercholesterolemia and Liver Fibrosis in Patients with Metabolic-Associated (Non-Alcoholic) Fatty Liver Disease. Gastrointest. Disord. 2023, 5, 464–473. [Google Scholar] [CrossRef]
- Dai, X.; Hou, H.; Zhang, W.; Liu, T.; Li, Y.; Wang, S.; Wang, B.; Cao, H. Microbial Metabolites: Critical Regulators in NAFLD. Front. Microbiol. 2020, 11, 567654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsai, H.J.; Hung, W.C.; Hung, W.W.; Lee, Y.J.; Chen, Y.C.; Lee, C.Y.; Tsai, Y.C.; Dai, C.Y. Circulating Short-Chain Fatty Acids and Non-Alcoholic Fatty Liver Disease Severity in Patients with Type 2 Diabetes Mellitus. Nutrients 2023, 15, 1712. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rinaldi, R.; De Nucci, S.; Castellana, F.; Di Chito, M.; Giannuzzi, V.; Shahini, E.; Zupo, R.; Lampignano, L.; Piazzolla, G.; Triggiani, V.; et al. The Effects of Eight Weeks’ Very Low-Calorie Ketogenic Diet (VLCKD) on Liver Health in Subjects Affected by Overweight and Obesity. Nutrients 2023, 15, 825. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Nucci, S.; Bonfiglio, C.; Donvito, R.; Di Chito, M.; Cerabino, N.; Rinaldi, R.; Sila, A.; Shahini, E.; Giannuzzi, V.; Pesole, P.L.; et al. Effects of an Eight Week Very Low-Calorie Ketogenic Diet (VLCKD) on White Blood Cell and Platelet Counts in Relation to Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in Subjects with Overweight and Obesity. Nutrients 2023, 15, 4468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crabtree, C.D.; Kackley, M.L.; Buga, A.; Fell, B.; LaFountain, R.A.; Hyde, P.N.; Sapper, T.N.; Kraemer, W.J.; Scandling, D.; Simonetti, O.P.; et al. Comparison of Ketogenic Diets with and without Ketone Salts versus a Low-Fat Diet: Liver Fat Responses in Overweight Adults. Nutrients 2021, 13, 966. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cunha, G.M.; Guzman, G.; Correa De Mello, L.L.; Trein, B.; Spina, L.; Bussade, I.; Marques Prata, J.; Sajoux, I.; Countinho, W. Efficacy of a 2-Month Very Low-Calorie Ketogenic Diet (VLCKD) Compared to a Standard Low-Calorie Diet in Reducing Visceral and Liver Fat Accumulation in Patients with Obesity. Front. Endocrinol. 2020, 11, 607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watanabe, M.; Risi, R.; Camajani, E.; Contini, S.; Persichetti, A.; Tuccinardi, D.; Ernesti, I.; Mariani, S.; Lubrano, C.; Genco, A.; et al. Baseline HOMA IR and Circulating FGF21 Levels Predict NAFLD Improvement in Patients Undergoing a Low Carbohydrate Dietary Intervention for Weight Loss: A Prospective Observational Pilot Study. Nutrients 2020, 12, 2141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Abbondanza, M.; Ministrini, S.; Pucci, G.; Nulli Migliola, E.; Martorelli, E.E.; Gandolfo, V.; Siepi, D.; Lupattelli, G.; Vaudo, G. Very Low-Carbohydrate Ketogenic Diet for the Treatment of Severe Obesity and Associated Non-Alcoholic Fatty Liver Disease: The Role of Sex Differences. Nutrients 2020, 12, 2748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vilar-Gomez, E.; Athinarayanan, S.J.; Adams, R.N.; Hallberg, S.J.; Bhanpuri, N.H.; McKenzie, A.L.; Campbell, W.W.; McCarter, J.P.; Phinney, S.D.; Volek, J.S.; et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: An open-label, non-randomised controlled study. BMJ Open 2019, 9, e023597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arab, J.P.; Díaz, L.A.; Dirchwolf, M.; Mark, H.E.; Lazarus, J.V.; Vaughan, E.; Méndez-Sánchez, N.; Oliveira, C.P.; Gadano, A.; Arrese, M. NAFLD: Challenges and opportunities to address the public health problem in Latin America. Ann. Hepatol. 2021, 24, 100359. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J. Putting non-alcoholic fatty liver disease on the radar for primary care physicians: How well are we doing? BMC Med. 2018, 16, 148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bergqvist, C.J.; Skoien, R.; Horsfall, L.; Clouston, A.D.; Jonsson, J.R.; Powell, E.E. Awareness and opinions of non-alcoholic fatty liver disease by hospital specialists. Intern. Med. J. 2013, 43, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.J.; Banh, X.; Horsfall, L.U.; Hayward, K.L.; Hossain, F.; Johnson, T.; Stuart, K.A.; Brown, N.N.; Saad, N.; Clouston, A.; et al. Underappreciation of non-alcoholic fatty liver disease by primary care clinicians: Limited awareness of surrogate markers of fibrosis. Intern. Med. J. 2018, 48, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Blais, P.; Husain, N.; Kramer, J.R.; Kowalkowski, M.; El-Serag, H.; Kanwal, F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am. J. Gastroenterol. 2015, 110, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.G.; Soriano, C.; Schreibman, I.; Rivas, G.; Hummer, B.; Yoo, E.; Schmitz, K.; Sciamanna, C. Breaking Down Barriers to Physical Activity in Patients with Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2021, 66, 3604–3611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kwon, O.Y.; Kim, S.U.; Ahn, S.H.; Jang, Y. Self-Management and Associated Factors among Patients with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 20, 667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fortunka, K.D. Health education as an effective element of prevention carried out by medical staff-review work. J. Ofeducation Health Sport 2020, 10, 137–142. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, J.A.; Olarte-Arias, Y.A.; Glasserman-Morales, L.D. Educational Processes for Health and Disease Self-Management in Public Health: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 6448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hughes, M.; Addala, A.; Buckingham, B. Digital Technology for Diabetes. N. Engl. J. Med. 2023, 389, 2076–2086. [Google Scholar] [CrossRef]
- Kosmalski, M.; Śliwińska, A.; Drzewoski, J. Non-Alcoholic FattyLiver Disease or Type 2 DiabetesMellitus—The Chicken or the Egg Dilemma. Biomedicines 2023, 11, 1097. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Shang, T.; Koliwad, S.K.; Klonoff, D.C. Continuous Ketone Monitoring: A New Paradigm for Physiologic Monitoring. J. Diabetes Sci. Technol. 2021, 15, 775–780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toro-Ramos, T.; Michaelides, A.; Anton, M.; Karim, Z.; Kang-Oh, L.; Argyrou, C.; Loukaidou, E.; Charitou, M.M.; Sze, W.; Miller, J.D. Mobile delivery of the diabetes prevention program in people with prediabetes: Randomized controlled trial. JMIR mHealth uHealth 2020, 8, e17842. [Google Scholar] [CrossRef] [PubMed]
- Faust, A.; Stine, J.G. Time to Step It Up: Mobile Health Intervention for Lifestyle Modification in Patients with Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2022, 67, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Johal, J.; Ong, K.W.; Han, C.Y.; Chan, Y.H.; Lee, Y.M.; Loo, W.M. Lifestyle Intervention Enabled by Mobile Technology on Weight Loss in Patients with Nonalcoholic Fatty Liver Disease: Randomized Controlled Trial. JMIR mHealth uHealth 2020, 8, e14802. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tincopa, M.A.; Lyden, A.; Wong, J.; Jackson, E.A.; Richardson, C.; Lok, A.S. Impact of a Pilot Structured Mobile Technology Based Lifestyle Intervention for Patients with Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2022, 67, 481–491. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valinskas, S.; Aleknavicius, K.; Jonusas, J. KetoCycle mobile app for ketogenic diet: A retrospective study of weight loss and engagement. BMC Nutr. 2022, 8, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falkenhain, K.; Locke, S.R.; Lowe, D.A.; Lee, T.; Singer, J.; Weiss, E.J.; Little, J.P. Use of an mHealth Ketogenic Diet App Intervention and User Behaviors Associated with Weight Loss in Adults with Overweight or Obesity: Secondary Analysis of a Randomized Clinical Trial. JMIR mHealth uHealth 2022, 10, e33940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shalabi, H.; Alotaibi, A.; Alqahtani, A.; Alattas, H.; Alghamdi, Z. Ketogenic Diets: Side Effects, Attitude, and Quality of Life. Cureus 2021, 13, e20390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bostock, E.C.S.; Kirkby, K.C.; Taylor, B.V.; Hawrelak, J.A. Consumer Reports of “Keto Flu” Associated with the Ketogenic Diet. Front. Nutr. 2020, 7, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).