Cognition in Patients with Spinocerebellar Ataxia 1 (SCA1) and 2 (SCA2): A Neurophysiological and Neuropsychological Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
Clinical Data
2.3. Neuropsychological Testing
- Mini-mental State Examination (MMSE): Test for brief screening of the cognitive status, where a score below the cut-off of 24 indicates cognitive impairment [20].
- Frontal Assessment Battery (FAB): Widely used to assess executive functions. It consists of 6 subtests exploring conceptualization, mental flexibility, motor programming, sensitivity to interference, inhibitory control (go–no-go), and environmental autonomy [21].
- Verbal fluency test (F-A-S letters): This a lexical retrieval test in which the subject is invited to produce as many words as possible using phonemic criteria in one minute; it requires processing speed, and is considered to assess executive functions [22].
- Trail Making Test (TMT) A-B: In the TMT-A, it is required to connect twenty-five numbered circles with a direct line in ascending order; this part assesses selective attention and motor speed. In TMT-B, it is required to alternate thirteen numbers and letters, also implicating attentional shifting. The B-A score is considered as a measure of executive functions [23].
- Raven Colored Progressive Matrices (RCPMs): In this test, it is required to choose, from six items, the missing element that completes a matrix 2 × 2. It assesses non-verbal reasoning ability and visuospatial processing skills [24].
- Stroop Test: This consists of three parts which demand word reading, color naming, and color–word reading. This involves many cognitive functions such as selective attention, sensitivity to interference, and inhibitory control; thus, it is also considered a test for executive functions [25].
- Rey–Osterrieth Complex Figure (ROCF): This consists of two different parts: the first investigates the ability of construction practice and visuospatial planning, and the second explores the visual memory by the recall of the model after fifteen minutes [26].
- Prose memory test (Babcock’s short tale—BST): A short story has to be stored and recalled after ten minutes. This test measures the verbal-episodic memory [27].
- Emotion Attribution Task (EAT): This test demands the recognition of the emotions of a subject in 58 short stories which can elicit basic emotions (i.e., happiness, sadness, anger, fear, envy, embarrassment, and disgust). The test is considered to assess social cognition [28].
- Visual Analogue Test for Anosognosia for motor impairment (VATA-m): A questionnaire that compares patient self-evaluation to his/her caregiver’s evaluation of the patient’s abilities in a series of motor tasks [29].
2.4. Electrophysiological Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whaley, N.R.; Fujioka, S.; Wszolek, Z.K. Autosomal dominant cerebellar ataxia type I: A review of the phenotypic and genotypic characteristics. Orphanet J. Rare Dis. 2011, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Donato, S.D.; Mariotti, C.; Taroni, F. Spinocerebellar ataxia type 1. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 103, pp. 399–421. [Google Scholar]
- Schöls, L.; Bauer, P.; Schmidt, T.; Schulte, T.; Riess, O. Autosomal dominant cerebellar ataxias: Clinical features, genetics, and pathogenesis. Lancet Neurol. 2004, 3, 291–304. [Google Scholar] [CrossRef]
- Sokolovsky, N.; Cook, A.; Hunt, H.; Giunti, P.; Cipolotti, L. A preliminary characterisation of cognition and social cognition in spinocerebellar ataxia types 2, 1, and 7. Behav. Neurol. 2010, 23, 17–29. [Google Scholar] [CrossRef]
- Bürk, K.; Globas, C.; Bösch, S.; Klockgether, T.; Zühlke, C.; Daum, I.; Dichgans, J. Cognitive deficits in spinocerebellar ataxia type 1, 2, and 3. J. Neurol. 2003, 250, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, A.; Cook, A.; Hunt, H.; Adams, M.E.; Cipolotti, L.; Giunti, P. A longitudinal investigation into cognition and disease progression in spinocerebellar ataxia types 1, 2, 3, 6, and 7. Orphanet J. Rare Dis. 2016, 11, 82. [Google Scholar] [CrossRef]
- Klinke, I.; Minnerop, M.; Schmitz-Hübsch, T.; Hendriks, M.; Klockgether, T.; Wüllner, U.; Helmstaedter, C. Neuropsychological features of patients with spinocerebellar ataxia (SCA) types 1, 2, 3, and 6. Cerebellum 2010, 9, 433–442. [Google Scholar] [CrossRef]
- Ma, J.; Wu, C.; Lei, J.; Zhang, X. Cognitive impairments in patients with spinocerebellar ataxia types 1, 2 and 3 are positively correlated to the clinical severity of ataxia symptoms. Int. J. Clin. Exp. Med. 2014, 7, 5765–5771. [Google Scholar]
- Gigante, A.F.; Lelli, G.; Romano, R.; Pellicciari, R.; Di Candia, A.; Mancino, P.V.; Pau, M.; Fiore, P.; Defazio, G. The Relationships Between Ataxia and Cognition in Spinocerebellar Ataxia Type 2. Cerebellum 2020, 19, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Fancellu, R.; Paridi, D.; Tomasello, C.; Panzeri, M.; Castaldo, A.; Genitrini, S.; Soliveri, P.; Girotti, F. Longitudinal study of cognitive and psychiatric functions in spinocerebellar ataxia types 1 and 2. J. Neurol. 2013, 260, 3134–3143. [Google Scholar] [CrossRef]
- Orsi, L.; D’Agata, F.; Caroppo, P.; Franco, A.; Caglio, M.M.; Avidano, F.; Manzone, C.; Mortara, P. Neuropsychological picture of 33 spinocerebellar ataxia cases. J. Clin. Exp. Neuropsychol. 2011, 33, 315–325. [Google Scholar] [CrossRef]
- Van Dinteren, R.; Arns, M.; Jongsma, M.L.; Kessels, R.P. P300 development across the lifespan: A systematic review and meta-analysis. PLoS ONE 2014, 9, e87347. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Solomon, E.S.; Haase, L.; Wang, M.; Morgan, C.D. Olfaction in aging and Alzheimer’s disease: Event-related potentials to a cross-modal odor-recognition memory task discriminate ApoE epsilon4+ and ApoE epsilon 4- individuals. Ann. N. Y. Acad. Sci. 2009, 1170, 647–657. [Google Scholar] [CrossRef]
- Gilbert, P.E.; Murphy, C. The effect of the ApoE epsilon4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer’s disease, probable Alzheimer’s disease, and healthy elderly controls. J. Clin. Exp. Neuropsychol. 2004, 26, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Golob, E.J.; Ringman, J.M.; Irimajiri, R.; Bright, S.; Schaffer, B.; Medina, L.D.; Starr, A. Cortical event-related potentials in preclinical familial Alzheimer disease. Neurology 2009, 73, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Contaldi, E.; Sensi, M.; Colucci, F.; Capone, J.G.; Braccia, A.; Nocilla, M.R.; Diozzi, E.; Contini, E.; Pelizzari, A.C.; Tugnoli, V. Electrophysiological and neuropsychological assessment of cognition in spinocerebellar ataxia type 1 patients: A pilot study. Neurol. Sci. 2023, 44, 1597–1606. [Google Scholar] [CrossRef]
- Rodríguez-Labrada, R.; Velázquez-Pérez, L.; Ortega-Sánchez, R.; Peña-Acosta, A.; Vázquez-Mojena, Y.; Canales-Ochoa, N.; Medrano-Montero, J.; Torres-Vega, R.; González-Zaldivar, Y. Insights into cognitive decline in spinocerebellar Ataxia type 2: A P300 event-related brain potential study. Cerebellum Ataxias 2019, 6, 3. [Google Scholar] [CrossRef]
- Kremlacek, J.; Valis, M.; Masopust, J.; Urban, A.; Zumrova, A.; Talab, R.; Kuba, M.; Kubova, Z.; Langrova, J. An electrophysiological study of visual processing in spinocerebellar ataxia type 2 (SCA2). Cerebellum 2011, 10, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Hübsch, T.; du Montcel, S.T.; Baliko, L.; Berciano, J.; Boesch, S.; Depondt, C.; Giunti, P.; Globas, C.; Infante, J.; Kang, J.S.; et al. Scale for the assessment and rating of ataxia: Development of a new clinical scale. Neurology 2006, 66, 1717–1720. [Google Scholar] [CrossRef]
- Magni, E.; Binetti, G.; Bianchetti, A.; Rozzini, R.; Trabucchi, M. Mini-Mental State Examination: A normative study in Italian elderly population. Eur. J. Neurol. 1996, 3, 198–202. [Google Scholar] [CrossRef]
- Appollonio, I.; Leone, M.; Isella, V.; Piamarta, F.; Consoli, T.; Villa, M.L.; Forapani, E.; Russo, A.; Nichelli, P. The Frontal Assessment Battery (FAB): Normative values in an Italian population sample. Neurol. Sci. 2005, 26, 108–116. [Google Scholar] [CrossRef]
- Carlesimo, G.A.; Caltagirone, C.; Gainotti, G. The Mental Deterioration Battery: Normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur. Neurol. 1996, 36, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Giovagnoli, A.R.; Del Pesce, M.; Mascheroni, S.; Simoncelli, M.; Laiacona, M.; Capitani, E. Trail making test: Normative values from 287 normal adult controls. Ital. J. Neurol. Sci. 1996, 17, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.; Capitani, E.; Laiacona, M. Raven’s coloured progressive matrices: Normative values on 305 adult normal controls. Funct. Neurol. 1987, 2, 189–194. [Google Scholar] [PubMed]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. A short version of the Stroop test: Normative data in an Italian population sample. Nuova Riv. Neurol. 2002, 12, 111–115. [Google Scholar]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. Rey-Osterrieth complex figure: Normative values in an Italian population sample. Neurol. Sci. 2002, 22, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Spinnler, H.; Tognoni, G. Standardizzazione e taratura italiana di test neuropsicologici. Ital. J. Neurol. Sci. 1987, 6 (Suppl. 8), 1–120. (In Italian) [Google Scholar]
- Prior, M.; Marchi, S.; Sartori, G. Cognizione Sociale e Comportamento. Uno Strumento per la Misurazione; Upsel Domeneghini Editore: Padova, Italy, 2003; Volume 1. (In Italian) [Google Scholar]
- Della Sala, S.; Cocchini, G.; Beschin, N.; Cameron, A. VATA-m: Visual-Analogue Test assessing Anosognosia for motor impairment. Clin. Neuropsychol. 2009, 23, 406–427. [Google Scholar] [CrossRef] [PubMed]
- Remijn, G.B.; Hasuo, E.; Fujihira, H.; Morimoto, S. An introduction to the measurement of auditory event-related potentials (ERPs). Acoust. Sci. Technol. 2014, 35, 229–242. [Google Scholar] [CrossRef]
- Polich, J. Clinical application of the P300 event-related brain potential. Phys. Med. Rehabil. Clin. N. Am. 2004, 15, 133–161. [Google Scholar] [CrossRef]
- Patel, S.H.; Azzam, P.N. Characterization of N200 and P300: Selected studies of the Event-Related Potential. Int. J. Med. Sci. 2005, 2, 147–154. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Sherman, J.C. The cerebellar cognitive affective syndrome. Brain 1998, 121 Pt 4, 561–579. [Google Scholar] [CrossRef]
- Van Harskamp, N.J.; Rudge, P.; Cipolotti, L. Cognitive and social impairments in patients with superficial siderosis. Brain 2005, 128 Pt 5, 1082–1092. [Google Scholar] [CrossRef]
- Guell, X.; Gabrieli, J.D.E.; Schmahmann, J.D. Triple representation of language, working memory, social and emotion processing in the cerebellum: Convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage 2018, 172, 437–449. [Google Scholar] [CrossRef]
- Higuchi, S.; Imamizu, H.; Kawato, M. Cerebellar activity evoked by common tool-use execution and imagery tasks: An fMRI study. Cortex 2007, 43, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Ito, M. Movement and thought: Identical control mechanisms by the cerebellum. Trends Neurosci. 1993, 16, 448–450, discussion 453–454. [Google Scholar] [CrossRef]
- Leiner, H.C.; Leiner, A.L.; Dow, R.S. Reappraising the cerebellum: What does the hindbrain contribute to the forebrain? Behav. Neurosci. 1989, 103, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D.; Guell, X.; Stoodley, C.J.; Halko, M.A. The Theory and Neuroscience of Cerebellar Cognition. Annu. Rev. Neurosci. 2019, 42, 337–364. [Google Scholar] [CrossRef] [PubMed]
- Vandervert, L. The prominent role of the cerebellum in the learning, origin and advancement of culture. Cerebellum Ataxias 2016, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Metoki, A.; Wang, Y.; Olson, I.R. The Social Cerebellum: A Large-Scale Investigation of Functional and Structural Specificity and Connectivity. Cereb. Cortex 2022, 32, 987–1003. [Google Scholar] [CrossRef]
- Tamaš, O.; Kostić, M.; Kačar, A.; Stefanova, E.; Ðokić, B.S.; Stanisavljević, D.; Milovanović, A.; Ðorđević, M.; Glumbić, N.; Dragašević-Mišković, N. Social Cognition in Patients with Cerebellar Neurodegenerative Disorders. Front. Syst. Neurosci. 2021, 15, 664223. [Google Scholar] [CrossRef]
- Kawai, Y.; Suenaga, M.; Watanabe, H.; Sobue, G. Cognitive impairment in spinocerebellar degeneration. Eur. Neurol. 2009, 61, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D. The cerebellum and cognition. Neurosci. Lett. 2019, 688, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Van Overwalle, F.; Baetens, K.; Mariën, P.; Vandekerckhove, M. Cerebellar areas dedicated to social cognition? A comparison of meta-analytic and connectivity results. Soc. Neurosci. 2015, 10, 337–344. [Google Scholar] [PubMed]
- Le Pira, F.; Zappalà, G.; Saponara, R.; Domina, E.; Restivo, D.; Reggio, E.; Nicoletti, A.; Giuffrida, S. Cognitive findings in spinocerebellar ataxia type 2: Relationship to genetic and clinical variables. J. Neurol. Sci. 2002, 201, 53–57. [Google Scholar] [CrossRef]
- Le Pira, F.; Giuffrida, S.; Maci, T.; Marturano, L.; Tarantello, R.; Zappalà, G.; Nicoletti, A.; Zappia, M. Dissociation between motor and cognitive impairments in SCA2: Evidence from a follow-up study. J. Neurol. 2007, 254, 1455–1456. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, M.; Manto, M. Cerebellar and afferent ataxias. Contin. Lifelong Learn. Neurol. 2013, 19, 1312–1343. [Google Scholar] [CrossRef] [PubMed]
- Van Prooije, T.; Knuijt, S.; Oostveen, J.; Kapteijns, K.; Vogel, A.P.; van de Warrenburg, B. Perceptual and Acoustic Analysis of Speech in Spinocerebellar ataxia Type 1. Cerebellum 2024, 23, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Clausi, S.; Olivito, G.; Siciliano, L.; Lupo, M.; Bozzali, M.; Masciullo, M.; Molinari, M.; Romano, S.; Leggio, M. The neurobiological underpinning of the social cognition impairments in patients with spinocerebellar ataxia type 2. Cortex 2021, 138, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Giocondo, F.; Curcio, G. Spinocerebellar ataxia: A critical review of cognitive and socio-cognitive deficits. Int. J. Neurosci. 2018, 128, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Kish, S.J.; el-Awar, M.; Schut, L.; Leach, L.; Oscar-Berman, M.; Freedman, M. Cognitive deficits in olivopontocerebellar atrophy: Implications for the cholinergic hypothesis of Alzheimer’s dementia. Ann. Neurol. 1988, 24, 200–206. [Google Scholar] [CrossRef]
- Van den Stock, J.; Vandenbulcke, M.; Zhu, Q.; Hadjikhani, N.; de Gelder, B. Developmental prosopagnosia in a patient with hypoplasia of the vermis cerebelli. Neurology 2012, 78, 1700–1702. [Google Scholar] [CrossRef] [PubMed]
- Guerrier, L.; Le Men, J.; Gane, A.; Planton, M.; Salabert, A.S.; Payoux, P.; Dumas, H.; Bonneville, F.; Péran, P.; Pariente, J. Involvement of the Cingulate Cortex in Anosognosia: A Multimodal Neuroimaging Study in Alzheimer’s Disease Patients. J. Alzheimer’s Dis. 2018, 65, 443–453. [Google Scholar] [CrossRef]
- Dodich, A.; Cerami, C.; Canessa, N.; Crespi, C.; Iannaccone, S.; Marcone, A.; Realmuto, S.; Lettieri, G.; Perani, D.; Cappa, S.F. A novel task assessing intention and emotion attribution: Italian standardization and normative data of the Story-based Empathy Task. Neurol. Sci. 2015, 36, 1907–1912. [Google Scholar] [CrossRef]
- Velázquez-Pérez, L.; Rodríguez-Labrada, R.; González-Garcés, Y.; Vázquez-Mojena, Y.; Pérez-Rodríguez, R.; Ziemann, U. Neurophysiological features in spinocerebellar ataxia type 2: Prospects for novel biomarkers. Clin. Neurophysiol. 2022, 135, 1–12. [Google Scholar] [CrossRef]
- Koziol, L.F.; Budding, D.; Andreasen, N.; D’Arrigo, S.; Bulgheroni, S.; Imamizu, H.; Ito, M.; Manto, M.; Marvel, C.; Parker, K.; et al. Consensus paper: The cerebellum’s role in movement and cognition. Cerebellum 2014, 13, 151–177. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Chan, S.H.; Khan, S.; Schneider, A.; Nanakul, R.; Teichholtz, S.; Niu, Y.Q.; Seritan, A.; Tassone, F.; Grigsby, J.; et al. Neural substrates of executive dysfunction in fragile X-associated tremor/ataxia syndrome (FXTAS): A brain potential study. Cereb. Cortex 2013, 23, 2657–2666. [Google Scholar] [CrossRef] [PubMed]
- Annanmaki, T.; Palmu, K.; Murros, K.; Partanen, J. Altered N100-potential associates with working memory impairment in Parkinson’s disease. J. Neural Transm. 2017, 124, 1197–1203. [Google Scholar] [CrossRef]
- Tachibana, H.; Aragane, K.; Sugita, M. Event-related potentials in patients with cerebellar degeneration: Electrophysiological evidence for cognitive impairment. Brain Res. Cogn. Brain Res. 1995, 2, 173–180. [Google Scholar] [CrossRef]
- Globas, C.; du Montcel, S.T.; Baliko, L.; Boesch, S.; Depondt, C.; DiDonato, S.; Durr, A.; Filla, A.; Klockgether, T.; Mariotti, C.; et al. Early symptoms in spinocerebellar ataxia type 1, 2, 3, and 6. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 2232–2238. [Google Scholar] [CrossRef]
| SCA1 | SCA2 | p | |
|---|---|---|---|
| Number of participants | 16 | 18 | |
| Sex (males/females) | 9/7 | 8/10 | |
| Age (years), mean (SD) | 47.69 (8.16) | 44.77 (12.47) | ns |
| Educational level (years), mean (SD) | 12.5 (3.03) | 12.6 (2.3) | ns |
| Disease duration (years), mean (SD) | 6.47 (3.62) | 9.44 (5.87) | =0.06 |
| Age at onset (years), mean (SD) | 41.27 (8.50) | 35.61 (10.49) | <0.05 |
| Parental inheritance (Paternal/maternal) | 4/12 | 6/12 | |
| Number of repeats expanded allele, mean (SD) | 47.73 (6.44) | 40.5 (5.16) | |
| Symptom onset (N) | |||
| 13 | 10 | |
| 2 | 2 | |
| 1 | 0 | |
| 0 | 6 | |
| Comorbidities (N) | |||
| 0 | 1 | |
| 1 | 2 | |
| 1 | 0 | |
| 1 | 2 | |
| 1 (Ovarian) | 0 | |
| 1 (Hashimoto thyroiditis) | 0 |
| Test (Pathological Cut-Off) | SCA1 a | SCA2 a | p a | SCA1 b | SCA2 b | p b | SCA1 c | SCA2 c | p c |
|---|---|---|---|---|---|---|---|---|---|
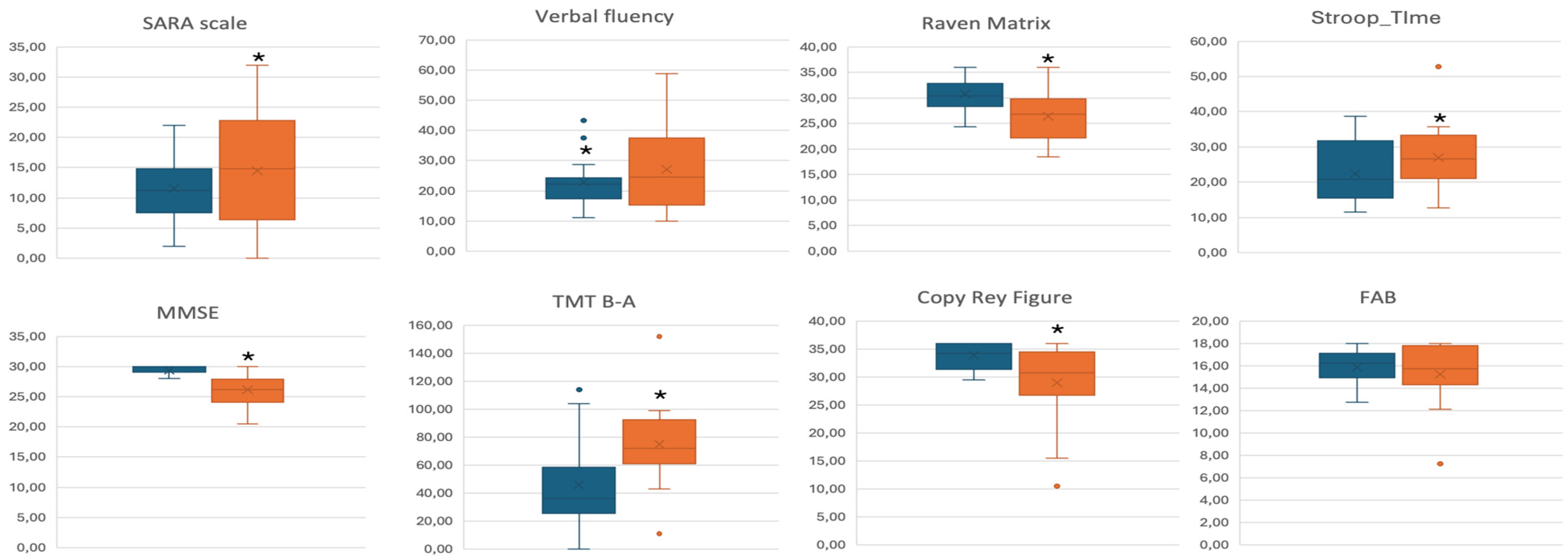
| SARA | 11.53 (5.13) | 14.44 (9.26) | ns | 12.59 (1.74) | 13.50 (1.64) | <0.05 | 12.49 (1.80) | 13.59 (1.70) | <0.05 |
| MMSE (<24) | 29.33 (0.82) | 26.16 (2.80) | <0.01 | 29.11 (0.52) | 26.37 (0.50) | <0.01 | 29.01 (0.52) | 26.47 (0.50) | <0.01 |
| FAB (<13.4) | 15.79 (1.37) | 15.23 (2.79) | ns | 15.58 (0.53) | 15.50 (0.52) | ns | 15.40 (0.51) | 15.69 (0.50) | ns |
| Verbal Fluency (<17.3) | 22.90 (8.46) | 27.09 (8.46) | ns | 22.58 (3.00) | 27.40 (2.90) | ns | 21.23 (2.70) | 28.65 (2.61) | <0.05 |
| TMT A (>94) | 68.93 (28.04) | 116.31 (58.16) | =0.01 | 75.98 (11.50) | 110.14 (10.72) | <0.01 | 74.15 (11.71) | 102.79 (12.10) | <0.05 |
| TMT B (>187) | 121.08 (37.0) | 190.42 (65.30) | <0.01 | 123.51 (14.31) | 187.78 (14.90) | <0.01 | 125.90 (13.64) | 169.23 (14.93) | <0.01 |
| TMT B_A (>187) | 45.93 (31.14) | 74.92 (33.84) | <0.05 | 45.66 (8.85) | 75.23 (9.56) | <0.01 | 48.19 (6.66) | 71.47 (12.20) | <0.01 |
| Stroop Test Time (>36.92) | 21.57 (8.52) | 26.94 (9.76) | ns | 22.56 (2.59) | 26.84 (2.41) | ns | 23.75 (2.32) | 25.81 (2.16) | <0.05 |
| Raven’s Matrices (<18.96) | 30.75 (3.29) | 26.44 (4.86) | <0.01 | 30.55 (1.10) | 26.52 (1.06) | <0.05 | 30.02 (0.96) | 27.12 (0.93) | <0.01 |
| Rey’s Figure Copy (<28.53) | 33.79 (2.43) | 28.97 (7.37) | <0.05 | 32.85 (1.47) | 29.74 (1.32) | <0.01 | 33.09 (1.53) | 29.53 (1.37) | <0.05 |
| Rey’s Figure Recall (<9.46) | 13.16 (5.47) | 10.57 (5.64) | ns | 13.07 (1.60) | 10.64 (1.49) | ns | 12.17 (1.50) | 11.43 (1.29) | ns |
| Prose memory test (Babcock’s tale) (<8.2) | 8.87 (3.37) | 12.45 (14.54) | ns | 8.11 (2.76) | 13.16 (2.67) | ns | 7.97 (2.85) | 13.30 (2.75) | ns |
| Emotions Test (<44.19) | 48.83 (8.49) | 44.25 (7.80) | ns | 47.82 (2.22) | 45.01 (1.91) | <0.05 | 47.89 (2.29) | 44.96 (1.97) | <0.05 |
| VATA-m | 6.00 (5.16) | 1.60 (3.25) | <0.05 | 6.20 (1.23) | 1.43 (1.14) | <0.05 | 6.03 (1.25) | 1.58 (1.15) | =0.057 |
| SCA1 a | SCA2 a | pa | SCA1 b | SCA2 b | pb | SCA1 c | SCA2 c | pc | |
|---|---|---|---|---|---|---|---|---|---|
| P300 | |||||||||
| Latency | 390.18 (58.32) | 386.11 (37.94) | 0.814 | 394.82 (11.94) | 381.99 (1.23) | 0.179 | 393.82 (12.40) | 382.88 (11.63) | 0.318 |
| Amplitude | 3.99 (4.36) | 4.26 (3.78) | 0.849 | 4.06 (1.03) | 4.19 (0.97) | 0.699 | 3.48 (0.98) | 4.71 (0.91) | 0.073 |
| N100 | |||||||||
| Latency | 101.32 (6.32) | 101.78 (11.31) | 0.887 | 101.31 (2.37) | 101.78 (2.24) | 0.990 | 101.45 (2.48) | 101.66 (2.33) | 0.994 |
| Amplitude | 6.04 (2.51) | 7.05 (3.24) | 0.324 | 6.18 (0.68) | 6.92 (0.64) | 0.043 | 5.66 (0.58) | 7.38 (0.55) | 0.0003 |
| N200 | |||||||||
| Latency | 260.25 (41.32) | 219.61 (19.30) | 0.002 | 261.18 (7.82) | 218.79 (7.37) | 0.001 | 263.49 (7.96) | 216.73 (7.48) | 0.002 |
| Amplitude | 3.61 (1.93) | 4.94 (3.19) | 0.155 | 3.66 (0.67) | 4.90 (0.63) | 0.239 | 3.42 (0.68) | 5.11 (0.64) | 0.163 |
| P300 Latency | P300 Amplitude | N200 Latency | N200 Amplitude | N100 Latency | N100 Amplitude | SARA | Disease Duration | ||
|---|---|---|---|---|---|---|---|---|---|
| MMSE | Pearson’s Coefficient | −0.171 | 0.047 | 0.322 | 0.707 | 0.026 | 0.169 | −0.370 | −0.517 |
| Sig. | 0.527 | 0.864 | 0.224 | 0.002 | 0.924 | 0.532 | 0.158 | 0.040 | |
| FAB | Pearson’s Coefficient | −0.029 | 0.144 | −0.086 | 0.520 | 0.106 | 0.346 | −0.495 | −0.548 |
| Sig. | 0.916 | 0.593 | 0.750 | 0.039 | 0.696 | 0.189 | 0.051 | 0.028 | |
| Verbal Fluency | Pearson’s Coefficient | 0.094 | −0.201 | −0.274 | −0.171 | −0.030 | 0.093 | −0.337 | −0.100 |
| Sig. | 0.729 | 0.456 | 0.305 | 0.527 | 0.911 | 0.731 | 0.202 | 0.713 | |
| Stroop_Time | Pearson’s Coefficient | −0.149 | 0.056 | 0.119 | 0.495 | −0.076 | −0.221 | −0.224 | −0.066 |
| Sig. | 0.581 | 0.837 | 0.661 | 0.051 | 0.781 | 0.411 | 0.404 | 0.808 | |
| Raven_Matrix | Pearson’s Coefficient | 0.070 | 0.047 | −0.130 | 0.272 | −0.265 | −0.057 | −0.270 | −0.244 |
| Sig. | 0.796 | 0.862 | 0.632 | 0.308 | 0.321 | 0.834 | 0.312 | 0.362 | |
| Copy_Rey_ Figure | Pearson’s Coefficient | −0.160 | 0.412 | −0.367 | 0.192 | −0.060 | 0.317 | −0.621 | −0.486 |
| Sig. | 0.554 | 0.113 | 0.162 | 0.492 | 0.826 | 0.231 | 0.010 | 0.056 | |
| Recall_Rey_ Figure | Pearson’s Coefficient | 0.310 | −0.243 | −0.104 | 0.029 | −0.021 | −0.131 | −0.044 | 0.176 |
| Sig. | 0.262 | 0.382 | 0.711 | 0.916 | 0.940 | 0.643 | 0.877 | 0.531 | |
| Prose_Memory_Test | Pearson’s Coefficient | −0.424 | 0.605 | −0.296 | 0.493 | −0.021 | 0.368 | −0.400 | −0.383 |
| Sig. | 0.102 | 0.053 | 0.266 | 0.053 | 0.937 | 0.161 | 0.125 | 0.143 | |
| Emotion_test | Pearson’s Coefficient | −0.376 | 0.472 | 0.077 | −0.119 | 0.000 | 0.522 | −0.355 | −0.778 |
| Sig. | 0.151 | 0.065 | 0.776 | 0.673 | 1.000 | 0.038 | 0.177 | <0.001 | |
| VATAm | Pearson’s Coefficient | 0.155 | −0.190 | −0.212 | −0.791 | 0.341 | 0.130 | 0.193 | 0.295 |
| Sig. | 0.582 | 0.497 | 0.449 | <0.001 | 0.214 | 0.644 | 0.492 | 0.286 | |
| TMT_A | Pearson’s Coefficient | 0.228 | −0.329 | 0.215 | −0.143 | 0.373 | −0.065 | 0.656 | 0.579 |
| Sig. | 0.395 | 0.213 | 0.423 | 0.598 | 0.155 | 0.811 | 0.006 | 0.019 | |
| TMT_B | Pearson’s Coefficient | −0.091 | 0.047 | 0.225 | 0.237 | 0.627 | 0.363 | 0.474 | 0.225 |
| Sig. | 0.780 | 0.885 | 0.482 | 0.458 | 0.029 | 0.246 | 0.119 | 0.481 | |
| TMT_B-A | Pearson’s Coefficient | 0.010 | 0.429 | −0.274 | 0.351 | 0.365 | 0.037 | −0.088 | 0.115 |
| Sig. | 0.976 | 0.164 | 0.388 | 0.263 | 0.243 | 0.909 | 0.787 | 0.722 | |
| SARA | Pearson’s Coefficient | 0.308 | −0.275 | 0.541 | 0.037 | 0.007 | −0.142 | 0.528 | |
| Sig. | 0.214 | 0.270 | 0.020 | 0.883 | 0.978 | 0.575 | 0.024 | ||
| Disease_ Duration | Pearson’s Coefficient | 0.736 | −0.540 | 0.124 | −0.688 | −0.033 | −0.468 | 0.528 | |
| Sig. | 0.0001 | 0.021 | 0.624 | 0.002 | 0.898 | 0.050 | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colucci, F.; Stefanelli, S.; Contaldi, E.; Gozzi, A.; Marchetti, A.; Pugliatti, M.; Laudisi, M.; Antenucci, P.; Capone, J.G.; Gragnaniello, D.; et al. Cognition in Patients with Spinocerebellar Ataxia 1 (SCA1) and 2 (SCA2): A Neurophysiological and Neuropsychological Approach. J. Clin. Med. 2024, 13, 4880. https://doi.org/10.3390/jcm13164880
Colucci F, Stefanelli S, Contaldi E, Gozzi A, Marchetti A, Pugliatti M, Laudisi M, Antenucci P, Capone JG, Gragnaniello D, et al. Cognition in Patients with Spinocerebellar Ataxia 1 (SCA1) and 2 (SCA2): A Neurophysiological and Neuropsychological Approach. Journal of Clinical Medicine. 2024; 13(16):4880. https://doi.org/10.3390/jcm13164880
Chicago/Turabian StyleColucci, Fabiana, Sara Stefanelli, Elena Contaldi, Andrea Gozzi, Alessia Marchetti, Maura Pugliatti, Michele Laudisi, Pietro Antenucci, Jay Guido Capone, Daniela Gragnaniello, and et al. 2024. "Cognition in Patients with Spinocerebellar Ataxia 1 (SCA1) and 2 (SCA2): A Neurophysiological and Neuropsychological Approach" Journal of Clinical Medicine 13, no. 16: 4880. https://doi.org/10.3390/jcm13164880





